Abstract
Cocaine and alcohol are commonly co-abused for reasons that are incompletely understood. Laboratory animal studies have suggested that, although the reinforcing effects of low cocaine doses are increased following chronic ethanol (EtOH) consumption, acute EtOH administration does not consistently alter cocaine self-administration. The present study examined whether EtOH influences another abuse-related effect of cocaine: reinstatement of extinguished responding. Rhesus monkeys that had previously consumed EtOH for 8 weeks (2.0 g/kg over one hour, 5 days per week) self-administered up to 10 injections per day of 0.1 mg/kg cocaine under a fixed-interval 300-second schedule. After responding had been extinguished by substituting saline for cocaine, a pre-session infusion of saline or EtOH (0.5 or 1.0 g/kg, i.v. over 10 minutes) was followed by a “priming” injection of saline or cocaine (i.v.). Responding was increased significantly by priming injections of cocaine, but not saline. EtOH infusions neither reinstated behavior when administered prior to a saline prime nor altered the priming effect of cocaine. The inability of EtOH to alter the response-reinstating ability of cocaine provides further evidence for a lack of acute behavioral interactions between cocaine and ethanol.
Keywords: cocaine, ethanol, nonhuman primates, reinstatement, self-administration, rhesus monkey
Introduction
Estimates indicate that up to 90% of cocaine abusers also abuse alcohol (Grant & Harford, 1990; Helzer & Pryzbeck, 1988; Kampman et al. 2013). The pharmacological and behavioral mechanisms that drive co-abuse of these drugs is poorly understood. Laboratory studies of various endpoints in animals and humans have produced mixed results. For example, in humans, cocaine and EtOH can enhance each other’s cardiovascular effects, while effects on cognitive performance may be attenuated (Farre et al. 1993; Foltin and Fischman, 1988; Foltin et al. 1993; Higgins et al. 1992). In laboratory animals, synergistic effects have been observed on motor endpoints such as locomotion and rates of lever pressing under schedules of reinforcement (Aston-Jones et al. 1984; Masur et al. 1989; Misra et al. 1989; Rech et al. 1978). However, in nonhuman primate studies that model substance abuse, acute EtOH administration does not consistently alter the reinforcing effects of cocaine (Aspen and Winger, 1997; Winger et al. 2007; Czoty, 2015).
The present study was designed to examine cocaine-EtOH interactions using another paradigm—the extinction/reinstatement procedure (Gerber and Stretch, 1975). The extinction/reinstatement model represents a relatively simple, widely used model to study the behavioral pharmacology of cocaine. Under this procedure, cocaine self-administration is extinguished by substituting saline injections until rates of responding decrease to near zero. Responding can be reinstated by administering a priming injection of cocaine as well as by exposing the animal to a stimulus that has been associated with cocaine or to a stress-inducing stimulus (Bossert et al., 2013). One interpretation of the effects observed using this procedure is that the reinstatement of behavior is driven by the ability of the drug prime to produce cocaine-like interoceptive effects (e.g., Gerber and Stretch, 1975; Banks et al., 2007; Keiflin et al., 2008). By extension, another drug may alter cocaine prime-induced reinstatement through an interaction with cocaine’s discriminative stimulus effects. Thus, the present study can be viewed as an assessment of the similarity of discriminative stimulus effects of EtOH and cocaine.
Methods
Subjects
Three adult male rhesus monkeys (Macaca mulatta) served as subjects. All had experience self-administering cocaine and drinking EtOH in the home cage as described previously (Czoty et al., 2015). In that study, a cocaine self-administration dose-effect curve was determined in monkeys with extensive experience self-administering cocaine (several years). Next, self-administration sessions were suspended and monkeys were allowed to drink 2.0 g/kg (in a solution of 4% EtOH and 6% Tang in water) over one hour in the home cage 5–7 days per week. After ~8 weeks, cocaine self-administration was re-introduced 5 days per week. Note, however, that exposure to cocaine and EtOH was separated across the day such that no EtOH was on board when cocaine was available and vice versa. All procedures were approved by the Wake Forest University Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Self-administration procedure
While seated in a primate chair (Primate Products, Redwood City, CA) placed within a standard operant behavioral chamber (Med Associates, St. Albans, VT), monkeys pressed a response key under a 300-second fixed-interval (FI 300-sc) schedule of intravenous (IV) cocaine injection as described previously (see Czoty, 2015). Briefly, during illumination of a green stimulus light above a photo-optic switch, the first response recorded after 300 seconds elapsed produced a 10-second i.v. injection of 0.1 mg/kg cocaine, initiated a 60-s timeout period (TO) during which responses had no scheduled consequences and changed the illuminated stimulus light from green to red for 10 seconds. The chamber was dark for the duration of the TO. If, after the 300-s interval elapsed, 10 additional seconds elapsed without a response, the green light was extinguished and the 60-s TO was initiated. Sessions ended after 10 presentations of the FI 300-s schedule.
Once responding was deemed stable (three consecutive days with ±1 infusions and no upward or downward trend, saline was substituted for cocaine until responding declined (<2 infusions for 3 days. On the next day, monkeys received an i.v. infusion of saline, 0.5 g/kg EtOH or 1.0 g/kg EtOH over 10 minutes. Immediately thereafter, monkeys received an i.v. infusion of saline or cocaine (a “prime”) and were placed into the chamber for a self-administration session under extinction conditions. The priming dose of cocaine was the lowest tested dose that produced a significant increase in responding during the reinstatement test (0.1 mg/kg for two monkeys and 1.0 mg/kg in a third). No more than two tests were conducted before cocaine self-administration was re-established. Each infusion + prime combination was assessed at least twice in each monkey.
Drugs and data analysis
(−)-Cocaine HCl was obtained from the National Institute on Drug Abuse (Bethesda, MD). Ethanol (95% ethyl alcohol) was obtained from The Warner-Graham Company (Cockeysville, MD) and diluted each morning. The primary dependent variable was the number of infusions delivered. Data were analyzed with a two-way ANOVA with repeated measures using infusion and “prime” as factors, followed by post-hoc multiple comparisons testing using the Holm-Sidak method. Differences were considered significant at the 95% level of confidence (p < 0.05).
Results
When saline injections replaced cocaine and monkeys’ behavior stabilized, on average fewer than 2 injections were delivered. Administration of saline injection prior to the self-administration session did not affect the number of injections delivered, regardless of whether that injection had been preceded by an i.v. infusion of saline or 0.5 or 1.0 g/kg EtOH (Fig. 1; 3 bars above “sal”). Similarly, the ability of an injection of 0.1 mg/kg cocaine to significantly increase responding was not significantly affected by a preceding infusion of saline or EtOH (Fig. 1, bars above “coc”). Two-way, repeated-measures ANOVA indicated a significant main effect of cocaine vs. saline prime (F1,2=28.7, p<0.05) but no significant main effect of the pre-prime infusion, and no significant interaction. Post-hoc tests confirmed that responding after a cocaine prime was significantly different from responding after the saline prime for each infusion, and that responding after a saline prime or a cocaine prime did not differ according to the preceding infusion. Data for individual subjects are shown in Table 1. There appeared to be a dose-related decrease in overall behavior associated with EtOH infusion in R-1606, and perhaps in R-1505. Overall, however, individual subject data reflect the conclusion that cocaine increased responding that had been extinguished regardless of the preceding infusion.
Figure 1.
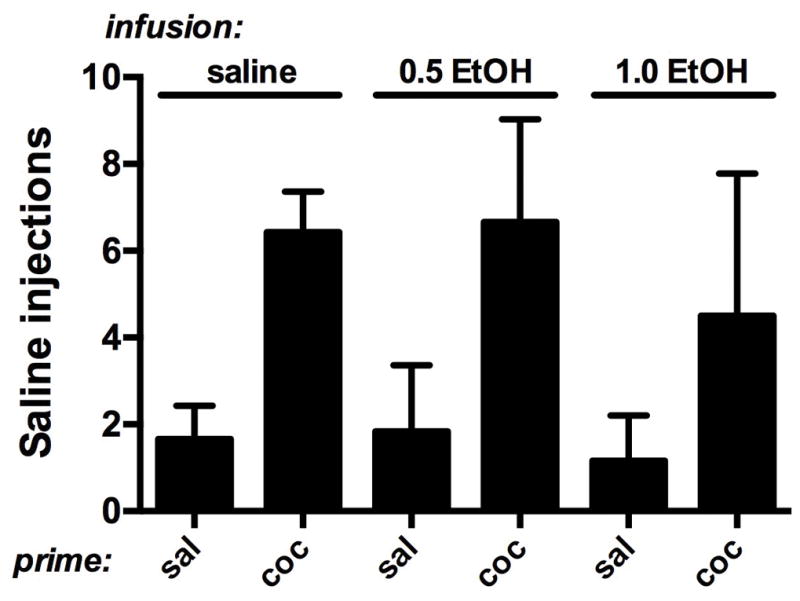
Effects of a 10-min infusion of saline or EtOH (0.5 or 1.0 g/kg) on reinstatement produced by priming injections of saline or cocaine. Horizontal axis: priming injection. Vertical axis: number of saline injections self-administered under reinstatement conditions. Bars represent mean + SEM (n=3).
Table 1.
Effects of a 10-min infusion of saline or EtOH (0.5 or 1.0 g/kg) on reinstatement produced by a priming injection of saline or cocaine. Data are mean (SD) number of self-administered saline injections (upper half) and responses (lower half) for individual subjects.
| INJECTIONS | |||
|---|---|---|---|
| infusion: | saline | 0.5 EtOH | 1.0 EtOH |
| prime: | saline, cocaine | saline, cocaine | saline, cocaine |
| subject | |||
| R-1605 | 1.5 (0.7), 7.5 (0.7) | 3.5 (0.7), 8.5 (2.1) | 1.5 (2.1), 4.0 (1.7) |
| R-1606 | 2.5 (0.7), 6.0 (0.0) | 0.5 (0.7), 4.0 (2.8) | 0.0 (0.0), 1.5 (0.7) |
| R-1758 | 1.0 (0.0), 5.8 (1.5) | 1.5 (0.7), 7.5 (0.7) | 2.0 (2.8), 8.0 (2.8) |
| RESPONSES | |||
| infusion: | saline | 0.5 EtOH | 1.0 EtOH |
| prime: | saline, cocaine | saline, cocaine | saline, cocaine |
| subject | |||
| R-1605 | 93.5 (36.1), 640 (236.2) | 191.0 (227.7), 822.5 (576.3) | 53.0 (70.7), 478.0 (303.2) |
| R-1606 | 28.5 (6.4), 250.5 (55.9) | 44.0 (62.2), 211.5 (150.6) | 2.5 (3.5), 177.5 (113.8) |
| R-1758 | 223.0 (171.1), 1471.8 (227.8) | 93.5 (24.7), 1469.5 (255.3) | 265.0 (318.2), 2576.5 (1866.1) |
Discussion
One noteworthy result of this experiment was that noncontingently administered EtOH did not reinstate extinguished responding that had previously been maintained by cocaine. These data extend findings in rodents (de Wit and Stewart, 1981; Wise et al., 1990) to monkeys with a history of cocaine and ethanol self-administration. Moreover, EtOH infusions did not alter the ability of cocaine to reinstate extinguished responding. The lack of an effect of an acute EtOH infusion on the behavioral effects of cocaine using the reinstatement/extinction procedure parallels its lack of effect on active cocaine self-administration in monkeys (Aspen and Winger, 1997; Winger et al. 2007; Czoty, 2015).
Although the extinction/reinstatement procedure has been used widely to model relapse to cocaine use (de Wit and Stewart, 1981; Bossert et al., 2013), the validity of this interpretation has been questioned (e.g. Katz and Higgins 2003; Epstein et al., 2006). For example, the critical method of decreasing behavior, extinction, does not occur in humans. Moreover, this view assumes that the non-contingent cocaine injection increases extinguished responding due to its reinforcing effects, although this hypothesis has not been supported when tested directly (Banks et al., 2007; Keiflin et al., 2008). Nonetheless, to the extent that the model recapitulates characteristics of relapse, the data suggest that alcohol consumption is not likely to lead an abstinent cocaine user to relapse. Moreover, the data suggest that the tendency of use of a small amount of cocaine in an abstinent person to lead to relapse would not be exacerbated by concurrent alcohol use.
As an alternative to the relapse interpretation of the extinction/reinstatement paradigm, recent evidence supports the historical view that the ability of a drug prime to increase extinguished behavior involves its production of cocaine-like interoceptive effects (e.g., Gerber and Stretch, 1975; Banks et al., 2007; Keiflin et al., 2008). From this perspective, the present data suggest that EtOH does not produce discriminative stimulus effects that overlap those of cocaine. This conclusion is largely supported by previous studies of EtOH substitution in animals trained to discriminate cocaine from vehicle and vice versa (Emmett-Oglesby, 1990; Grant et al., 1991; Schechter, 1994). Studies of drug combinations, however, have produced discrepant results. One study in rats trained to discriminate 10.0 mg/kg cocaine from saline reported that the cocaine-like discriminative stimulus effects of a lower cocaine dose (2.5 mg/kg), which produced 35% cocaine-appropriate responding when given alone, were increased to 71% by co-administering 0.6 g/kg EtOH (which, alone, produced 29% cocaine-appropriate responding; Schechter, 1994). However, in a subsequent study that more completely characterized EtOH/cocaine interactions, EtOH produced a maximum of 26% cocaine-appropriate responding and dose-dependently attenuated the cocaine-like discriminative stimulus effects of a range of cocaine doses (Gatch et al., 2003). The results reported here are more consistent with the latter study.
Regarding co-abuse of cocaine and EtOH, the results support the view that the frequent co-administration of alcohol and cocaine is not driven primarily by acute pharmacological interactions between the drugs. Other behavioral and pharmacological mechanisms should be investigated in future studies. Moreover, a focus on chronic treatment conditions likely will improve translation to the clinical condition, as chronic ethanol exposure may modify some aspects of cocaine’s reinforcing effects. For example, a previous study in rhesus monkeys (Czoty, 2015) showed that chronic EtOH consumption (2.0 g/kg per day over 8 weeks) in the absence of cocaine self-administration increased in self-administration of low doses of cocaine that previously lacked reinforcing effects. Attention should also be paid to alternative types of behavioral interaction. For example, it has been suggested that some individuals may use EtOH to mitigate the negative effects of cocaine binges such as insomnia, anxiety and agitation (e.g. Chitwood, 1985).
Acknowledgments
The author thanks Phillip Epperly for excellent technical assistance.
Source of funding: National Institute on Drug Abuse grant DA 21658
Footnotes
Conflicts of interest: None declared
References
- Aspen JM, Winger G. Ethanol effects on self-administration of alfentanil, cocaine and nomifensine in rhesus monkeys. Psychopharmacology. 1997;130:222–227. doi: 10.1007/s002130050232. [DOI] [PubMed] [Google Scholar]
- Aston-Jones S, Aston-Jones G, Koob GF. Cocaine antagonizes the anxiolytic effects of ethanol. Psychopharmacology. 1984;84:28–31. doi: 10.1007/BF00432019. [DOI] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Nader MA. The influence of reinforcing effects of cocaine on cocaine-induced increases in extinguished responding in cynomolgus monkeys. Psychopharmacology. 2007;192:449–456. doi: 10.1007/s00213-007-0732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DD. Patterns and consequences of cocaine use. NIDA Res Monogr. 1985;61:111–29. [PubMed] [Google Scholar]
- Czoty PW. Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 2015;153:278–285. doi: 10.1016/j.drugalcdep.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Emmett-Oglesby MW. Tolerance to the discriminative stimulus effects of ethanol. Behav Pharmacol. 1990;1:497–503. [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Cami J. Alcohol and cocaine interactions in humans. J Pharmacol Exp Ther. 1993;266:1364–1373. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Ethanol and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1988;31:877–883. doi: 10.1016/0091-3057(88)90399-1. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Youngblood BD, Forster MJ. Effects of ethanol on cocaine discrimination in rats. Pharmacol Biochem Behav. 2003;75:837–844. doi: 10.1016/s0091-3057(03)00158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: results of a national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Knisely JS, Tabakoff B, Barrett JE, Balster RL. Ethanol-like discriminative stimulus effects of non-competitive n-methyl-d-aspartate antagonists. Behav Pharmacol. 1991;2:87–95. [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol. 1988;49:219–224. doi: 10.15288/jsa.1988.49.219. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133:94–99. doi: 10.1016/j.drugalcdep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. 2003 [Google Scholar]
- Keiflin R, Isingrini E, Cador M. Cocaine-induced reinstatement on rats: evidence for a critical role of cocaine stimulus properties. Psychopharmacology. 2008;197:649–660. doi: 10.1007/s00213-008-1083-1. [DOI] [PubMed] [Google Scholar]
- Masur J, Souza-Formigoni ML, Pires ML. Increased stimulatory effect by the combined administration of cocaine and alcohol in mice. Alcohol. 1989;6:181–182. doi: 10.1016/0741-8329(89)90015-3. [DOI] [PubMed] [Google Scholar]
- Misra AL, Pontani RB, Vadlamani NL. Interactions of cocaine with barbital, pentobarbital and ethanol. Arch Int Pharmacodyn Ther. 1989;299:44–54. [PubMed] [Google Scholar]
- Rech RH, Vomachka MK, Rickert DE. Interactions between depressants (alcohol-type) and stimulants (amphetamine-type) Pharmacol Biochem Behav. 1978;8:143–151. doi: 10.1016/0091-3057(78)90331-3. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Discriminative stimulus effects of cocaethylene in rats trained to discriminate cocaine or ethanol. Life Sci. 1994;55:1033–1043. doi: 10.1016/0024-3205(94)00638-5. [DOI] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR. Modification of ethanol’s reinforcing effectiveness in rhesus monkeys by cocaine, flunitrazepam, or gamma-hydroxybutyrate. Psychopharmacology. 2007;193:587–598. doi: 10.1007/s00213-007-0809-9. [DOI] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology. 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]


