Abstract
Background
Ragweed is a major cause of seasonal allergy, affecting millions of people worldwide. Several allergens have been defined based on IgE reactivity, but their relative immunogenicity in terms of T cell responses has not been studied.
Objective
We comprehensively characterized T cell responses from atopic, ragweed-allergic subjects to Amb a 1, Amb a 3, Amb a 4, Amb a 5, Amb a 6, Amb a 8, Amb a 9, Amb a 10, Amb a 11, and Amb p 5, and examined their correlation with serological reactivity and sequence conservation in other allergens.
Methods
Peripheral blood mononuclear cells (PBMCs) from donors positive for IgE toward ragweed extracts after in vitro expansion for secretion of IL-5 (a representative Th2 cytokine) and IFNγ (Th1) in response to a panel of overlapping peptides spanning the above listed allergens.
Results
Three previously identified dominant T cell epitopes (Amb a 1 176–191, 200–215, and 344–359) were confirmed and three novel dominant epitopes (Amb a 1 280–295, 304–319, and 320–335) were identified. Amb a 1, the dominant IgE allergen, was also the dominant T cell allergen, but dominance patterns for T cell and IgE responses for the other ragweed allergens did not correlate. Dominance for T cell responses correlated with conservation of ragweed epitopes with sequences of other well-known allergens.
Conclusion and clinical relevance
These results provide the first assessment of the hierarchy of T cell reactivity in ragweed allergens, which is distinct from that observed for IgE reactivity and influenced by T cell epitope sequence conservation. The results suggest that ragweed allergens associated with lesser IgE reactivity and significant T cell reactivity may be targeted for T cell immunotherapy, and further support the development of immunotherapies against epitopes conserved across species to generate broad reactivity against many common allergens.
INTRODUCTION
Ragweed pollen, one of the most frequent inhaled outdoor allergens worldwide and a potent inducer of seasonal asthma and rhinitis, is widely distributed throughout North America, including most of the United States and Canada [1]. Ragweed is also found in Europe, with the highest levels present in Italy, France, Croatia, and Hungary [2, 3]. Its spread and pollen release are dependent upon climate [4, 5]. Major allergens in ragweed are associated with several species of the Artemisia genus. Ambrosia artemisiifolia (Amb a; common ragweed) is the species most prevalently associated with ragweed allergies and is allergenic at very low pollen concentrations [5]. To date, 14 Amb allergens have been described. The Amb a 1 allergen has been described as particularly dominant; up to 97% of allergic subjects test positive for IgE to this allergen [6–8] while the other Amb allergens are associated with lower levels of IgE reactivity [7, 9–12].
In contrast to the relatively large amount of data available in terms of serological reactivity, surprisingly little is known regarding T cell reactivity to ragweed allergens. Previous studies have described three main Amb a 1 T cell epitopes [13]. However, the study was limited to only epitopes of the Amb a 1.3 isoform, one of twelve Amb a 1 isoforms, and the epitope identification screen utilized proliferation assays and peptides of 12 residues in size. As the most common size of class II epitopes is 13–15 amino acids, some epitopes may have been missed. A single T cell epitope has been described for Amb a 3 [14] and three for Amb t 5 [15].
As mentioned, the available data indicates that Amb a 1 is dominant in terms of IgE titers, but no data is available in terms of the relative levels of T cell reactivity of Amb a 1 and other Amb allergens. Several recent studies have shown that T cell and IgE reactivity only loosely correlate, and the most dominant allergens at the level of T cell reactivity are not necessarily predictable on the basis of IgE reactivity [15–17]. To be able to consistently predict which allergens are dominant for T cell responses would be of interest in the context of the development of immunotherapeutic regimens, since modulation of T cell responses in AIT has been demonstrated and may indicate successful AIT [18–20].
The molecular basis for immunodominance at either the T cell or IgE level is not clear, but several hypotheses have been proposed, including interaction with innate immunity pathways [21] and release patterns from pollen particles [17]. No consistent biological function is associated with allergenicity in general [22, 23]. Similarly, ragweed allergens are associated with diverse biological functions. Amb a 1 is a pectate lyase, while other allergens are plastocyanins (Amb a 3, Amb a 7), actin-binding (Amb a 8, Amb t 8) or calcium-binding proteins (Amb a 9, Amb a 10), cysteine proteases (Amb a 11), defensins (Amb a 4), and function in lipid transfer (Amb a 6).
Another recent hypothesis hinges on the observation that T cell responses are influenced by sequence variation and conservation [24–26]. Furthermore, data from our laboratory indicates that conservation amongst other allergens is important in determining allergenicity, and sequence conservation in several related allergen sequences seems to increase immunogenic potential [27]. As mentioned, the Amb a 1 allergen exists in several different isoforms [22, 23], yet only a few have thus far been analyzed at the level of T cell specificity. Other ragweed species such as Ambrosia psilostachya (Amb p; western ragweed) and Ambrosia trifida (Amb t; giant ragweed) are also associated with allergic reactions; extensive cross-reactivity exists between Amb a allergens and those encoded by other species such as mugwort and cypress [1, 8, 28].
Thus, a comprehensive characterization of the epitopes recognized by human T cells in the context of ragweed allergy is still lacking. The precise definition and exact mapping of the epitopes involved, antigens of origin, patterns of associated T helper cell responses and potential impact of sequence conservation would contribute to a better understanding of pathogenic immune responses and the mechanisms associated with immunodominance in allergen responses. Accordingly, in the present study we undertook a comprehensive analysis of the T cell response to Amb a ragweed allergens.
METHODS
Study population
Ethical approval for this study was obtained from the LJI institutional review board (protocol number VD-112-0216). PBMCs and plasma were obtained from blood donors from San Diego, CA and Dallas, TX. Both common and western ragweed-specific IgE titers, as well as those against other common plant allergens (see Supplemental Table 2) were determined from plasma using the ImmunoCAP system (Thermo Fisher, Uppsala, Sweden). Those with IgE titers of 0.35 kUA/L or more were considered to have an atopic background and were therefore included, yielding a cohort of 25 donors (21 from San Diego and 4 from Dallas). In this group, 13 were female, 8 male, and 4 did not include gender information because of anonymous donors (52%, 32%, 16%), and the average age was 37 years (range, 18–62). The donors were 85% Caucasian and 15% Hispanic or Latino. Serum IgE specific for ragweed pollen extracts was 2.3 kU/L (median), with a range of 0.55 to 59.9 kUA/L (Supplemental Table 1). PBMCs were isolated from whole blood by density gradient centrifugation according to manufacturers’ instructions (Ficoll-Hypaque, Amersham Biosciences, Uppsala, Sweden).
Selection of peptides from ragweed allergen sequences
Sequences of all known Amb a and Amb p isoforms were obtained from the WHO/IUIS Allergen Nomenclature database (www.allergen.org) [22]. Sets of peptides of 16 amino acids in length, overlapping by 8 residues, were generated to cover the entire allergen protein sequences. As Amb a 1 is comprised of 12 known isoforms, all peptides existing in the 1.0101 isoform were synthesized. Overall, a total of 279 peptides were assembled in 47 pools of 10 peptides, on average, and screened for their immunogenicity.
Peptide synthesis
Peptides were purchased from A and A (San Diego, CA) as crude material on a small (1 mg) scale. Individual peptides were resuspended in DMSO at a final concentration of 0.25% (v/v) DMSO (corresponding to the percentage of DMSO in the pools/peptides) as a control.
Stimulation and expansion of ragweed-specific T cells
For in vitro expansion of ragweed-specific T cells, PBMCs of ragweed-sensitized individuals were stimulated with a 1:1 mixture of common (ALK-Abello A/S, Horsholm, Denmark) and western (Greer, Lenoir, NC) ragweed extracts at 2 μg/mL. Cells were cultured in RPMI 1640 supplemented with 5% human AB serum in 24 well plates (BD Biosciences, San Diego, CA) at a density of 2 × 106/ml and incubated at 37 °C. IL-2 (10 U/mL, Ebioscience, San Diego, USA) was added every 3 days after initial stimulation. Cells were harvested on day 14 and screened for IFNγ and IL-5 production by ELISPOT.
Dual ELISPOT assays
The production of IFNγ and IL-5 from cultured PBMCs in response to antigenic stimulation was assessed by dual ELISPOT assays as described previously [29]. Flat-bottom 96-well plates were coated with 5 μg/mL of both anti-human IFNγ (Clone 1-D1K; Mabtech, Cincinnati, OH) and anti-human IL-5 (clone TRFK5; Mabtech). Cells were then incubated at a density of 1 × 105 cells/well with either peptide pools (5 μg/mL), individual peptides (10 μg/mL), or common and western ragweed extracts (2 μg/mL each), PHA (10 μg/mL), or medium containing 0.25% (v/v) DMSO (corresponding to the percentage of DMSO in the pools/peptides) as a control. Spot-forming cells (SFC) were counted by computer-assisted image analysis (KS-ELISPOT reader, Zeiss, Munich, Germany). Each assay was performed in triplicate. Student’s t-test, using the mean of triplicate values of the response against the extract, pool, or individual peptides when compared to the response against medium control, was applied to calculate statistical significance. As previously described, criteria for positivity were 20 SFCs per 106 PBMCs, P <0.05, and a stimulation index >2 [30]. Positive pools were deconvoluted to identify the individual epitopes inducing the response. Peptide pools that were associated with positive responses on a given day were deconvoluted on the following day(s) by individually testing each peptide contained in the pool and determining which were associated with a positive response. Whenever possible the same cell cultures used to test the peptide pools were used to test the individual peptides. Rarely, if not enough cells were available, peptide pools were deconvoluted utilizing cells from additional frozen aliquots.
HLA typing and restriction
HLA typing for Class I (HLA A, B, and C) and Class II (DQA1; DQB1, DRB1, 3, 4, 5; DPB1) was performed by a laboratory accredited by the American Society for Histocompatibility and Immunogenetics at Murdoch University (Western Australia) using locus-specific PCR amplification on genomic DNA. The assay was adapted from previously published protocol for barcoded PCR with modifications to the primer sequences [31]. Briefly, 11 amplifications per sample were set up with primers for a given patient sample tailed with a specific barcode tag sequence. Amplified products were quantitated, normalized and pooled by subject and up to 48 subjects were pooled. The pooled and normalised PCR reactions were purified using Agencourt AMPure XP beads (Beckman Coulter). Samples were prepared for sequencing on either a FLX 454 or Illumina MiSeq using the manufacturer’s standard library preparation protocol. Libraries were quantified using Kapa universal QPCR library quantification kits (Kapa Biosystems). Sequencing was performed using either a Roche 454 FLX+ sequencer with titanium chemistry (Roche) or an Illumina MiSeq using a 2 × 300 paired-end chemistry kit (Illumina). Reads were quality-filtered and passed through a proprietary allele calling algorithm and analysis pipeline using the latest IMGT HLA allele database as a reference [32]. Potential HLA-epitope restriction odds ratios and relative frequencies were calculated using the RATE program [33].
Transcriptome homology analysis
Conservation of each synthesized Amb peptide was calculated against the proteomes of 10 additional pollens (Pla p, Phl p, Ant o, Lol p, Poa p, Cyn d, Bet v, Fra e, Ole e, and Que e). Putative transcriptomes of these ten pollen species were obtained by transcriptomes as described earlier [27, 34]. Briefly, RNA was isolated from pollen and sequenced on a HiSeq 2500 Sequencer (Illumina, San Diego, CA). After processing, the reads were assembled de novo into transcripts using Trinity (r2012-10-05). The ten pollens in our study were selected based on the availability of de novo (without a reference genome) transcriptome assemblies. Based on these transcriptome assemblies, ORFs in all 6 reading frames that were long enough to encode at least 15 amino acids were included to form the putative proteome for each species. All ORFs in all 6 reading frames that were 15 aa or longer were included in the proteome for each species. In addition, all known allergen sequences from each of these species were retrieved from the IUIS database [22]. Using an in-house script, each Amb peptide was compared against all possible peptides in each proteome for its maximal sequence identity. All allergen peptides with one or two substitutions compared to the Amb peptides were recorded, and the peptide with the lowest number of substitutions was retained as the best match. A p value for the difference in average peptide conservation among the two Amb allergen groups (reactive and nonreactive) was obtained using a two-sided Student’s t test. P values for conservation among the three groups of peptides (non, weak, and dominant epitopes) across transcriptomes were obtained using a Mann-Whitney test, followed by Benjamini-Hochberg adjustment for multiple testing.
RESULTS
Overall T cell and IgE reactivity to ragweed extract in sensitized donors
To assess T cell responses, we used a strategy previously utilized to study various common allergens including timothy grass, house dust mite and cockroach allergens [15, 34–36]. PBMCs from each donor were stimulated in vitro with a mixture of common and western ragweed extract. After 14 days, the extract mixture was tested using ELISPOT assays for its capacity to elicit IFNγ and/or IL-5 responses. IL-5 and IFNγ were chosen as representative of Th2 and Th1 cytokines, respectively. Vigorous responses to the ragweed extracts used as positive control were detected in 23/25 donors. Total IL-5 and IFNγ responses are summarized in Supplemental Table 1.
Plasma from the same donors was also assayed for reactivity to a panel of eleven grass and tree allergens (Supplemental Table 2). The percentage of these pollens to which each donor displayed IgE reactivity (polysensitization) is shown for each donor in Supplemental Table 1. No significant correlation between overall response magnitude to ragweed extract and degree of polysensitization was detected (data not shown).
Breadth and immunodominance of T cell responses to Amb a 1 allergen
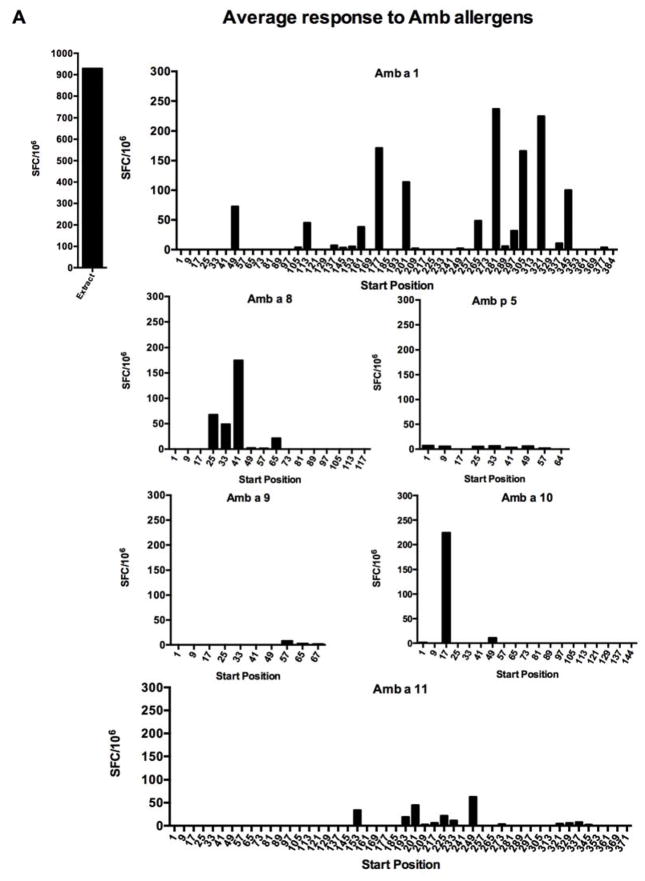
Next, we characterized T cell reactivity of the donor panel to the Amb a 1 allergen at the epitope level. A panel of 16-mer peptides, overlapping by 8 residues and spanning the Amb a 1 sequence, was generated; in instances where more than one isoform sequence was identified, the most frequent isoform was considered. These peptides were assembled in pools of 10, on average, and screened for their T cell-activating capacity in IFNγ and IL-5 ELISPOT assays; cells were first stimulated for 14 days with ragweed extract. Next, positive pools were deconvoluted to identify individual epitopes eliciting responses. The average and individual reactivity (SFC) to each peptide detected in the course of these experiments is shown in Figure 1.
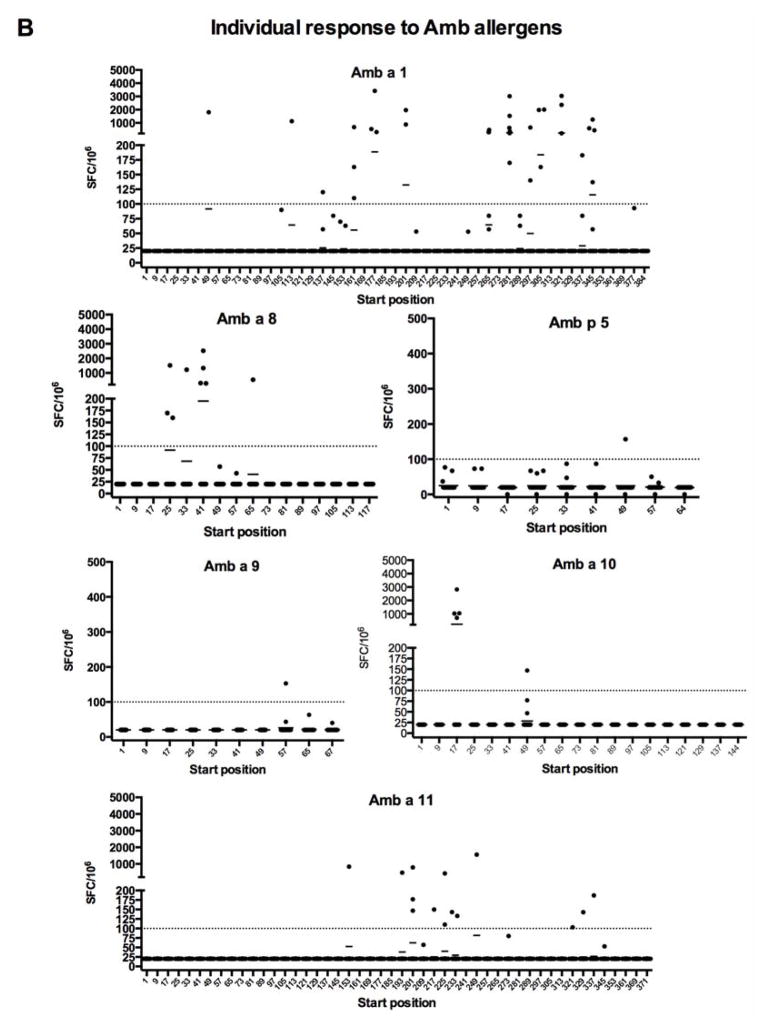
Figure 1. Average SFC response (total SFC/number of donors tested, n = 25) to individual 16-mer peptides spanning Amb allergens.
PBMCs from ragweed-sensitized individuals were stimulated with ragweed extract and, after 14 days expansion with IL-2, production of IFNγ and IL-5 was assessed by dual ELISPOT. A, average total SFC detected for each peptide from each antigen; B, individual responses of each donor. The detection threshold was 20 SFC/106.
Average reactivity at an arbitrary threshold of 100 SFCs (where IFNγ and IL-5-positive spots were added together) or more was detected for 6 peptides (Amb a 1 177–192, 201-216, 281–296, 305–320, 321–336, and 345–360). Three of these peptides roughly correspond to three dominant epitopes reported by Jahn-Schmid and coworkers (Amb a 1 178–189, 199–216, and 343–357) and three additional epitopes, Amb a 1 281–296, 305–320, and 321–336, represent novel epitopes [13]. The corresponding overall frequency of donor recognition of each peptide, shown in Supplemental Figure 1, reveals a hierarchy of immunodominance similar to but clearly distinct from the one observed for response magnitude. More specifically, two of the three Amb a 1 peptides recognized in 15% or more of the donors were also recognized with magnitude above 100 SFC.
Reactivity against other Amb a and Amb p allergens
In parallel, to determine whether Amb a 1 is dominant not only for IgE responses but also T cell responses, we assayed in the same donors and cultures reactivity to similar panels of peptides spanning the Amb a 3, Amb a 4, Amb a 5, Amb a 6, Amb a 8, Amb a 9, Amb a 10, Amb a 11, and Amb p 5 allergens. Amb p 5 was included because it is frequently described in the literature and therefore of potential relevance.
The pattern of reactivity detected is also shown in Figure 1, which depicts the average total SFC detected for each peptide from each antigen. Reactivity above 100 SFC per 106 cells was detected for the three allergens Amb a 1, Amb a 8, and Amb a 10. Low-level reactivity against several peptides was detected in the case of the Amb p 5, Amb a 9, and Amb a 11 allergens. Strikingly, we found that Amb a 3, Amb a 4 and Amb a 6 were totally nonreactive (data not shown). The reactivity to each peptide tested can be found in the Immune Epitope Database (submission ID in progress; www.iedb.org). The corresponding overall frequencies of donor recognition are also shown in Supplemental Figure 1. As seen for Amb a 1, analysis of response frequencies reveals a hierarchy of immunodominance similar to the one observed for response magnitude. That is, the only Amb a 8 and Amb a 10 peptides recognized in 15% or more of the donors corresponded to the only peptides for each antigen recognized at a magnitude above 100 SFC.
Relationship between T cell reactivity and previously reported IgE reactivity
The above results indicate that Amb a 1 is dominant for both IgE and T cell responses. To further examine the correlation between IgE reactivity and T cell reactivity, we tabulated the total T cell reactivity detected against the various allergens (defined as the sum of SFC for all donors and all peptides for each allergen tested in Figure 1; Table 1). At the same time, we also tabulated IgE reactivity from published reports [7, 9–12, 37].
Table 1.
Allergens ranked by T cell response, IgE reactivity, and conservation. IgE response values based on the literature [3–8].
| Allergen | T cell response (total SFC) | T cell rank | IgE % response | IgE rank | Mean % conserved peptides | Conservation rank |
|---|---|---|---|---|---|---|
| Amb a 8 | 6,874 | 2 | 50% | 4 | 61% | 1 |
| Amb a 9 | 300 | 5 | 0% | 8 | 33% | 2 |
| Amb a 1 | 32,288 | 1 | 97% | 1 | 30% | 3 |
| Amb a 10 | 5,918 | 3 | 10% | 7 | 21% | 4 |
| Amb a 11 | 5,571 | 4 | 66% | 2 | 15% | 5 |
| Amb a 3 | 0 | 6 | 51% | 3 | 13% | 6 |
| Amb a 5 | 0 | 6 | 17% | 6 | 9% | 7 |
| Amb a 4 | 0 | 6 | 0% | 8 | 1% | 8 |
| Amb a 6 | 0 | 6 | 21% | 5 | 0% | 9 |
Three out of the four antigens that elicit the greatest T cell reactivity (Amb a 1, Amb a 8 and Amb a 11) are among the four most IgE-reactive allergens. Interestingly, Amb a 3, despite being associated with IgE reactivity in many donors, is not recognized by T cells, while Amb a 10, despite being recognized by T cells, was infrequently IgE-reactive. Although IgE reactivity to Amb a 6 has previously been observed in 21% of patients, it was not recognized by T cells. Amb a 4 was nonreactive for both T cells and IgE. This trend towards a correlation between IgE and T cell reactivity was not statistically significant, with a Spearman rho value of 0.42 and a two-tailed p value of 0.25.
T cell reactivity of Amb isoform sequence variants
As mentioned above, we synthesized and tested all sequences found in at least 25% of the isoforms retrieved from public databases, corresponding to a total of 104 peptides and 49 sets of two or more isoform sequences. When these sequences were tested, we found that most were either uniformly negative, in that none of the isoform sequences was positive in any donor, or associated with relatively weak responses. Table 2 shows the seven pairs of isoform sequences where at least one of the isoforms was recognized with an average of > 100 SFC, along with their relative frequency and pattern of reactivity.
Table 2.
Frequency and reactivity of isoform pairs having reactivity >100 SFC/106 PBMCs.
| Allergen | Start position | Relative frequency* | Sequence | Responding donors | Mean SFC |
|---|---|---|---|---|---|
| Amb a 1 | 177 | 0.58 | PGGMIKSNDGPPILRQ | 12% | 171 |
| Amb a 1 | 177 | 0.08 | PGGLIKSNDGPAAPRQ | 8% | 103 |
|
| |||||
| Amb a 1 | 201 | 0.58 | VAGSSQIWIDHCSLSK | 8% | 114 |
| Amb a 1 | 201 | 0.08 | ISGSSQIWIDHCSLSK | 24% | 69 |
|
| |||||
| Amb a 1 | 281 | 0.58 | FGFFQVVNNNYDRWGT | 24% | 237 |
| Amb a 1 | 281 | 0.08 | HGFFQVVNNNYDKWGS | 24% | 161 |
|
| |||||
| Amb a 1 | 305 | 0.42 | PTILCQGNRFLAPDDQ | 12% | 166 |
| Amb a 1 | 305 | 0.08 | PTILSQGNRFCAPDER | 20% | 200 |
|
| |||||
| Amb a 1 | 321 | 0.42 | IKKNVLARTGTGAAES | 12% | 225 |
| Amb a 1 | 321 | 0.08 | SKKNVLGRHGEAAAES | 12% | 181 |
|
| |||||
| Amb a 1 | 345 | 0.42 | DKDLLENGAIFVTSGS | 20% | 100 |
| Amb a 1 | 345 | 0.08 | NKDVLENGAIFVASGV | 24% | 126 |
|
| |||||
| Amb a 8 | 41 | 0.5 | PEFKPDEINAIIKEFS | 12% | 175 |
| Amb a 8 | 41 | 0.5 | PEFKPDEINAIIKEFD | 8% | 167 |
Relative frequency indicates the frequency of that particular sequence within the sequences of the 12 isoforms from the WHO/IUIS Allergen Nomenclature database (www.allergen.org).
In general, the reactivity of these epitopes was not greatly affected by sequence variation associated with isoform variants. In 6 of the 7 pairs, the reactivity of the different isoforms varied within a two-fold range. No consistent relationship between relative frequency and frequency of response was apparent (data not shown).
Immunodominance and polarization in Amb epitope responses
Relatively few epitope regions accounted for a large fraction of the total response. Table 3 lists the main antigenic epitope regions defined for each allergen, including any region accounting for 1% or more of the total reactivity to the corresponding antigen. For each epitope the percentage of donors responding is tabulated, along with the percentage of the average SFC response accounted for by each epitope. In summary, 17 epitopes are sufficient to account for 90% or more of the reactivity to the Amb antigens tested.
Table 3.
Main antigenic T cell epitope regions of ragweed allergens
| Allergen | Start position | Sequence | Responding donors (%) | Mean SFC | IL-5/IFNγ ratio* | % Overall SFC | Significant HLA associations | p value |
|---|---|---|---|---|---|---|---|---|
| Amb a 1 | 49 | YNIIDGCWRGKADWAE | 4 | 72.4 | +++ | 3.5% | N/A | - |
| Amb a 1 | 113 | QNRPLWIIFKNDMVIN | 4 | 45.2 | 2.1 | 2.2% | N/A | - |
| Amb a 1 | 161 | KNIIIHNINIHDVKVL | 12 | 38.2 | 14.2 | 1.8% | DRB1*03:01 & DRB1*08:01 &DRB1*08:03 | 0.015 |
| Amb a 1 | 177 | PGGMIKSNDGPPILRQ | 12 | 171.1 | 2.4 | 8.3% | DRB1*08:01 & (DRB1*1501 or DRB5*01:01) | 0.015 |
| Amb a 1 | 201 | VAGSSQIWIDHCSLSK | 8 | 113.9 | 4.2 | 5.5% | N/A | - |
| Amb a 1 | 265 | FNMFTDNVDQRMPRCR | 20 | 48.5 | 2.6 | 2.3% | DRB1*03:01 & DRB1*04:05 | 0.021 |
| Amb a 1 | 281 | FGFFQVVNNNYDRWGT | 24 | 236.5 | 5.0 | 11.4% | DRB1*08:02 & DRB1*11:11 & DRB1*13:03 & DRB08:03 | 0.001 |
| Amb a 1 | 305 | PTILCQGNRFLAPDDQ | 12 | 166.2 | 4.5 | 8.0% | DRB1*15:01 & DRB5*01:01 | 0.009 |
| DRB1*11:01 & DRB1*11:11 & | ||||||||
| Amb a 1 | 321 | IKKNVLARTGTGAAES | 12 | 224.8 | 1.9 | 10.8% | DRB1*13:02 | - |
| Amb a 1 | 345 | DKDLLENGAIFVTSGS | 20 | 99.6 | 5.2 | 4.8% | DRB1*11:04 & DRB1*13:02 & DQB1*06:04 & (DRB1*04:04 or DRB1*08:01 or DRB3*03:01) | 0.028 |
| Amb a 8 | 25 | AIFGTDGAVWAKSGSF | 8 | 67.7 | 2.9 | 3.2% | DQB1*0301 | 0.04 |
| Amb a 8 | 33 | VWAKSGSFPEFKPDEI | 4 | 49.0 | 9.0 | 2.4% | N/A | - |
| Amb a 8 | 41 | PEFKPDEINAIIKEFD | 16 | 167.2 | 18.3 | 8.1% | DRB1*03:01 | 0.003 |
| Amb a 10 | 17 | VTKIFNRFDTNGDGQI | 16 | 224.3 | 3.1 | 10.8% | DRB1*04:05 | 0.008 |
| Amb a 11 | 153 | GSCWAFAAVVALEGIN | 4 | 33.4 | 4.3 | 1.6% | N/A | - |
| Amb a 11 | 201 | AFTYVIKHGGIAPEAS | 12 | 44.6 | 1.4 | 2.1% | DRB1*04:04 & DRB1*08:02 & (DRB1*11:01 or DRB1*11:04) | 0.002 |
| Amb a 11 | 249 | EALRKAVAHQPVATGI | 4 | 62.5 | 9.9 | 3.0% | N/A | - |
+++ indicates that no IFNγ was detected
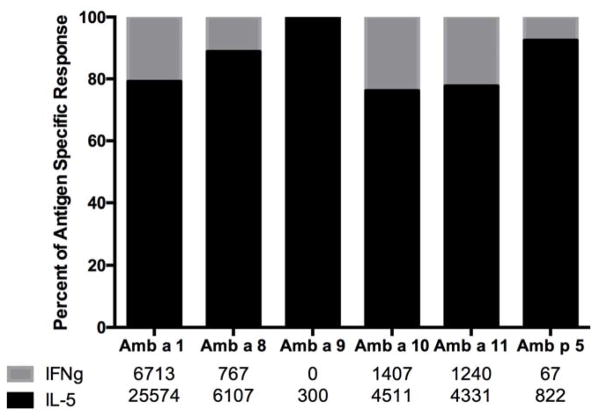
Next, polarization of Th responses was determined from the above ELISPOT results. As expected, because of the atopic status of the donors, Th2 responses dominated Th1 responses. As shown in Figure 2, regardless of the allergen, a similar pattern of IL-5 dominance was observed. Further analysis of responses to each of the main antigenic regions revealed similar patterns of polarization regardless of the antigenic region considered (see Table 3).
Figure 2. IL-5/IFNγ balance for each Amb antigen.
Pooled data from 25 donors are shown as percentage of total antigen-specific response (SFC) attributable to IL-5 (black) and IFNγ (gray).
Inferred HLA restriction of dominant epitopes
A previous report described several Amb a 1 epitopes with a rather heterogeneous set of HLA restrictions [13]. That is, while DR-restricted responses were the most prevalent, DQ and DP restrictions were also identified, and some epitopes were also restricted by multiple loci. A similar pattern of restriction was reported in the context of responses to timothy grass (TG; Phl p allergens) [29]. Here, to identify potential HLA restrictions, and to facilitate the design and use of HLA tetramers, we used the RATE program [33] to calculate the relative frequency and significance of association between all the epitopes/regions and HLA alleles (or combinations thereof) expressed in responding donors.
A detailed account of the results of the RATE analysis is shown in Supplemental Table 3, which gives the number of donors that responded (R+) or did not respond (R−) to a given peptide, and expressed (A+) or did not express the given HLA(s) (A−). For example, the Amb a 1 161 epitope has 100% of the responders express the HLA molecules DRB1*08:01, DRB1*03:01, and DRB1*08:03, while only 3/18 (17%) of the non-responders express the same HLAs (p=0.015).
This analysis allowed inference of potential restrictions for a majority (11/17) of the main epitopes, while for the remaining six no significant HLA association was detected. These inferred epitope-HLA restrictions are listed in Table 3. Of the eleven cases where restrictions could be inferred, eight were promiscuous, i.e. the epitope is inferred to be potentially restricted by multiple HLAs, thus confirming and extending the previous results [9, 23].
Ragweed allergens differ in the degree of conservation across other allergen species
Conservation amongst other allergens is important in determining allergenicity [24] and earlier reports have demonstrated cross-reactivity at the cellular and serological level between ragweed and mugwort epitopes [1, 28]. Here we compared the Amb peptides to the proteomes of 10 additional relatively unrelated common allergens.
A peptide was considered to be conserved if it was present in the proteome of another allergen’s sequence with two or fewer substitutions (87.5% sequence identity). Table 4 indicates, for each allergen species, the percentage of synthesized Amb peptides that are conserved. Amb a 8, followed by Amb a 9 and Amb a 1, were the most highly conserved. This would indicate a trend or correlation with the pattern of dominance for T cell responses, which showed that Amb a 1 and Amb a 8 were the most dominant T cell allergens.
Table 4.
Conservation of ragweed allergen peptides across phylogenetically distant plant allergens. Numbers represent percentages.
| Allergen | No. of peptides | Amb p | Pla p | Phl p | Ant o | Lol p | Poa p | Cyn d | Bet v | Frae | Olee | Que a | Mean % conserved |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amb a 1 | 104 | 75 | 16 | 56 | 54 | 19 | 12 | 15 | 2 | 20 | 35 | 25 | 30 |
| Amb a 3 | 12 | 25 | 33 | 0 | 25 | 0 | 0 | 0 | 0 | 25 | 33 | 0 | 13 |
| Amb a 4 | 20 | 0 | 0 | 5 | 0 | 0 | 5 | 5 | 0 | 0 | 0 | 0 | 1 |
| Amb a 5 | 5 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Amb a 6 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amb a 8 | 26 | 92 | 46 | 85 | 100 | 42 | 27 | 46 | 38 | 69 | 81 | 46 | 61 |
| Amb a 9 | 13 | 100 | 15 | 23 | 100 | 15 | 0 | 0 | 0 | 15 | 77 | 15 | 33 |
| Amb a 10 | 19 | 100 | 0 | 5 | 95 | 0 | 0 | 5 | 5 | 5 | 5 | 5 | 21 |
| Amb a 11 | 48 | 100 | 0 | 0 | 40 | 0 | 0 | 4 | 4 | 4 | 17 | 0 | 15 |
| Amb p 5 | 18 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
Numbers given for each allergen species indicate the percentage of peptides conserved with the Amb a allergen. A peptide was considered to be conserved in a related species if a homolog was identified with 2 or fewer mismatches.
Ragweed epitope conservation across other allergen species
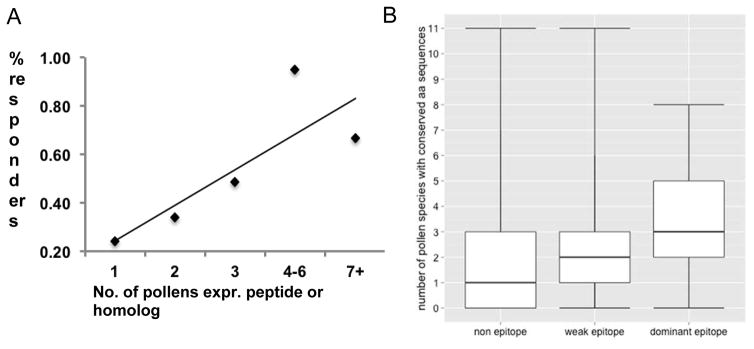
We next undertook a computational analysis assessing conservation of the sequences corresponding to dominant T cell epitopes in the transcriptome of the other allergens. Specifically, we examined the relationship between the average number of responders, defined as the average number of individuals with a positive IL-5 or IFNγ response (as described in the Methods section on dual ELISPOT assays), and the number of species in which the peptide was conserved. Figure 3a shows a positive correlation between these two variables, further supporting the hypothesis that sequence conservation can be associated with increased T cell allergenicity.
Figure 3. T cell response correlates with epitope conservation.
A. Proportion of donors with T cell responses as a function of peptide conservation. Peptides were binned into 5 similarly sized groups according to the number of pollens expressing a homolog. B. Epitopes are significantly more conserved than non-epitopes across transcriptomes of common allergens.
Next, we classified the Amb peptides tested in this study as non-epitopes (never recognized in any donor), weak epitopes (recognized in one or two donors), or dominant epitopes (recognized in three or more donors, corresponding to the epitopes from Table 3) (classification of each peptide is in Supplemental Table 4). Figure 3b shows that weak and dominant epitopes, on average, tend to be more conserved in the proteomes of more allergen species than non-epitopes. Removing weak epitopes from the comparison, the difference in conservation across allergen species between non-epitopes (median=1) and dominant epitopes (median=3) achieves statistical significance with a Benjamini-Hochberg-adjusted P value of 0.002 by a Mann-Whitney test.
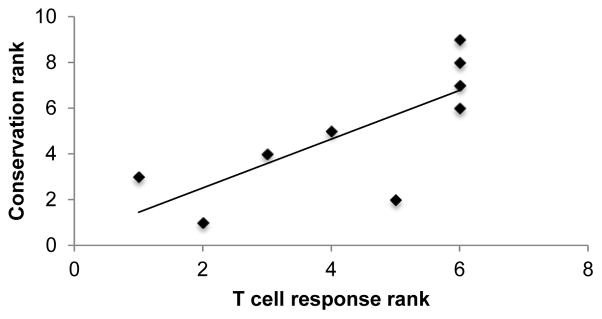
To examine whether the degree of allergen conservation correlated with T cell reactivity (Table 1), we again used the Spearman rho test. We found that allergen conservation significantly correlated with T cell reactivity with an R value of 0.82 and a two-tailed p value of 0.007 (Figure 4). Thus, we conclude that allergen conservation is more strongly correlated than IgE reactivity with immunodominance for ragweed-specific T cell responses.
Figure 4. Correlation between T cell reactivity and allergen conservation rankings.
Each of the Amb allergens were ranked according to their T cell response (total IL-5 + IFNγ SFC among all donors) as well as their degree of conservation among all allergen species studied. A peptide was considered to be conserved in a related species if a homolog was identified with 2 or fewer mismatches. The mean conservation over all species examined is plotted here and reported in
DISCUSSION
In the present study, we investigated patterns of immunodominance in T cell recognition to several ragweed allergens. For the first time we compared side-by-side the reactivity of human T cells to various ragweed allergens, and compared T cell reactivity to the known prevalence of IgE responses against the same allergens. We further mapped T cell epitopes, assigned inferred restriction elements, and characterized the Th1/Th2 balance in T cell responses directed against the different epitopes. Finally, we assessed the impact of sequence variation by analyzing reactivity against common isoforms and epitope conservation in the transcriptome of several common allergens.
Our data demonstrate that Amb a 1 dominates not only IgE reactivity, but also T cell reactivity. This suggests that approaches aimed at desensitizing T cell responses utilizing peptide epitopes derived from Amb a 1 might be particularly desirable, since this antigen accounts for a majority of T cell responses. Further, the use of peptide epitopes would be less likely than whole Amb a 1 antigen to lead to adverse reactions caused by IgE binding. At the molecular level, it is still unclear why the allergen repertoire of ragweed is remarkably narrow in terms of the number of different epitopes required to account for a majority of the T cell responses. In the case of a recent analysis of cockroach epitopes, for example, a total of 90% of the responses was encompassed by the top 164 epitopes [36]. Remarkably, in the present study, a number of as few as 17 ragweed epitopes accounted for 90% of the overall T cell response. One speculative hypothesis is that this difference is due to exposure patterns or kinetics of release of proteins from the allergen particle.
While Amb a 1 is dominant for both T cell and IgE responses, overall there is no statistically significant correlation between dominance patterns for T cell and IgE responses. This is consistent with what has been observed in other allergen sources, namely cockroach and timothy grass [15, 29].
In terms of the specific epitopes recognized within the Amb a 1 allergen, our data confirm recognition of three epitopes identified as dominant in a previous study utilizing proliferation assays and 12-mer peptides. The definition of immune dominance at the epitope level is based on which peptides are most frequently/vigorously recognized in the subjects studied. Here, utilizing more sensitive assays, and peptides that more closely comport with optimal HLA class II ligand sizes, we describe three additional epitopes associated with similar levels of reactivity that were not previously described as immunodominant. This result highlights the need to utilize longer peptides in epitope identification screens. At the same time the use of ELISPOT cytokine assays allowed confirmation that the Th2 phenotype dominates over Th1 in the ragweed-specific responses. In the current study, we measured IL-5 as a representative Th2 cytokine. In other studies where the issue was addressed it was found that the same immunodominant allergy peptides do stimulate IL-4, IL-5 and IL-13 [15]. In two recently published studies, we measured IL-17 and IL-10 in addition to IFNγ and IL-5, but found that the contribution of those two cytokines to overall responses was very modest [35, 36]. Accordingly, these two cytokines were not measured in this study.
Further, the use of the RATE approach allowed inference of restriction elements for several of the more dominant epitopes. These data will be of use in the production of tetrameric staining reagents to be used in future studies characterizing ragweed-specific T cell responses in disease and immunotherapy contexts.
The immunodominant epitopes that were found in an earlier study were somewhat different [13]. It is possible that differences in HLA type, or more likely, differences in patterns of exposure between the USA and Europe, might play a role in the differences observed.
Our study also evaluated to what extent T cell reactivity is influenced by sequence variation associated with different isoforms and conservation among different common allergens. In general, we found that isoform-associated variation did not greatly impact T cell recognition for the most dominant epitopes. In terms of conservation in different common allergens, in good agreement with what was previously reported in timothy grass, epitopes tended to be more conserved than non-epitopes. Indeed, we show that a statistically significant correlation exists between dominance patterns for T cell responses and conservation of allergen sequences. This observation suggests that allergen conservation could be of broad utility in predicting T cell allergens.
A limitation of the current study is that plasma and PBMCs were obtained from donors that had limited or no clinical characterization. Levels of specific IgE against ragweed and other allergens therefore served as indicators of atopic background. While the present study was not designed to monitor the relationship between T cell responses and clinical manifestations, additional studies addressing these issues would be key to understanding molecular mechanisms involved in the pathogenesis of ragweed allergy.
Vigorous responses to the ragweed extracts used as positive control were detected in 23/25 donors. This corresponds to a response rate of greater than 90%, and is in line with what we routinely observe in extract stimulation assays. We do not have a clear explanation for this phenomenon, but possibilities include low sensitization, problems with cell quality or culture conditions, or potential in vitro toxicity of the extract preparations.
Our studies suggest that Amb a 8 (profilin) and Amb a 10 pan-allergens may be promising for immunotherapy. Future studies may address whether they can be used to induce broad tolerance via regulatory mechanisms when administered in an immunotherapy setting.
Our analysis further supports the notion that by focusing the response on a set of broadly conserved epitopes, it might be possible to develop immunotherapeutic regimens with broad activity against different common allergens. In particular, Amb a 8 and Amb a 10 might represent reasonable candidates for inducing regulatory T cells, since they are recognized by T cells but only marginally by IgE responses. The high degree of conservation of the Amb a 8 antigen across several pollen allergens supports the notion that this allergen could be of potential use of this allergen as a immunotherapeutic with broad activity against a number of different pollen allergies.
Supplementary Material
Supplemental Figure 1. Frequency of responding donors to individual 16-mer peptides spanning Amb allergens shown as percentage of donors investigated (n = 25). PBMCs from ragweed-sensitized individuals were stimulated with ragweed extract and, after 14 days expansion with IL-2, production of IFNγ and IL-5 was assessed by dual ELISPOT.
Supplemental Table 1. Subject demographics, IgE and T cell responses, and polysensitization.
Supplemental Table 2. Polysensitization to grass and and tree pollen allergen sources.
Supplemental Table 3. Detailed account of RATE analysis.
Acknowledgments
The authors thank April Frazier for help with clinical recruitment and project management of the study, and Jessica Moore for editing and review of the manuscript. Funding was provided in part by ALK-Abello A/S (Horsholm, Denmark) and with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under grant number U19 AI100275.
Footnotes
Conflict of interest
Alessandro Sette and Bjoern Peters are consultants for ALK-Abelló A/S Bøge Allé 6 DK-2970 Hørsholm, Denmark.
References
- 1.Pichler U, Hauser M, Wolf M, Bernardi ML, Gadermaier G, Weiss R, Ebner C, Yokoi H, Takai T, Didierlaurent A, Rafaiani C, Briza P, Mari A, Behrendt H, Wallner M, Ferreira F. Pectate lyase pollen allergens: sensitization profiles and cross-reactivity pattern. PLoS ONE. 2015;10:e0120038. doi: 10.1371/journal.pone.0120038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laaidi M, Laaidi K, Besancenot JP, Thibaudon M. Ragweed in France: an invasive plant and its allergenic pollen. Ann Allergy Asthma Immunology. 2003;91:195–201. doi: 10.1016/S1081-1206(10)62177-1. [DOI] [PubMed] [Google Scholar]
- 3.Dechamp C. Ambrosia pollinosis. Revue des maladies respiratoires. 2013;30:316–27. doi: 10.1016/j.rmr.2012.10.632. [DOI] [PubMed] [Google Scholar]
- 4.Hamaoui-Laguel L, Vautard R, Liu L, Solmon F, Viovy N, Khvorostyanov D, Essl F, Chuine I, Colette A, Semenov MA, Schaffhauser A, Storkey J, Thibaudon M, Epstein MM. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nature Clim Change. 2015;5:766–71. [Google Scholar]
- 5.El-Qutob D. Vaccine development and new attempts of treatment for ragweed allergy. Therapeutic Adv Vaccines. 2015;3:41–7. doi: 10.1177/2051013614565354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King TP, Norman PS, Tao N. Chemical modifications of the major allergen of ragweed pollen, antigen E. Immunochemistry. 1974;11:83–92. doi: 10.1016/0019-2791(74)90321-8. [DOI] [PubMed] [Google Scholar]
- 7.Adolphson C, Goodfriend L, Gleich GJ. Reactivity of ragweed allergens with IgE antibodies. Analyses by leukocyte histamine release and the radioallergosorbent test and determination of cross-reactivity. J Allergy Clin Immunol. 1978;62:197–210. doi: 10.1016/0091-6749(78)90208-7. [DOI] [PubMed] [Google Scholar]
- 8.Jahn-Schmid B, Hauser M, Wopfner N, Briza P, Berger UE, Asero R, Ebner C, Ferreira F, Bohle B. Humoral and cellular cross-reactivity between Amb a 1, the major ragweed pollen allergen, and its mugwort homolog Art v 6. J Immunol. 2012;188:1559–67. doi: 10.4049/jimmunol.1102445. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh B, Perry MP, Rafnar T, Marsh DG. Cloning and expression of immunologically active recombinant Amb a V allergen of short ragweed (Ambrosia artemisiifolia) pollen. J Immunol. 1993;150:5391–9. [PubMed] [Google Scholar]
- 10.Roebber M, Hussain R, Klapper DG, Marsh DG. Isolation and properties of a new short ragweed pollen allergen, Ra6. J Immunol. 1983;131:706–11. [PubMed] [Google Scholar]
- 11.Bouley J, Groeme R, Le Mignon M, Jain K, Chabre H, Bordas-Le Floch V, Couret MN, Bussieres L, Lautrette A, Naveau M, Baron-Bodo V, Lombardi V, Mascarell L, Batard T, Nony E, Moingeon P. Identification of the cysteine protease Amb a 11 as a novel major allergen from short ragweed. J Allergy Clin Immunol. 2015;136:1055–64. doi: 10.1016/j.jaci.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Bordas-Le Floch V, Le Mignon M, Bouley J, Groeme R, Jain K, Baron-Bodo V, Nony E, Mascarell L, Moingeon P. Identification of Novel Short Ragweed Pollen Allergens Using Combined Transcriptomic and Immunoproteomic Approaches. PLoS ONE. 2015;10:e0136258. doi: 10.1371/journal.pone.0136258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahn-Schmid B, Wopfner N, Hubinger G, Asero R, Ebner C, Ferreira F, Bohle B. The T-cell response to Amb a 1 is characterized by 3 dominant epitopes and multiple MHC restriction elements. J Allergy Clin Immunol. 2010;126:1068–71. 71 e1–2. doi: 10.1016/j.jaci.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Rothbard JB, Lechler RI, Howland K, Bal V, Eckels DD, Sekaly R, Long EO, Taylor WR, Lamb JR. Structural model of HLA-DR1 restricted T cell antigen recognition. Cell. 1988;52:515–23. doi: 10.1016/0092-8674(88)90464-3. [DOI] [PubMed] [Google Scholar]
- 15.Oseroff C, Sidney J, Tripple V, Grey H, Wood R, Broide DH, Greenbaum J, Kolla R, Peters B, Pomes A, Sette A. Analysis of T cell responses to the major allergens from German cockroach: epitope specificity and relationship to IgE production. J Immunol. 2012;189:679–88. doi: 10.4049/jimmunol.1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wopfner N, Jahn-Schmid B, Schmidt G, Christ T, Hubinger G, Briza P, Radauer C, Bohle B, Vogel L, Ebner C, Asero R, Ferreira F, Schwarzenbacher R. The alpha and beta subchain of Amb a 1. the major ragweed-pollen allergen show divergent reactivity at the IgE and T-cell level. Mol Immunol. 2009;46:2090–7. doi: 10.1016/j.molimm.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Ghiani A, Aina R, Asero R, Bellotto E, Citterio S. Ragweed pollen collected along high-traffic roads shows a higher allergenicity than pollen sampled in vegetated areas. Allergy. 2012;67:887–94. doi: 10.1111/j.1398-9995.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- 18.Schulten V, Tripple V, Aasbjerg K, Backer V, Lund G, Wurtzen PA, Sette A, Peters B. Distinct modulation of allergic T cell responses by subcutaneous vs. sublingual allergen-specific immunotherapy. Clin Exp Allergy. 2016;46:439–48. doi: 10.1111/cea.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulten V, Tripple V, Sidney J, Greenbaum J, Frazier A, Alam R, Broide D, Peters B, Sette A. Association between specific timothy grass antigens and changes in TH1- and TH2-cell responses following specific immunotherapy. J Allergy Clin Immunol. 2014;134:1076–83. doi: 10.1016/j.jaci.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wambre E. Effect of allergen-specific immunotherapy on CD4+ T cells. Curr Opin Allergy Clin Immunol. 2015;15:581–7. doi: 10.1097/ACI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karp CL. Guilt by intimate association: what makes an allergen an allergen? J Allergy Clin Immunol. 2010;125:955–60. doi: 10.1016/j.jaci.2010.03.002. quiz 61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radauer C, Nandy A, Ferreira F, Goodman RE, Larsen JN, Lidholm J, Pomes A, Raulf-Heimsoth M, Rozynek P, Thomas WR, Breiteneder H. Update of the WHO/IUIS Allergen Nomenclature Database based on analysis of allergen sequences. Allergy. 2014;69:413–9. doi: 10.1111/all.12348. [DOI] [PubMed] [Google Scholar]
- 23.Mari A, Rasi C, Palazzo P, Scala E. Allergen databases: current status and perspectives. Curr Allergy Asthma Rep. 2009;9:376–83. doi: 10.1007/s11882-009-0055-9. [DOI] [PubMed] [Google Scholar]
- 24.Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, Crotty S, Peters B, Sette A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Nat Acad Sci U S A. 2015;112:E4256–63. doi: 10.1073/pnas.1505956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindestam Arlehamn CS, Paul S, Mele F, Huang C, Greenbaum JA, Vita R, Sidney J, Peters B, Sallusto F, Sette A. Immunological consequences of intragenus conservation of Mycobacterium tuberculosis T-cell epitopes. Proc Nat Acad Sci U S A. 2015;112:E147–55. doi: 10.1073/pnas.1416537112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu C, McCausland M, Sidney J, Duh FM, Rouphael N, Mehta A, Mulligan M, Carrington M, Wieland A, Sullivan NL, Weinberg A, Levin MJ, Pulendran B, Peters B, Sette A, Ahmed R. Broadly reactive human CD8 T cells that recognize an epitope conserved between VZV. HSV and EBV. PLoS Pathogens. 2014;10:e1004008. doi: 10.1371/journal.ppat.1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westernberg L, Schulten V, Greenbaum JA, Natali S, Tripple V, McKinney DM, Frazier A, Hofer H, Wallner M, Sallusto F, Sette A, Peters B. T-cell epitope conservation across allergen species is a major determinant of immunogenicity. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asero R, Bellotto E, Ghiani A, Aina R, Villalta D, Citterio S. Concomitant sensitization to ragweed and mugwort pollen: who is who in clinical allergy? Ann Allergy Asthma Immunol. 2014;113:307–13. doi: 10.1016/j.anai.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Oseroff C, Sidney J, Kotturi MF, Kolla R, Alam R, Broide DH, Wasserman SI, Weiskopf D, McKinney DM, Chung JL, Petersen A, Grey H, Peters B, Sette A. Molecular determinants of T cell epitope recognition to the common Timothy grass allergen. J Immunol. 2010;185:943–55. doi: 10.4049/jimmunol.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, Grey H, Sette A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–9. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 31.Erlich RL, Jia X, Anderson S, Banks E, Gao X, Carrington M, Gupta N, DePristo MA, Henn MR, Lennon NJ, de Bakker PI. Next-generation sequencing for HLA typing of class I loci. BMC Genomics. 2011;12:1–13. doi: 10.1186/1471-2164-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh Steven GE. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43:D423–D31. doi: 10.1093/nar/gku1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul S, Dillon MB, Lindestam Arlehamn CS, Huang H, Davis MM, McKinney DM, Scriba TJ, Sidney J, Peters B, Sette A. A population response analysis approach to assign class II HLA-epitope restrictions. J immunol. 2015;194:6164–76. doi: 10.4049/jimmunol.1403074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulten V, Greenbaum JA, Hauser M, McKinney DM, Sidney J, Kolla R, Lindestam Arlehamn CS, Oseroff C, Alam R, Broide DH, Ferreira F, Grey HM, Sette A, Peters B. Previously undescribed grass pollen antigens are the major inducers of T helper 2 cytokine-producing T cells in allergic individuals. Proc Nat Aca Sci U S A. 2013;110:3459–64. doi: 10.1073/pnas.1300512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinz D, Oseroff C, Pham J, Sidney J, Peters B, Sette A. Definition of a pool of epitopes that recapitulates the T cell reactivity against major house dust mite allergens. Clin Exp Allergy. 2015;45:1601–12. doi: 10.1111/cea.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dillon MB, Schulten V, Oseroff C, Paul S, Dullanty LM, Frazier A, Belles X, Piulachs MD, Visness C, Bacharier L, Bloomberg GR, Busse P, Sidney J, Peters B, Sette A. Different Bla-g T cell antigens dominate responses in asthma versus rhinitis subjects. Clin Exp Allergy. 2015 doi: 10.1111/cea.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodfriend L, Choudhury AM, Klapper DG, Coulter KM, Dorval G, Del Carpio J, Osterland CK. Ra5G, a homologue of Ra5 in giant ragweed pollen: isolation, HLA-DR-associated activity and amino acid sequence. Mol Immunol. 1985;22:899–906. doi: 10.1016/0161-5890(85)90076-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Frequency of responding donors to individual 16-mer peptides spanning Amb allergens shown as percentage of donors investigated (n = 25). PBMCs from ragweed-sensitized individuals were stimulated with ragweed extract and, after 14 days expansion with IL-2, production of IFNγ and IL-5 was assessed by dual ELISPOT.
Supplemental Table 1. Subject demographics, IgE and T cell responses, and polysensitization.
Supplemental Table 2. Polysensitization to grass and and tree pollen allergen sources.
Supplemental Table 3. Detailed account of RATE analysis.