Abstract
Behavioral interventions for autism have gained prominence in recent years; however, the neural-systems-level targets of these interventions remain poorly understood. We use a novel Bayesian framework to extract network-based differences before and after a 16-week Pivotal Response Treatment (PRT) regimen. Our results suggest that functional changes induced by PRT localize to the posterior cingulate and are marked by a shift in connectivity from the orbitofrontal cortex to the occipital temporal cortex. Our results illuminate a potential PRT-induced learning mechanism, whereby the neural circuits involved during social perception shift from sensory and attentional systems to higher-level object and face processing areas.
Keywords: fMRI, Bayesian Analysis, Pivotal Response Training, Autism Spectrum Disorders
Introduction
Autism Spectrum Disorders (ASD) are characterized by social and communication deficits. Theories of ASD have postulated both reduced social motivation and atypical reward processing [1] [2] as well as difficulty in predicting real-world events [3]. Given the universality of social deficits in ASD, dysfunction in brain systems subservient to social perception are central to research in the field [4] [5]. Moreover, core social-communication symptoms are natural targets for the development of pharmacological and behavioral interventions.
Behavioral therapies, such as Pivotal Response Treatment (PRT) [6], have shown promise in reducing core symptoms of ASD [7]. PRT is structured as a series of play-based sessions, during which children are reinforced for utilizing appropriate social communication skills. Several randomized control trials of PRT have shown significant improvement in language and social functioning [8] [9], and a recent open-label trial of PRT from our group demonstrated reduction in restricted and repetitive behavior following a 16-week PRT regimen [7].
While it is believed that behavioral interventions for ASD stimulate socially-responsive areas of the brain, little is known about the neural underpinnings of such therapies, nor their short- and long-term effects on neural systems. To this end, Voos et al. [10] demonstrated that two high functioning children with ASD showed increased activation from baseline to treatment endpoint in key brain regions associated with social functioning. In a follow-up study, Ventola et al. [7] illustrated that the neural systems supporting social perception in an additional ten children with ASD were malleable through implementation of PRT; specifically, neural responses were more similar to those of typically developing (TD) children following treatment. Using a similar treatment approach called the Early Start Denver Model (ESDM) [11], Dawson and colleagues [12] measured the neural correlates of response via EEG after two years of treatment. Following the intervention, children in the ESDM group showed a shorter Nc latency and increased cortical activation (decreased α power and increased θ power) when viewing faces, compared to a group of children who received only community-based intervention. This study, however, did not include a baseline time point, so it is not possible to evaluate whether and/or how the groups differed prior to the onset of ESDM treatment.
Understanding of the neural mechanisms of treatment response is crucial to mitigating the core social-communication deficits in ASD, from the development of novel and adaptive treatment approaches to behavioral and pharmacological therapies which target specific neural circuitries. Here, we leverage an unbiased probabilistic model for functional magnetic resonance imaging (fMRI) that aggregates group-level changes in functional synchrony before and after treatment in order to localize a compact subset of affected regions, i.e., treatment foci.
Materials and Methods
Participants
We studied 19 children with a primary diagnosis of ASD (age = 5.87±1.09 years, 13 males). All participants were high functioning (IQ ≥ 70) and met the DSM-5 diagnostic criteria for ASD [13] by expert clinician judgment, as confirmed by the gold-standard Autism Diagnostic Interview-Revised (ADI-R) [14] and Autism Diagnostic Observation Schedule (ADOS) [15]. Further details about the patient demographics and clinical measures are provided in Table 1. Written informed consent was obtained from each set of parents and verbal assent was attained from each child. This study was approved by the Human Investigations Committee at Yale University and is registered at ClinicalTrials.gov (ID: NCT01908686).
Table 1.
Snapshot of participant demographics and clinical characteristics (19 subjects)
| Variable | Mean (SD) |
|---|---|
| Pretreatment Age (years) | 5.87 (1.09) |
| Gender, male (0=f, 1=m) | 0.68 (0.48) |
| DAS-II General Conceptual Ability* (IQ) | 104.53 (16.78) |
| Handedness, (1=right, 0=ambi, −1=left) | 0.68 (0.67) |
| ADOS (Calibrated Severity Score) | 7.74 (2.13) |
| CELF-P-2 Core Language* | 91.32 (24.18) |
| Pre-treatment SRS-parent Total Raw Score | 81.68 (22.65) |
| Post-treatment SRS-parent Total Raw Score | 66.53 (23.52) |
| Pre-treatment Head Motion (mm) | 1.38 (1.35) |
| Post-treatment Head Motion (mm) | 0.46 (0.44) |
Standard Score
Note: Treatment outcome is the residual change of SRS-parent total raw score, i.e., the delta change (POST-PRE) minus the predicted change, as specified by the group-wise linear trend.
Participants received 16 weeks of Pivotal Response Treatment (PRT) [6] [7], which involved five hours of direct intervention with the clinician and two hours of parental guidance at home per week. PRT is designed to increase the child’s social motivation via naturalistic reinforcement and goal-oriented tasks. The sessions were play-based and targeted pivotal behaviors, such as social initiation and responsiveness. It is believed that improvements in these domains will lead to more widespread and generalized improvements across development. All clinicians involved in the present study were extensively trained in PRT. Fidelity was maintained by videotaping and reviewing randomly-selected time intervals during each patient’s sessions. Overall, this sample represents 2,128 hours of direct therapeutic intervention, 1,064 family visits, and 57 clinical evaluations, in addition to the MRI protocol, as described below.
Image Acquisition & Preprocessing
Each child underwent MRI scanning before and after the PRT intervention. Participants were scanned on a Siemens MAGNETOM 3T Tim Trio scanner at Yale. We acquired a T1-weighted scan (MPRAGE, TR=1900ms, TE=2.96ms, flip angle=9°, resolution=1mm3 and an fMRI scan (BOLD, TR=2000ms, TE=25ms, flip angle=60°, resolution=3.44×3.44×4mm3) for each patient.
The fMRI paradigm featured coherent and scrambled point-light animations, presented in an alternating block-design framework (24s per block). The coherent biological motion depicts a point-light figure performing movements relevant to early childhood experiences [4]. Scrambled animations combine the trajectories of 16 randomly selected points from the coherent displays.
We processed the anatomical images using Freesurfer [16]. Region boundaries were derived from the built-in Desikan-Killany atlas, which segments the brain into 86 cortical and subcortical regions, roughly corresponding to Broadmann areas. The fMRI data were preprocessed using FSL [17] v5.0.8 according to the processing steps outlined by the creators of ICA-AROMA [18]. The pipeline consists of the following steps: (1) motion correction using MCFLIRT, (2) interleaved slice timing correction, (3) BET brain extraction, (4) global mean intensity normalization for the whole 4D data set, (5) spatial smoothing with FWHM=5mm, (6) denoising with ICA-AROMA [18], (7) nuisance regression of WM and CSF signals to remove physiological noise, and (8) high-pass temporal filtering. The first four volumes were discarded, and preprocessed data were then pre-whitened using FSL FILM to remove time series autocorrelation. Both the functional and anatomical data were registered to the MNI152 standard brain for subsequent analysis. The pairwise fMRI measures are computed as the Pearson correlation coefficients between the mean time courses of the two regions. We center the correlation distribution of each patient in order to model relative deviations from the subject-specific baseline functional synchrony.
Bayesian Analysis
Unlike traditional connectomics, which compares either pairwise correlation coefficients or average node-based measures between groups [19], our framework explicitly models the altered network topology while simultaneously adapting to both noise and subject variability [20] [21]. Within a Bayesian setting, we estimate a latent or hidden graph that characterizes the spread of altered functional connectivity from the region foci. This latent template subsequently explains the observable differences in fMRI correlation values. Hence, our model effectively translates connectivity information into estimates of the brain regions associated with PRT. Our approach is completely data-driven and does not impose spatial constraints on the region foci or altered functional pathways. By examining brain activity during a social perception task, we focus on functional connectivity during social information processing, a key area of deficit in people with ASD and target of PRT.
We consider two forms of model validation. First, we evaluate the reliability of the detected region foci via bootstrapping. Bootstrapping is a statistical technique, by which we subsample the data in order to derive robust estimates of a given model characteristic (ex. the network foci). In this work, we infer the model parameters while omitting either one or two subjects from the analysis. By aggregating the network results across subsets of the patient cohort, we can speculate on the generalizability of our network results for potentially larger PRT datasets. Second, we regress the POST-treatment fMRI correlation values implicated by the inferred Bayesian network with the residualized change in Social Responsiveness Scale (SRS) [22] before and after PRT. SRS was the primary outcome measure for our clinical trial and provides a link between our neuroimaging markers and the observed behavioral improvement.
Results
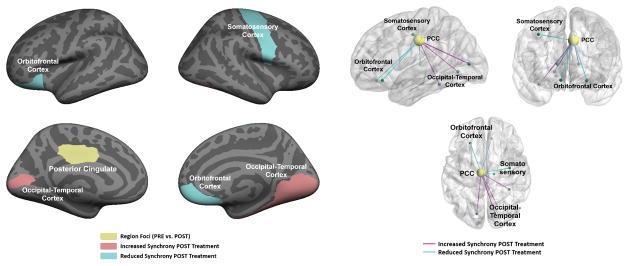
Figure 1 illustrates the model results when comparing the PRE- and POST-treatment functional synchrony across the scrambled and coherent biological motion conditions. As seen, the aggregate differences localize to the posterior cingulate cortex (PCC), depicted in yellow. We also observe a reduction in connectivity to orbital frontal cortex (blue) as well as an increase in connectivity to the occipital-temporal cortex (magenta).
Figure 1.
Network differences before (PRE) and after (POST) the 16-week PRT regimen. Left: Region membership in the altered network. The functional differences localize to the posterior cingulate (yellow). The pink and blue areas denote increased and reduced functional synchrony to the PCC after treatment. Right: 3D diagram of the altered functional synchrony. Each node corresponds to one of the predefined regions, and each edge represents a functional connection. Blue lines signify reduced functional synchrony after treatment; magenta lines denote increased functional synchrony.
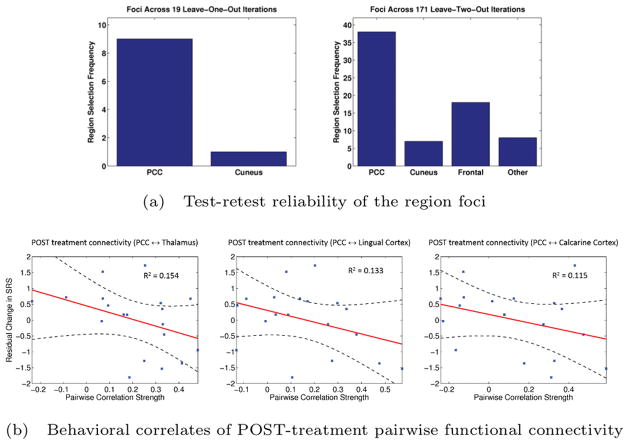
Figure 2(a) highlights the selection frequency of each cortical area across the 19 unique leave-one-out (excluding roughly 5% of the data) subsets and 171 unique leave-two-out subsets (excluding roughly 10% of the available data). Despite the small sample-size, our region foci consistently localize to the PCC based on 18 patients and to either the PCC or frontal cortex based on only 17 patients. These findings indicate that nearly all identifiable networks were centered on the PCC. Such reproducibility further strengthens the clinical relevance of our results.
Figure 2.
Model validation for the PRT analysis. (a) Reproducibility of the region foci when omitting one (top) and two (bottom) subjects from the analysis. Results are aggregated across all 19 and 171 bootstrapping configurations, respectively. (b) Linear regression between the pairwise fMRI correlation values and the residual change in SRS. The subject data is plotted in blue, and the linear fit and confidence intervals are overlaid in red and black, respectively.
Figure 2(b) reports the connections with the largest coefficients of determination (R2) values. While our sample size was too small to test the significance of these correlations, the effect sizes are encouraging and suggest that the networks we identified are related to the therapeutic processes engaged by PRT.
Discussion
It is striking that the PRT-induced changes in connectivity involve both a reduction in connectivity between the PCC and orbital frontal cortex and an increase in connectivity between the PCC and regions of ventral occipital temporal extrastriate cortex. The PCC is well known for its roles in social cognition [23]. The orbital frontal cortex is generally implicated in assessing the reward value of stimuli in the environment [1]. In contrast, sectors of ventral occipital and temporal cortex are well-known for processing various socially meaningful stimuli including faces and biological motion [24]. PRT seems to facilitate a process by which the brain shifts from a strong reliance on an orbital frontal—PCC circuit to a PCC—ventral occipital-temporal cortex circuit [7].
Our conclusions are also supported by the broader fMRI literature, as cataloged by the Neurosynth meta-analytic database (www.neurosynth.org). Broadly, Neurosynth aggregates both the spatial activation coordinates and the psychological words and phrases used to describe these effects across nearly 10,000 published fMRI studies. The web-based system leverages the power of large datasets to compute whole-brain posterior probabilities P(Feature|Coordinate) for individual psychological terms at each spatial coordinate [25]. Figure 3 illustrates the top eight “features” implied by the regions with increased (pink) and reduced (blue) synchrony to the posterior cingulate after PRT. As seen, there is a general shift from sensory topics, such as eye, movement and finger to higher-level constructs, such as scene, identity and face recognition. Hence, our results seem to support a PRT-induced social learning process by which children with ASD initially rely on motivational and attentional systems during social perception, as indicated by the preponderance of connectivity with orbital frontal cortex. Following PRT, social perception begins to engage higher-level systems involved in the recognition and classification of both social and non-social objects, supported by regions of temporal-occipital cortex.
Figure 3.
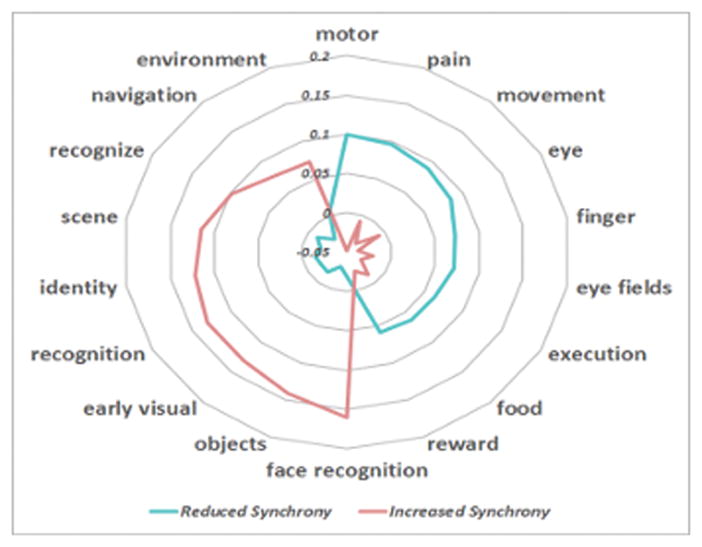
Specificity of neurocognitive functions derived from the Neurosynth meta-analytic database. Blue corresponds to the regions with reduced synchrony to the posterior cingulate after PRT. The constructs implicate basic sensory functions. Pink denotes the regions with increased synchrony to the posterior cingulate and maps onto scene and object-recognition domains.
These PRT-induced changes in connectivity are the first steps towards the goal of targeted, precision medicine for core social communication deficits in ASD. As a whole, the work towards precision medicine in ASD has been hindered by a lack of sensitive, objective biomarkers of treatment response. By objectively illustrating changes in connectivity and by revealing the key neuroanatomical circuits implicated in response to treatment, we are providing the crucial and much needed foundation to individualized treatment approaches and the development of novel/adaptive treatments that target specific neural circuits. Furthermore, these biological markers can be used towards the development of objective early efficacy indicators of treatment response. In time, these neural systems-based biomarkers may also be able to be tied to behavioral indicators, which will increase the scalability of the approach.
Despite the significance of the results presented here, there are clear limitations. The sample size, although consistent with other behavioral trials of children with ASD, is small. Additionally, we did not have a typically developing control group for comparison. Nonetheless, the results are highly impactful and amongst the first to demonstrate changes in connectivity following treatment for ASD.
Acknowledgments
Funding for the PRT study came from the Autism Science Foundation, the Simons Foundation, the Women’s Health Research at Yale, the Deitz Family, Esme Usdan & Family, and the Dwek Family. The analysis was supported in part by R01 NS035193 (NINDS), R01 MH100028 (NIMH) and the Yale Biomedical High Performance Computing Center (NIH RR19895 & RR029676-01). Author D. Yang is also supported by the Autism Speaks Meixner Postdoctoral Fellowship in Translational Research (#9284).
Footnotes
Competing Interests: The authors declare that they have no competing financial interests.
Author Contributions: AV developed the Bayesian network model, analyzed the PRT data, generated the figures, and drafted the manuscript. DY preprocessed the fMRI data and obtained the Neurosynth results. KAP and PV participated in the design of the study and helped to draft the manuscript. ND, LHS and JSD facilitated the research and discussion. All authors have read and approved the final document.
References
- 1.Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward Processing in Autism. Autism Research. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelphrey KA, Yang DYJ, McPartland JC. Building a Social Neuroscience of Autism Spectrum Disorder. Current Topics in Behavioral Neuroscience. 2014;16:215–233. doi: 10.1007/7854_2013_253. [DOI] [PubMed] [Google Scholar]
- 3.Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, Diamond SP, Held RM. Autism as a Disorder of Prediction. Proceedings of the National Academy of Sciences. 2014;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiser MD, Hudac CM, Schultz S, Lee SM, Cheung C, Berken AM, Deen B, Pitskel NB, Sugrue DR, Voos AC, Saulnier CA, Ventola P, Wolf JM, Klin A, Vander Wyk BC, Pelphrey KA. Neural Signatures of Autism. Proceedings of the National Academy of Sciences. 2010;107(49):21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson G, Webb SJ, McPartland J. Understanding the Nature of Face Processing Impairment in Autism: Insights from Behavioral and Electrophysiological Studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 6.Koegel RL, Schreffirnan L, Good A, Cerniglia L, Murphy C, Koegel LK. How to Teach Pivotal Behaviors to Children with Autism: a Training Manual. San Diego, CA: UC Graduate School of Education; 1989. [Google Scholar]
- 7.Ventola P, Friedman HE, Anderson LC, Wolf JM, Oosting D, Foss-Feig J, McDonald N, Volkmar F, Pelphrey KA. Improvements in Social and Adaptive Functioning Following Short-Duration {PRT} Program: a Clinical Replication. Journal of Autism and Developmental Disorders. 2014;44(11):2862–2870. doi: 10.1007/s10803-014-2145-3. [DOI] [PubMed] [Google Scholar]
- 8.Hardan AY, Gengoux GW, Berquist KL, Libove RA, Ardel CM, Phillips J, Frazier TW, Minjarez MB. A Randomized Controlled Trial of Pivotal Response Treatment Group for Parents of Children with Autism. Journal of Child Psychology and Psychiatry. 2015;58(8):884–892. doi: 10.1111/jcpp.12354. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadzaheri F, Koegel LK, Rezaee M, Rafiee SM. A Randomized Clinical Trial Comparison Between Pivotal Response Treatment (PRT) and Structured Applied Behavior Analysis (ABA) Intervention for Children with Autism. Journal of Autism Developmental Disorders. 2014;44(11):2769–2777. doi: 10.1007/s10803-014-2137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voos AC, Pelphrey KA, Tirrell J, Bolling DZ, Vander Wyk B, Kaiser MD, McPartland JC, Volkmar FR, Ventola P. Neural Mechanisms of Improvements in Social Motivation after Pivotal Response Treatment: Two Case Studies. Journal of Autism and Developmental Disorders. 2013;43(1):1–10. doi: 10.1007/s10803-012-1683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Princiotta D, Goldstein S. Interventions for Autism Spectrum Disorders: Translating Science into Practice. New York: Springer; 2013. Early Start Denver Model: An Intervention for Young Children with Autism Spectrum Disorders; pp. 59–73. [Google Scholar]
- 12.Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, Kamara D, Murias M, Greenson J, Winter J, Smith M, Rogers SJ, Webb SJ. Early Behavioral Intervention is Associated with Normalized Brain Activity in Young Children with Autism. Journal of American Academy of Child and Adolescent Psychiatry. 2013;51(11):1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A. P. Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 14.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 15.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, Di Lavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: a Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 16.Fischl B, Salat D, van der Kouwe AJ, Makri N, Segonne F, Quinn BT, Dale AM. Sequence-Independent Segmentation of Magnetic Resonance Images. Neuro Image. 2004;23:69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Bern H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in Functional and Structural MR Image Analysis and Implementation as FSL. Neuro Imag. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 18.Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A Robust ICA-Based Strategy for Removing Motion Artifacts from fMRI Data. Neuro Image. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 19.Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K. The Autism Brain Imaging Data Exchange: Towards a Large-Scale Evaluation of the Intrinsic Brain Architecture in Autism. Molecular Psychiatry. 2014;19(6):659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkataraman A, Kubicki M, Golland P. From Brain Connectivity Models to Region Labels: Identifying Foci of a Neurological Disorder. IEEE Transactions on Medical Imaging. 2013;32(11):2078–2098. doi: 10.1109/TMI.2013.2272976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkataraman A, Duncan JS, Yang D, Pelphrey KA. An Unbiased Bayesian Approach to Functional Connectomics Implicates Social-Communication Networks in Autism. Neuro Image Clinical. 2015;8:356–366. doi: 10.1016/j.nicl.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Constantino JM. Social Responsiveness Scale. 2. Torrance, CA: WPS; 2012. [Google Scholar]
- 23.Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain’s Default Network Anatomy, Function, and Relevence to Disease. Annals of the NY Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 24.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarsk P, Lacadie C, Cohen DJ, Gore JC. {Abnormal Ventral Temporal Cortical Activity During Face Discrimination Among Individuals With Autism and Asperger Syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 25.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wagner TD. Large-Scale Automated Synthesis of Human Functional Neuroimaging Data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]