Abstract
Anxiety disorders are common across the lifespan, cause severe distress and impairment, and usually have their onset in childhood. Substantial clinical and epidemiological research has demonstrated the existence of links between anxiety and its disorders in children and parents. Research on the pathways and mechanisms underlying these links has pointed to both behavioral and biological systems. This review synthesizes and summarizes several major aspects of this research. Behavioral systems include vicarious learning, social referencing, and modeling of parental anxiety; overly protective or critical parenting styles; and aspects of parental responses to child anxiety including family accommodation of the child’s symptoms. Biological systems include aspects of the prenatal environment affected by maternal anxiety, development and functioning of the oxytocinergic system, and genetic and epigenetic transmission. Implications for the prevention and treatment of child anxiety disorders are discussed, including the potential to enhance child anxiety treatment outcomes through biologically informed parent-based interventions.
Keywords: Anxiety disorders, Child, Mother–child relations, Oxytocin, Genetics
Introduction
Anxiety disorders are common in children and adults, and cause severe short- and long-term impairment (Canino et al. 2004; Costello et al. 2011; Essau et al. 2000; Langley et al. 2013; Lepine 2002; Mendlowicz and Stein 2000). Prevalence estimates for anxiety disorders in children and adolescents have varied across studies, due in part to different informants and measures, and populations. Despite the differences, time point prevalence rates appear to be between 2 and 5 %, and approximately one third of children will experience an anxiety disorder before adulthood (Costello et al. 2005, 2011; Merikangas et al. 2010). Adult anxiety disorder prevalence estimates have also varied across research methodologies and populations, with past-year prevalence estimates also as high as approximately one third of the population (Alonso et al. 2004; Baxter et al. 2013). Onset of anxiety disorders is most commonly in childhood, with a median onset as early as eleven years of age (Kessler et al. 2005).
Anxiety disorders are classified along domains of fear inducing stimuli (e.g., social situations; separation), and manifestations of anxiety (e.g., physical/autonomic arousal; persistent worry). Separation anxiety and specific phobias having the earliest average onset, followed by social phobia, generalized anxiety, and panic disorder (Kessler et al. 2012). Additional anxiety disorders included in the latest edition of the Diagnostic and Statistical Manual (DSM5) are agoraphobia, selective mutism, and illness anxiety disorder (American Psychiatric Association 2013).
A consistent finding in childhood anxiety and its disorders is the link between anxiety in children and parents. Children (used here to include both children and adolescents, unless specified otherwise) of anxious parents are more likely to exhibit elevated anxiety, and to meet criteria for an anxiety disorder than children of non-anxious parents (Beidel and Turner 1997; Biederman et al. 1991; Last et al. 1987; Silverman et al. 1988). Parents of children with anxiety disorders are also more likely to have a history of anxiety disorders than parents of children without anxiety disorders (Lieb et al. 2000; Weissman et al. 1984). A large scale population study of familial risk for anxiety concluded that the children of parents with anxiety disorders were significantly more likely to experience anxiety disorders, than children of parents without anxiety disorders, and this risk was elevated when both parents suffered from anxiety (Li et al. 2008). These results are supported by another large scale investigation of data from the Danish population registry (Steinhausen et al. 2009).
Elucidating the mechanisms involved in the cross-generational transmission of anxiety is of critical importance to the prevention and treatment of these disorders. Despite advances in behavioral and pharmacological treatments for childhood anxiety disorders over the past decades, a significant proportion of children remain symptomatic after treatment, and many still meet diagnostic criteria (Compton et al. 2004). An understanding of the factors that increase the risk of a child developing clinical anxiety could potentially inform preventative interventions that mitigate that risk. In one recent example of this, at-risk children who had at least one clinically anxious parent were significantly less likely to develop an anxiety disorder over a 1-year period if they were assigned to receive a preventative family-based intervention compared to an information-monitoring comparison control condition (Ginsburg et al. 2015). Future preventive interventions could be even more focused on particular pathways for the cross-generational transmission of anxiety, as the ability to identify, assess, and intervene around these pathways improves. In children already suffering from anxiety disorders, research on cross-generational factors that maintain the anxiety and/or moderate treatment response could inform treatment interventions and be useful in choosing the most effective treatment strategies.
The current review aims to provide a concise summary of major avenues of research into biological and behavioral factors that have been linked to the cross-generational transmission of anxiety and its disorders. A full exposition of all the relevant literature is beyond the scope of this review. Rather, we focus on a number of areas that have received major research attention, and in those areas we provide a synthesis of current knowledge. Inevitably, some lines of research, and many specific findings are omitted. The review focuses first on parental behaviors as a pathway for the cross-generational transmission of anxiety, through direct modeling of anxious behavior, critical and overprotective parenting style, and parents’ responses to anxiety symptoms in children. Biological mechanisms for anxiety transmission are reviewed next with the focus on prenatal influences of maternal stress, and the role of the oxytocinergic system for childhood anxiety. Following this is a brief review of genetic heritability of anxiety disorders, and key findings from epigenetic and gene by environment interaction research. While each of these domains of parental behavior, biological systems, and genetic or epigenetic heritability provides a unique lens for understanding the cross-generational transmission of anxiety, they are not separate or unconnected. Rather, they represent a tightly interwoven fabric of influences throughout the lifespan, with each methodology pulling at different threads that invariably reflect the action of several others. Significantly more research from each discipline, and emerging integrations across multiple disciplines, are the basis for eventually weaving these threads into a more whole understanding of cross-generational influences on childhood anxiety disorders.
Parent behavior
Vicarious learning, social referencing and modeling
Vicarious Learning was one of the pathways identified by Rachman and Costello (1961) over four decades ago, for the acquisition of fears, and is explained by social learning theory (Bandura 1969). Vicarious fear learning occurs when an individual acquires fear of a stimulus by observing the reactions of others to that stimulus. An early experimental demonstration of vicarious learning involved subjects observing another person reacting to (sham) electric shocks and acquiring fear of a sound that consistently accompanied the shocks (Berger 1962). Since that time, numerous studies using a variety of experimental methodologies have supported vicarious learning as a means of fear acquisition (Hygge 1976; Olsson and Phelps 2004; Vaughan and Lanzetta 1980). Alongside experimental designs, self-reports from clinically anxious individuals and their parents also indicate that vicarious learning plays a role in acquiring fears (Merckelbach et al. 1989; Muris et al. 1996; Ost 1985).
Children may possess an innate predisposition toward vicarious learning, and toward generalization of learned fears, because of the protective adaptive benefits inherent in learning about dangers from other, more experienced, adults and caregivers (Schiele et al. 2016). In one study of young Rhesus monkeys for example, observing a parent displaying fear of snakes led most of the subjects to develop a lasting fear of snakes (Mineka and Cook 1986). The salience and frequent presence of parents provide many opportunities for infants and children to vicariously acquire fear by observing them. In infants, social referencing refers to the tendency to assess danger based on the emotional reactions of others. Social referencing studies, using the classical ‘visual cliff’ paradigm, showed that parents’ facial expressions of fear caused infants to avoid crossing the cliff, whereas infants whose parent’s expressed other emotions were more likely to cross (Sorce et al. 1985). Other social referencing studies have demonstrated that parental expressions of fear can lead to infant fear or avoidance in response to toy snakes or spiders (Gerull and Rapee 2002) and strangers (de Rosnay et al. 2006). In the de Rosnay et al. (2006) study mothers were instructed to interact fearfully with one stranger and not fearfully with another stranger, while their infant child observed them. The infants were then found to react fearfully to the stranger that elicited the fear behavior in the mother, an effect that was magnified for children with anxious temperament. Further support for the intergenerational transmission of anxiety through social referencing comes from research showing that mothers with social phobia display more anxiety when their infants interact with strangers than do mothers without social phobia, and that these differences in turn were associated with differences in the infants’ social responsiveness (Murray et al. 2007).
Vicarious fear learning has also been demonstrated in school age children. Askew and Field (2007) showed children ages 7–9 images of unfamiliar animals together with faces showing either fear or happiness. Animals paired to fearful faces were later found to be associated with more fearful beliefs in the children. Burstein and Ginsburg (2010) randomly assigned mothers of 25 children to model either anxious or non-anxious behavior toward a spelling test their children were subsequently going to take. Maternal modeling of anxiety about the test led to children’s increased anxiety and desire to avoid the test. In another experiment, Lebowitz et al. (2015) used motion tracking to compare behavioral avoidance of spider and neutral images in mothers of clinically anxious children. Mothers’ avoidant behavior moderated the association between fear of spiders in mothers and in children such that children of spider phobic mothers showed elevated fear of spiders only when the mothers exhibited avoidant behavior.
Using an observational rather than experimental approach, Bevan et al. (1990) found that parental anxiety during a child’s preoperative anesthesia predicted the child’s anxiety both during anesthesia and 1 week post-surgery. Observations of verbal exchanges between mothers and children have also indicated that anxious children are more likely than non-anxious children to have mothers who provide anxious interpretations of ambiguous situations and who rely on avoidant solutions to hypothetical scenarios (Barrett et al. 1996; Dadds et al. 1996). Chorpita et al. (1996) analyzed verbalizations during mother–child interactions and found that children who discussed an ambiguous situation with their parents were more likely to revise their interpretations in a more anxious/avoidant direction when the parents verbalized more anxiety. Overall, there is considerable evidence for vicarious learning in childhood, and support for vicarious learning as a means of cross-generational transmission of anxiety from parent to child.
Parental behavior can also interact with innate predispositions toward fear acquisition. Non-associative models of anxiety disorders posit that fear of certain evolutionary-relevant stimuli and situations do not require associative learning to emerge, but rather are innately present and are overcome through gradual desensitization, extinction, and the learning of safety cues (Poulton and Menzies 2002). Most of the research on parental influences on anxiety disorders has focused on associative models of anxiety disorders, but research has also emerged supporting cross-generational influences on non-associative models. For example, a longitudinal study of separation anxiety disorder in youth from early childhood through adolescence found little support for associative learning processes, and tended to support a non-associative model (Poulton et al. 2001). Results from that study also indicated that parental factors, the combination of instigating separation experiences and strong environmental support, reduced the likelihood of separation anxiety disorder in youth.
Parenting style
Overprotective and critical parenting styles have been consistently linked to elevated risk of anxiety disorders in children, with the preponderance of research focusing on maternal behavior. Overprotective and controlling behavior in mothers was first empirically reported over eight decades ago (Levy 1931) and can be conceptualized as excessive protection of children from perceived physical or psychological threats, with correspondingly low encouragement of the child’s autonomy and independence. Overprotective parenting was first linked to the development of anxiety disorders through retrospective studies comparing childhood recollections in families of anxious and non-anxious individuals. Hersov (1960) found that mothers whose children were absent from school because of anxiety had a more overprotective style compared to mothers of children who were absent for other reasons, and to mothers of children who were not absent. Anxious adults also retrospectively report their parents as more overprotective than non-anxious adults (Parker 1981; Rapee 1997; Silove 1986). Observational research on the interactions between parents and children have also supported a link between overprotective parenting and childhood anxiety, with mothers of anxious children giving more help and behaving more intrusively while engaging in complex tasks with their children than mothers of non-anxious children (Hudson and Rapee 2001).
One way in which overprotective parenting may mediate the transmission of anxiety from parent to child is through information transfer (Rachman and Costello 1961). Information transfer, or instructional learning, refers to messages the child receives that either explicitly or implicitly convey a heightened sense of risk and threat. An anxious and overprotective parent is likely to provide both more frequent and more forceful messages with regard to possible risks, compared to a non-anxious and less protective parent. A poignant example from one of the authors’ clinical practice illustrates this possibility. An anxious father bringing his child to a routine medical checkup spontaneously explained to his 8-year-old that there was “no reason to suspect that the doctor would find a life threatening disease in so young a patient”. Messages like these may provide instruction to the child that routine activities are actually fraught with risk (Beidel and Turner 1997). Experimental support for this pathway comes from a series of studies in which children who were given negative information about a fictional animal by an adult developed more fear of the animal than children who were given positive information (Field et al. 2001), displayed more behavioral avoidance of the animal (Field and Lawson 2003), and the children’s fear of the animal persisted over several months (Field et al. 2008).
Overly critical parenting (and its inverse, parental acceptance) has similarly been linked to the risk of childhood anxiety disorders using various research methodologies. Siqueland et al. (1996) found that anxious children rated their parents as less accepting than non-anxious children. Moore et al. (2004), using systematically coded behavioral interactions, found that mothers of anxious children showed less warmth and were more critical compared to mothers of non-anxious children (see also, Wood et al. 2003).
There remain critical challenges in establishing the causal role of parenting style in the intergenerational transmission of anxiety. Alongside the evidence for parent-to-child effects, there are also indications that childhood anxiety symptoms may elicit certain behavioral phenotypes in parents. For example, Whaley et al. (1999) found that while maternal anxiety was the major predictor of maternal warmth (or lack thereof) during parent–child interactions, the child’s anxiety was the most predictive of maternal overprotectiveness. Another study using ratings scales from children, parents and spouses found that maternal overprotection was predominantly related to maternal anxiety, but that paternal overprotection was more strongly related to child anxiety (Bogels and van Melick 2004). The patterns of associations are likely to be more complicated than this, however. Mothers and fathers report not only different levels of anxiety in their children, but also different domains of child anxiety symptoms, suggesting that mothers and fathers are bringing different perspectives to the assessment process. Such findings highlight the need to carefully consider the ways that different informants view their own levels of anxiety as well their overprotection, and to work toward developing an integrated understanding of what the variance means. It also foreshadows the utility of moving toward other measurement approaches, including identifying more objective biological markers. The parent–child dyadic interactions over time likely create feedback loops that contribute to the development and maintenance of a child’s anxiety as well as the maintenance of the parent’s anxiety.
Parental responses to child anxiety symptoms
How parents react to symptoms of anxiety in their children appears to be influential in determining the course of the child’s anxiety. In children who are exhibiting nonclinical anxiety, parental responses can either amplify or diminish the child’s anxiety, self-efficacy, or other related constructs such as control. These effects could plausibly contribute to those normative experiences becoming increasingly severe and entrenched (Weems and Silverman 2006; Weems et al. 2003). Likewise, parental responses that increase the child’s fear of experiencing anxiety symptoms (i.e., anxiety sensitivity) could potentially lead to increased avoidance in the child, and to the onset of an anxiety disorder (Weems et al. 2002). In children who are already experiencing clinical anxiety symptoms, parental responses that reduce coping, increase anxious avoidance, or lower motivation to confront the fear could play a role in maintaining the disorder. To the extent that these parental reactions are influenced by parental anxiety, they represent probable pathways for the intergenerational transmission of anxiety. Ehlers (1993) examined the childhood recollections of adults with and without panic and other anxiety disorders. Participants with anxiety disorders had more recollections of experiencing somatic sensations of anxiety such as shortness of breath or dizziness accompanied by special attention from parents and instructions to refrain from physical or social activities, than participants without anxiety disorders. Adults with panic disorder in particular, recalled their parents treating them as sick or incapacitated due to the anxiety symptoms. In another retrospective study, high anxiety sensitivity in adults was linked to parents providing special attention and ‘sick role’ behavior, in response to somatic experiences of anxiety in childhood (Watt et al. 1998).
Family accommodation is one pattern of parental response to child anxiety that is increasingly being studied for its role in the development and maintenance of child anxiety disorders. Family accommodation refers to the myriad ways in which parents change their own behavior to help their child avoid or alleviate distress related to anxiety symptoms. Family accommodation was first studied in the context of obsessive–compulsive disorder (OCD) and greater family accommodation was found to predict more severe symptoms and worse impairment (Calvocoressi et al. 1995; Lebowitz et al. 2012). More recently, family accommodation has been studied in anxiety disorders, and has been found to be highly prevalent among parents of anxious children (Lebowitz et al. 2013; Norman et al. 2015). Examples of family accommodation in anxiety disorders are parents sleeping next to a child with separation anxiety, answering repeated reassurance seeking questions from a child with generalized anxiety, or speaking in place of a child with social phobia. Lebowitz et al. (2014b) compared family accommodation in mothers of children with anxiety, OCD, or no diagnosis and found that mothers of children in both clinical groups reported engaging in significantly more family accommodation than mothers of the non-anxious children.
Higher levels of family accommodation are associated with more severe anxiety symptoms and greater impairment, suggesting that these parental responses have the potential to impact the clinical course of childhood anxiety disorders (Lebowitz et al. 2013; Storch et al. 2015; Thompson-Hollands et al. 2014). Support for family accommodation as a pathway for cross-generational transmission of anxiety comes from data showing positive correlations between maternal anxiety and level of family accommodation (Storch et al. 2015; Thompson-Hollands et al. 2014). A study comparing child and mother ratings of family accommodation found that maternal anxiety moderates the link between the mother’s and child’s ratings, with more anxious mothers showing higher agreement with child ratings than less anxious mothers (Lebowitz et al. 2014a, b). Another study by Jones et al. (2015) directly examined whether family accommodation mediates the association between maternal anxiety and child anxiety and found significant mediation.
Parents’ anxiety can also influence their responses to their child’s anxiety by making the parent less likely to model effective coping strategies, and more likely to catastrophize the child’s symptoms. Some evidence for this hypothesis comes from studies of parental responses to children’s distress in stressful situations. LaMontagne et al. (1992) observed the behavior of parents toward children who were undergoing hospitalization in a pediatric critical care center. More anxious parents exhibited fewer problem solving coping strategies than less anxious parents, and were more focused on their own emotional response rather than on the child’s actual distress level. Likewise in an otherwise nonclinical sample of children undergoing surgery, parental anxiety predicted the child’s level of anxiety before surgery and likelihood of problems such as nightmares or separation anxiety after surgery (Kain et al. 1996).
Highly anxious parents are likely to catastrophize their child’s anxiety symptoms (i.e., view them as worse than they actually are), and anxious children may experience more severe anxiety as a result. Whaley et al. (1999) found that anxious mothers of anxious children were more likely to exhibit catastrophizing behavior than non-anxious mothers of non-anxious children. Moore et al. (2004), disentangling the influences of mother anxiety and child anxiety, reported that anxious mothers of anxious children displayed the most catastrophizing behavior, but that non-anxious mothers of anxious children were also more likely to catastrophize than non-anxious mothers of non-anxious children, underscoring the interaction between maternal and child anxiety. Clinical experience suggests that catastrophic parental cognitions such as ‘my child is falling apart’ or ‘my child won’t be able to live a normal life’ are common in response to child anxiety, and can contribute to a cascade of additional emotional and behavioral reactions in both mother and child. This is supported by empirical evidence from children exposed to severe stressors such as war conditions, showing that maternal anxiety and maternal difficulty in sensitively ‘containing’ the child’s anxious responses increased the risk of post traumatic symptoms in the children (Feldman and Vengrober 2011). Related research on children and natural disasters further highlights the mediating roles that life stressors and social support play in children’s anxiety (La Greca et al. 2010).
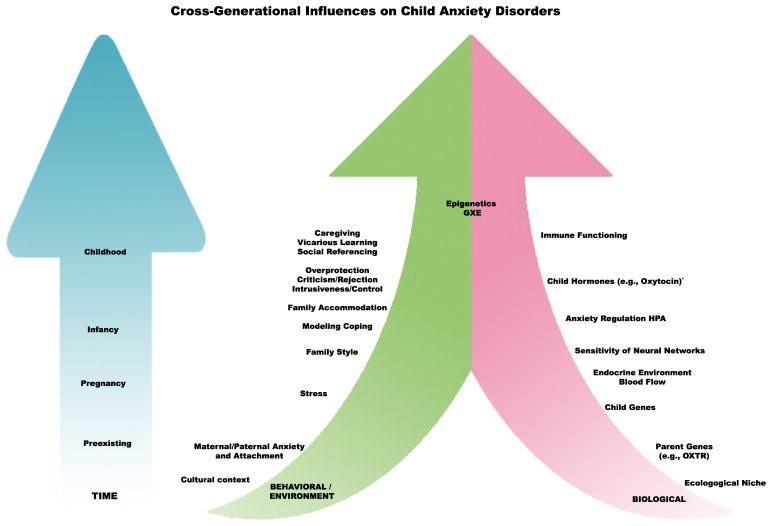
Overall, there is support for complex and multiple pathways linking parent and child anxiety through parental behavior. These pathways include (but are not limited to) vicarious learning through direct modeling and social referencing, information transfer, parenting styles of overprotection, control, and rejection, family accommodation, and other parental responses to child anxiety such as catastrophizing. Although some of these pathways are more trait-like and presumably exist independent of the child’s anxiety disorder, others are best understood in the context of the child’s symptoms. Drawing clear lines of distinction between the various parental behaviors is not always possible. A mother who is overwhelmed by her child’s distress and reacts protectively may be both catastrophizing and accommodating, for example. It is also clear that these paths interact with each other, as well as with the child’s anxiety. Modeling fear can lead to more anxiety in the child, which evokes more distress in the parent and leads to more accommodation. Considerably more research is needed before these influences can be reliably disentangled, and an understanding of underlying biological systems and mechanisms maintaining each of them may provide clues on the way to this disentanglement (Fig. 1).
Fig. 1.
Depicts cross-generational pathways and mechanisms impacting childhood anxiety disorders over time, including biological and environmental factors, and the epigenetic interactions between them
Biological mechanisms
Prenatal environment
Biological mechanisms for the intergenerational transmission of anxiety begin to exert their effects prenatally during gestation, and continue throughout development, up to and after the appearance of the child’s anxiety symptoms. The hypothesis that the prenatal environment affects the psychological functioning of offspring is well over a hundred years old (Wallace 1893), and decades of research now support it. Less timeworn is research highlighting specific biological mechanisms for these influences, and younger still are empirical data linking prenatal environment to childhood anxiety in particular.
Prospective studies linking maternal anxiety and stress during pregnancy to child development have focused on development in infants as young as 3 days, as well as children and adolescents. Rieger et al. (2004) found more regulation problems in 3–5 day old infants whose mothers had elevated scores on a measure of psychological distress around week 30 of pregnancy, and higher maternal cortisol during pregnancy predicted more difficulties in the infants habituating to new or aversive stimuli. Van den Bergh (1990) found that higher trait and state anxiety in mothers over the course of pregnancy predicted more difficult temperament, more crying, and less regular sleep at ages 10 weeks and 7 months. Several other studies have also reported associations between pregnant mothers’ distress or anxiety and signs of anxious temperament or high reactivity in infants (Brouwers et al. 2001; Field et al. 1985; Martin et al. 1999; Ponirakis et al. 1998).
In children, Van den Bergh and Marcoen (2004) found that maternal state anxiety during pregnancy predicted child self-rated anxiety at age nine, as well as externalizing problems. Viaux-Savelon et al. (2012) examined the effects of maternal stress specifically related to the wellbeing of their unborn child, on mother–child interactions after birth. Women in mid-pregnancy whose ultrasound indicated elevated risk of severe fetal abnormality, which was later ruled out in further screening, were compared to women with normal ultrasound screenings. The women in the ‘later ruled out risk’ group showed more signs of anxiety and had lower maternal sensitivity and more intrusive behavior during interactions with their newborn infants, potentially elevating the risk for childhood anxiety as well. Research supports the first two trimesters as being the most critical for the effects of maternal stress on childhood emotional problems but this has not been universally the case, and see Van den Bergh et al. (2005) for a comprehensive review. It is also becoming clear that maternal anxiety during gestation may have an impact on the child’s developing limbic system (Rifkin-Graboi et al. 2015).
Mechanisms for the intergenerational transmission of anxiety during pregnancy are not yet well understood. Hypotheses that have garnered preliminary empirical support include the possibility that the embryo is exposed to abnormally high maternal steroid hormones, and the possibility that the embryo is adversely affected by restricted uterine blood flow. Despite evidence for significant correlations between anxiety related hormone levels in pregnant mothers and fetuses (e.g., Gitau et al. 1998), and for poorer uterine flow in anxious pregnant mothers (Sjostrom et al. 1997; Teixeira et al. 1999) several questions still surround each of these mechanisms. For example, it is not clear how effectively candidate hormones such as cortisol or noradrenaline cross the placental barrier. This question is also being investigated in animal studies, and there is evidence that maternal exposure to testosterone during pregnancy can impact the limbic system of offspring in rats, and contribute to elevated anxiety-like behavior (Hu et al. 2015). It is also unclear to what extent changes in fetal blood flow, related to maternal anxiety, actually explain psychological outcomes in the babies.
A number of animal studies also provide robust evidence that prenatal stress can lead to persistent neuropsychological abnormalities in the offspring (Koehl et al. 2001). For example in rodents, exposure to prenatal stress can lead to a hypersensitivity to stressful stimuli (Wilson et al. 2013) and increased anxiety-like behaviors in the adult offspring (Takahashi et al. 1992; Zeng et al. 2015).
The oxytocinergic system and the biological transmission of anxiety
Systems for threat detection and anxiety regulation overlap in the mammalian brain with systems for social and affiliative behavior (Chang et al. 2013). Like most other mammals, human children are born highly dependent on parental caregivers for protection from threat and for regulation of anxious internal arousal. Evolutionarily shaped systems for detecting and responding to threat and for regulation of anxiety in childhood consequently involve both the child and the caregiver, and interact with systems for bonding and affiliative behavior in both (MacDonald and Feifel 2014). Biological systems that influence parental ability to appropriately detect, assess, and respond to child distress cues can influence the development of the child anxiety regulation systems, and are candidate mechanisms for the intergenerational transmission of anxiety.
The oxytocinergic system exemplifies the overlap in the brain between anxiety regulation systems and systems for bonding and affiliative behavior. The nonapeptide oxytocin evolved from an ancestral signaling peptide and appears in different variants across several phyla in the animal kingdom (Feldman et al. 2015). In mammals, alongside well-known physiological roles in uterus contraction and milk release, oxytocin plays important roles for anxiety regulation and social behavior (Insel 2010; Insel and Young 2001; Pedersen 1997). Animal studies, across a range of species and methodologies have supported an anxiolytic effect for oxytocin. A partial list of research methods implicating oxytocin in anxiety regulation in animals includes direct central or peripheral administration of oxytocin (Lee et al. 2005) or its agonists or antagonists (Smith and Wang 2014), genetically modified knockout animals (Amico et al. 2004), and blue-light axonal activation (Knobloch et al. 2012).
Oxytocin also affects social behavior in animals, and is particular important for the appearance and frequency of species-specific maternal behaviors (Olazabal and Young 2006). In rats, who respond with avoidance to the smell of pups prior to their own pregnancy and parturition, oxytocin suppresses this inhibitory effect and facilitates the expression of maternal behavior (Olazabal and Young 2006). Considerable research indicates that the oxytocinergic system is sensitive to variations in early parental care, supporting its role as a possible vehicle for cross-generational transmission of anxiety (Feldman et al. 2010b; Gimpl and Fahrenholz 2001; Keverne and Curley 2004; Meaney 2001; Ross and Young 2009).
Research in humans also implicates the oxytocinergic system in anxiety regulation and interpersonal behavior. Functional magnetic resonance imaging studies have found that oxytocin reduces activation in fear-related brain regions in response to anxiety provoking social cues such as angry faces (Kirsch et al. 2005; Labuschagne et al. 2010). Despite uncertainty about the validity of peripheral oxytocin as an indicator of central oxytocinergic activity, recent conceptualizations suggest coordination between central and peripheral oxytocin (Ross and Young 2009), and several studies have reported associations between peripheral oxytocin levels and symptoms of anxiety in human children (Carson et al. 2014) and adults (Lebowitz et al. 2016; Scantamburlo et al. 2007; Weisman et al. 2013). Most studies have supported a negative correlation, but certain inconsistencies remain, and the effects may be moderated by gender and the domain of anxiety. Weisman et al. (2013) for example, examined peripheral OT in 473 non-clinical adults (41.5 % males), and found that males showed a significant negative correlation between OT and trait anxiety. Trait anxiety was not associated with oxytocin levels in women, but a positive association was found in women between oxytocin levels and attachment anxiety. A single study has examined peripheral oxytocin levels in children with clinical anxiety disorders and found that low levels of salivary oxytocin were associated with the presence of separation anxiety disorder, and with higher levels of family accommodation (Lebowitz et al. 2016). Peripheral oxytocin levels were also negatively correlated with anxious behaviors exhibited by the children during interactions with their mothers.
Results of oxytocin administration studies have also underscored the complexity of oxytocin’s roles for both anxiety and interpersonal behavior. Using a behavioral avoidance paradigm in which participants responded to images of happy or angry faces Radke et al. (2013) found that oxytocin administration suppressed avoidance and increased approach to angry faces, but that this effect was limited to participants who were not high in social anxiety. Reducing avoidance motivation and increasing approach motivation in response to social stimuli may be one mechanism by which oxytocin facilitates parental caregiving (as in the case of pup aversion in nulliparous rats), and this mechanism may be influenced by parental anxiety, an hypothesis supported by neuroendocrinological and neurobiological data. Research on plasma hormonal profiles in mothers indicate that the oxytocin surges associated with breastfeeding are accompanied by decreases in the stress hormones ACTH and cortisol (Chiodera et al. 1991). Women who were administered intranasal oxytocin showed reduced activation of fear-related circuitry and increased activation in regions involved in empathy and prosocial behavior in response to the sound of infant cries, compared to women administered a placebo (Riem et al. 2011). But increased brain response to infant cries in regions associated with prosocial behavior after oxytocin administration was not found in women who reported high levels of maternal withdrawal in childhood (Riem et al. 2013). Likewise, Strathearn et al. (2009) found that activation of oxytocin-associated brain reward regions in response to infant cues, as well as peripherally measured oxytocin response, were lower in mothers with insecure attachment, compared to secure mothers. Several others studies have demonstrated that attachment anxiety moderates the effects of oxytocin administration, reducing rather than increasing social approach motivation (Bartz et al. 2015).
In light of these data, it is plausible to postulate that the oxytocin system may play a key role in the intergenerational transmission of anxiety from parent to child. High anxiety in parents is associated with disrupted oxytocinergic functioning, with potentially diminished motivation and/or ability to provide sensitive responses to child cues, including cues of fear or distress. Adverse effects on parental caregiving, during early life periods in which child oxytocinergic functioning is shaped through sensory and emotional caregiver input, could then contribute to the development of anxiety related problems in the child. Peripheral oxytocin levels, as well as risk alleles in the oxytocin receptor gene OXTR, have indeed been associated with a variety of parental behaviors in new parents including gaze, touch, verbalization, sensitivity, and parent–child synchrony (Bakermans-Kranenburg and van Ijzendoorn 2008; Elmadih et al. 2014; Feldman et al. 2010a, b, 2011a, b, 2012a, b, 2013; Gordon et al. 2010). Feldman et al. (2013) described a cross-generation gene by environment interaction, with maternal OXTR risk alleles interacting with maternal caregiving to predict lower peripheral oxytocin levels in children. At least three studies have reported interactions between familial risk for anxiety, parental caregiving, and OXTR variations in predicting the anxiety disorders in youth (Apter-Levy et al. 2013; Feldman et al. 2014; Thompson et al. 2011). Epigenetic studies in animals also support oxytocinergic functioning as a mechanism for intergenerational transmission of anxiety. Research on rats has shown that higher levels of maternal caregiving behavior in infancy is associated with alterations in OXTR expression, and that these rats also exhibit less anxiety behavior and more maternal caregiving themselves (Branchi et al. 2013; Champagne 2008; Pena et al. 2013).
Oxytocinergic functioning can also interact with other conditions that disrupt early maternal care, to exacerbate or mitigate the risk of childhood anxiety. Maternal depression is a major risk factor for a range of psychological problems in children, and in particular for childhood anxiety disorders (Conroy et al. 2012). Depressed mothers show less engagement with their children, potentially reducing the early life opportunities for stimulation of the oxytocinergic system during critical phases in its development (Lovejoy et al. 2000). Plasma oxytocin levels in patients with depression have been found to be significantly lower compared to non-depressed patients (Frasch et al. 1995), and to correlate with the severity of the depressive symptoms (Scantamburlo et al., 2007). An experimental oxytocin administration study recently provided an intriguing illustration of the interaction between oxytocin, maternal depression, and caregiving behavior (Mah et al. 2014). Mothers with postnatal depression who were administered intranasal oxytocin exhibited a more protective maternal reaction to an enthusiastic and socially intrusive (study confederate) stranger approaching their baby, compared to depressed mothers who were administered a placebo. Another recent study reported that both clinically depressed mothers and their children had lower salivary oxytocin levels, compared to non-depressed mothers (Apter-Levy et al. 2013). Notably, while children of chronically depressed mothers were significantly more likely to suffer from anxiety disorders than children of non-depressed mothers, the risk was significantly moderated by allelic variations on OXTR.
Genetic and epigenetic transmission
Evidence for the clustering of anxiety in families (e.g., Li et al. 2008) has provided the impetus for numerous studies exploring the genetic transmission of anxiety and its disorders. These have provided important information on the genetic heritability of anxiety, but have also highlighted important unanswered questions. Twin studies are a central tool for investigating genetic transmission of disorders and numerous twin studies, over the past 35 years, have examined the genetic heritability of anxiety disorders. A meta-analysis published around the turn of the century (Hettema et al. 2001) concluded that genetic heritability is the major source of familial risk for anxiety, and estimated heritability across the anxiety disorders at approximately 30–40 %. This represents a moderate level of heritability and leaves most individual differences in vulnerability to anxiety to environmental influences. In a recently updated review the authors reached similar conclusions, but emphasized that genetic heritability seems to be to pathological anxiety overall, rather than to specific anxiety disorders (Shimada-Sugimoto et al. 2015). Indeed, defining distinct phenotypes is one of the challenges faced by genetic research in general, and has been called the rate limiting factor in psychiatric genetic studies (Tsuang et al. 1993).
Current diagnostic nosology distinguishes between several anxiety disorders, but some studies have supported unidimensional factors underlying internalizing psychopathology including anxiety and depression. Other studies have supported a two factor model including distress (associated with depression and generalized anxiety) and fear (including most other anxiety disorders). The challenge is compounded by the fact that most of the research has focused on adults and developmental trajectories may be associated with different phenotypical structures. A recent investigation suggested different phenotypic and genetic structures for internalizing symptoms in childhood, adolescence, and young adulthood (Waszczuk et al. 2014). In childhood, one genetic factor influenced the various dimensions of anxiety, and a second factor influenced depression; in adolescence genetic factors appeared to be shared across internalizing symptoms; and in young adulthood data supported one factor for depression and anxiety symptoms, and a second genetic factor underlying fear. Recent reviews by Maron et al. (2010) and Shimada-Sugimoto et al. (2015) provide synopses of efforts to identify specific genes implicated in the inter-generational transmission of anxiety, focused mainly on panic disorder.
Modern genetic research is moving beyond direct linkage or association studies and increasingly tackling the complex question of intergenerational transmission through the lenses of gene X environment (GXE) interaction, and epigenetics. GXE interaction occurs when different genotypes respond differentially to variations in environmental variables, and epigenetic effects occur when environmental factors affect gene expression without changing the DNA sequence. By combining, rather than parsing, the environmental and genetic influences on anxiety vulnerability, these studies have the potential to elucidate mechanisms of intergenerational transmission. Several studies have reported GXE interactions between genes implicated in anxiety, and environmental factors in childhood. Specifically, variants of the anxiety-associated serotonin transporter gene (SLC6A4) and brain derived neurotrophin factor gene (BDNF) interact with environmental stressors in childhood in predicting anxiety symptoms (Gatt et al. 2009; Gunthert et al. 2007; Klauke et al. 2011; Laucht et al. 2009; Stein et al. 2008). A study by Hicks et al. (2009) examined the interplay between genetic vulnerability and six environmental risk factors including mother–child and father-child relations and concluded that there is evidence for a consistent pattern of GXE interaction in the emergence of anxiety and other internalizing symptoms in adolescence. Even more recently, evidence is emerging for epigenetic markers of response to psychotherapeutic interventions for anxiety disorders. A study by Ziegler et al. (2016) found that monoamine oxidase A methylation levels, which were lower in patients with panic disorder, compared to healthy controls, increased to normal levels in patients who responded to CBT, but not in patients who did not respond to treatment.
Epigenetic studies also support the interaction between genetic and environmental transmission of anxiety vulnerability. The most frequently studied mechanisms for epigenetic effects of environment on gene expression involve DNA methylation or chromatin modification. A comparison of DNA methylation in anxious and non-anxious individuals found significantly higher global methylation levels in anxious participants (Szyf 2015). Methylation alterations in anxiety disorders have been reported for several genes involved in anxiety regulation, including glutamate decarboxylase 1 (Domschke et al. 2013) and OXTR. In a study comparing adults with social anxiety disorder to matched controls decreased OXTR methylation was associated with social anxiety disorder diagnosis and severity, and with increased response to social anxiety triggers including cortisol response and amygdala activation (Ziegler et al. 2015). This research builds on epigenetic studies in animals showing that quality of maternal caregiving predicts alterations in offspring DNA methylation (Weaver et al. 2004), and that early life stress such as maternal separation is associated with alterations to methylation in several genes implicated in anxiety (Chen et al. 2012; Kember et al. 2012; Murgatroyd et al. 2010; Shimada-Sugimoto et al. 2015; Sotnikov et al. 2014; Wu et al. 2014). One study with particular bearing on the intergenerational transmission of anxiety used a cross-fostering model to expose infant rats to mothers who were subjected to stress and displayed abusive caregiving behaviors (Chen et al. 2012). Compared to rats that were exposed to positive caregiving from non-stressed mothers, the rats which were exposed to stressed mothers had significant alteration in BDNF DNA methylation and grew up to provide more negative caregiving themselves. Notably, the offspring of those rats also showed significantly increased BDNF methylation, illustrating the perpetuation of the negative effects of stress across generations. Studies in primates have also clearly documented that variations in patterns of maternal rearing can lead to distinct epigenetic signatures that effect how an individual’s DNA is read in specific brain regions compared to DNA present in blood cells (Fox et al. 2015; Provencal et al. 2012). Given the spatial and temporal complexity of gene expression during early human brain development (Kang et al. 2011), it is clear that maternal stress during pregnancy and beyond can have intergenerational consequences.
Summary
Numerous pathways and mechanisms contribute to the cross-generational transmission of anxiety and its disorders, exerting effects from prior to birth and across the lifespan. Among these are behavioral, neurobiological, neuroendocrinological, and genetic systems. Recent advances, including from the fields of epigenetic and GXE research, are beginning to tackle the formidable challenge of desegregating what have historically been largely isolated lines of research, and fusing them into what may ultimately be a unified understanding of the whole. Achieving this goal in its entirety will likely remain elusive but research aimed at advancing it provides important insights, with useful implications to the prevention and treatment of childhood anxiety.
One example is the possibility of improving the efficacy of parent-based behavioral interventions for childhood anxiety by building on insights from biological research. Involving parents in the treatment of childhood anxiety disorders has so far not been reliably shown to improve outcomes beyond what can be achieved through front line child treatment (Manassis et al. 2014; Reynolds et al. 2012; Silverman et al. 2008). This likely relates in part to the fact that, contrary to what may be expected, parent behavior is not necessarily differentially modified in the parent treatment arm compared with the individual child treatment arm (Silverman et al. 2009). Additionally, reduction in child anxiety is not only a result of parent change, but also can precede (and likely cause) changes in parental behavior. Disentangling the complex causal pathways leading from parent to child and from child to parent in the etiology, maintenance, and treatment of childhood anxiety disorders remains a challenge that will be addressed through better understanding of the relevant cross-generational influences.
The findings reported herein, pertaining to the oxytocinergic system as it relates to parental behavior and family accommodation suggests that peripheral oxytocin may be a useful biomarker, moderating treatment outcomes and potentially helping to identify cases most likely to benefit from interventions that can reduce family accommodation (Lebowitz 2013; Lebowitz et al. 2014a). The authors are currently conducting research including randomized controlled trials directed at unraveling these complexities, including a focus on parenting behavior and the oxytocinergic system. Another example is increasing the precision with which risk for the development of anxiety disorders in youth can be calculated by incorporating multiple units of analysis from several domains including behavioral observation of parental behavior, assessment of parent anxiety, examination of risk alleles for genetic vulnerability, and exposure to environmental risk factors. More precise risk assessment could lead to targeted preventative interventions, potentially reducing the cross-generational transmission, and reducing the overall risk of anxiety disorders.
Information from brain imaging studies, not covered in the current review, could further enhance understanding of cross-generational factors in fear acquisition, and the etiology of anxiety disorders. To cite one example, studies have implicated distinct brain regions in the acquisition of fear through social processes such as vicarious learning, and non-social processes such as direct conditioning (Olsson and Phelps 2007). Deeper understanding of the underlying mechanisms at work in social fear learning will likely also shed light on the role of parental behaviors and the cross-generational transmission of anxiety. Future studies are also needed to examine further the neural and hormonal basis of the dyadic interactions in anxious families (Lebowitz et al. 2016). Ideally, these studies would monitor simultaneously the neural activity of both parent and child in neutral and anxiogenic settings (Gordon et al. 2014). There are many hypotheses and experimental paradigms to be pursued, as research continues to reveal the pathways and mechanisms underlying the cross-generational transmission of anxiety.
References
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand. 2004;109(Supplementum 420):21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; Arlington: 2013. [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16(4):319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. Impact of maternal depression across the first 6 years of life on the child’s mental health, social engagement, and empathy: the moderating role of oxytocin. Am J Psychiatry. 2013;170(10):1161–1168. doi: 10.1176/appi.ajp.2013.12121597. [DOI] [PubMed] [Google Scholar]
- Askew C, Field AP. Vicarious learning and the development of fears in childhood. Behav Res Ther. 2007;45(11):2616–2627. doi: 10.1016/j.brat.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Principles of behavior modification. Holt, Rinehart, and Winston; New York: 1969. [Google Scholar]
- Barrett PM, Rapee RM, Dadds MM, Ryan SM. Family enhancement of cognitive style in anxious and aggressive children. J Abnorm Child Psychol. 1996;24(2):187–203. doi: 10.1007/BF01441484. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Lydon JE, Kolevzon A, Zaki J, Hollander E, Ludwig N, Bolger N. Differential effects of oxytocin on agency and communion for anxiously and avoidantly attached individuals. Psychol Sci. 2015;26(8):1177–1186. doi: 10.1177/0956797615580279. [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med. 2013;43(5):897–910. doi: 10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- Beidel DC, Turner SM. At risk for anxiety: I. psychopathology in the offspring of anxious parents. J Am Acad Child Adolesc Psychiatry. 1997;36(7):918–924. doi: 10.1097/00004583-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Berger SM. Conditioning through vicarious instigation. Psychol Rev. 1962;69(5):450–466. doi: 10.1037/h0046466. [DOI] [PubMed] [Google Scholar]
- Bevan JC, Johnston C, Haig MJ, Tousignant G, Lucy S, Kirnon V, et al. Preoperative parental anxiety predicts behavioural and emotional responses to induction of anaesthesia in children. Can J Anaesth. 1990;37(2):177–182. doi: 10.1007/BF03005466. [DOI] [PubMed] [Google Scholar]
- Biederman J, Rosenbaum JF, Bolduc EA, Faraone SV, Hirshfeld DR. A high risk study of young children of parents with panic disorder and agoraphobia with and without comorbid major depression. Psychiatry Res. 1991;37(3):333–348. doi: 10.1016/0165-1781(91)90068-z. [DOI] [PubMed] [Google Scholar]
- Bogels SM, van Melick M. The relationship between child-report, parent self-report, and partner report of perceived parental rearing behaviors and anxiety in children and parents. Personal Individ Differ. 2004;37(8):1583–1596. doi: 10.1016/j.paid.2004.02.014. [DOI] [Google Scholar]
- Branchi I, Curley JP, D’Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38(4):522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers EPM, van Baar AL, Pop VJM. Maternal anxiety during pregnancy and subsequent infant development. Infant Behav Dev. 2001;24(1):95–106. doi: 10.1016/s0163-6383(01)00062-5. [DOI] [Google Scholar]
- Burstein M, Ginsburg GS. The effect of parental modeling of anxious behaviors and cognitions in school-aged children: an experimental pilot study. Behav Res Ther. 2010;48(6):506–515. doi: 10.1016/j.brat.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvocoressi L, Lewis B, Harris M, Trufan SJ, Goodman WK, McDougle CJ, Price LH. Family accommodation in obsessive-compulsive disorder. Am J Psychiatry. 1995;152(3):441–443. doi: 10.1176/ajp.152.3.441. [DOI] [PubMed] [Google Scholar]
- Canino G, Shrout PE, Rubio-Stipec M, Bird HR, Bravo M, Ramirez R, et al. The DSM-IV rates of child and adolescent disorders in Puerto Rico. Arch Gen Psychiatry. 2004;61(1):85–93. doi: 10.1001/archpsyc.61.1.85. [DOI] [PubMed] [Google Scholar]
- Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry. 2015;20(9):1085–1090. doi: 10.1038/mp.2014.132. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29(3):386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. Neuroethology of primate social behavior. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10387–10394. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J Neuroendocrinol. 2012;24(7):1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodera P, Salvarani C, Bacchi-Modena A, Spallanzani R, Cigarini C, Alboni A, et al. Relationship between plasma profiles of oxytocin and adrenocorticotropic hormone during suckling or breast stimulation in women. Horm Res Paediatr. 1991;35(3–4):119–123. doi: 10.1159/000181886. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Albano AM, Barlow DH. Cognitive processing in children: relation to anxiety and family influences. J Clin Child Psychol. 1996;25(2):170–176. doi: 10.1207/s15374424jccp2502_5. [DOI] [Google Scholar]
- Compton SN, March JS, Brent D, Albano AMT, Weersing R, Curry J. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43(8):930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- Conroy S, Pariante CM, Marks MN, Davies HA, Farrelly S, Schacht R, Moran P. Maternal psychopathology and infant development at 18 months: the impact of maternal personality disorder and depression. J Am Acad Child Adolesc Psychiatry. 2012;51(1):51–61. doi: 10.1016/j.jaac.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clinics N Am. 2005;14(4):631–648. doi: 10.1016/j.chc.2005.06.003(vii). [DOI] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, Copeland W, Erkanli A, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Anxiety Disord Child Adolesc Res Assess Interv. 2011:56–75. doi: 10.1016/j.chc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Barrett PM, Rapee RM, Ryan S. Family process and child anxiety and aggression: an observational analysis. J Abnorm Child Psychol. 1996;24(6):715–734. doi: 10.1007/BF01664736. [DOI] [PubMed] [Google Scholar]
- de Rosnay M, Cooper PJ, Tsigaras N, Murray L. Transmission of social anxiety from mother to infant: an experimental study using a social referencing paradigm. Behav Res Ther. 2006;44(8):1165–1175. doi: 10.1016/j.brat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Domschke K, Tidowa N, Schrempf M, Schwarte K, Klauke B, Reif A, et al. Epigenetic signature of panic disorder: a role of glutamate decarboxylase 1 (GAD1) DNA hypomethylation? Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:189–196. doi: 10.1016/j.pnpbp.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Ehlers A. Somatic symptoms and panic attacks: a retrospective study of learning experiences. Behav Res Ther. 1993;31(3):269–278. doi: 10.1016/0005-7967(93)90025-p. [DOI] [PubMed] [Google Scholar]
- Elmadih A, Wai Wan M, Numan M, Elliott R, Downey D, Abel KM. Does oxytocin modulate variation in maternal caregiving in healthy new mothers? Brain Res. 2014;1580(1580):143–150. doi: 10.1016/j.brainres.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Essau CA, Conradt J, Petermann F. Frequency, comorbidity, and psychosocial impairment of anxiety disorders in German adolescents. J Anxiety Disord. 2000;14(3):263–279. doi: 10.1016/s0887-6185(99)00039-0. [DOI] [PubMed] [Google Scholar]
- Feldman R, Vengrober A. Posttraumatic stress disorder in infants and young children exposed to war-related trauma. J Am Acad Child Adolesc Psychiatry. 2011;50(7):645–658. doi: 10.1016/j.jaac.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010a;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm Behav. 2010b;58(4):669–676. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72(3):175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology. 2013;38(7):1154–1162. doi: 10.1038/npp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Vengrober A, Ebstein RP. Affiliation buffers stress: cumulative genetic risk in oxytocin-vasopressin genes combines with early caregiving to predict PTSD in war-exposed young children. Transl Psychiatry. 2014;4:e370. doi: 10.1038/tp.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Field AP, Lawson J. Fear information and the development of fears during childhood: effects on implicit fear responses and behavioural avoidance. Behav Res Ther. 2003;41(11):1277–1293. doi: 10.1016/s0005-7967(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Field T, Sandberg D, Garcia R, Vega-Lahr N, Goldstein S, Guy L. Pregnancy problems, postpartum depression, and early mother-infant interactions. Dev Psychol. 1985;21(6):1152–1156. doi: 10.1037/0012-1649.21.6.1152. [DOI] [Google Scholar]
- Field AP, Argyris NG, Knowles KA. Who’s afraid of the big bad wolf: a prospective paradigm to test Rachman’s indirect pathways in children. Behav Res Ther. 2001;39(11):1259–1276. doi: 10.1016/s0005-7967(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Field AP, Lawson J, Banerjee R. The verbal threat information pathway to fear in children: the longitudinal effects on fear cognitions and the immediate effects on avoidance behavior. J Abnorm Psychol. 2008;117(1):214–224. doi: 10.1037/0021-843X.117.1.214. [DOI] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, et al. Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci USA. 2015;112(29):9118–9122. doi: 10.1073/pnas.1508593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch A, Zetzsche T, Steiger A, Jirikowski GF. Reduction of plasma oxytocin levels in patients suffering from major depression. Adv Exp Med Biol. 1995;395:257–258. [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gerull FC, Rapee RM. Mother knows best: the effects of maternal modelling on the acquisition of fear and avoidance behaviour in toddlers. Behav Res Ther. 2002;40(3):279–287. doi: 10.1016/s0005-7967(01)00013-4. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Ginsburg GS, Drake KL, Tein JY, Teetsel R, Riddle MA. Preventing onset of anxiety disorders in offspring of anxious parents: a randomized controlled trial of a family-based intervention. Am J Psychiatry. 2015;172(12):1207–1214. doi: 10.1176/appi.ajp.2015.14091178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352(9129):707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Leckman JF, Berg DN. From attachment to groups: tapping into the neurobiology of our interconnectedness. J Am Acad Child Adolesc Psychiatry. 2014;53(2):130–132. doi: 10.1016/j.jaac.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Gunthert KC, Conner TS, Armeli S, Tennen H, Covault J, Kranzler HR. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: a daily process approach to gene-environment interaction. Psychosom Med. 2007;69(8):762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- Hersov LA. Refusal to go to school. J Child Psychol Psychiatry. 1960;1:137–145. doi: 10.1111/j.1469-7610.1960.tb01988.x. [DOI] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hicks BM, DiRago AC, Iacono WG, Mcgue M. Gene-environment interplay in internalizing disorders: consistent findings across six environmental risk factors. J Child Psychol Psychiatry. 2009;50(10):1309–1317. doi: 10.1111/j.1469-7610.2009.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015;112(46):14348–14353. doi: 10.1073/pnas.1507514112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JL, Rapee RM. Parent–child interactions and anxiety disorders: an observational study. Behav Res Ther. 2001;39(12):1411–1427. doi: 10.1016/s0005-7967(00)00107-8. [DOI] [PubMed] [Google Scholar]
- Hygge S. Information about the model’s unconditioned stimulus and response in vicarious classical conditioning. J Pers Soc Psychol. 1976;33(6):764–771. doi: 10.1037/0022-3514.33.6.764. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Jones JD, Lebowitz ER, Marin CE, Stark KD. Family accommodation mediates the association between anxiety symptoms in mothers and children. J Child Adolesc Ment Health. 2015;27(1):41–51. doi: 10.2989/17280583.2015.1007866. [DOI] [PubMed] [Google Scholar]
- Kain ZN, Mayes LC, O’Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150(12):1238–1245. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, et al. Spatiotemporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kember RL, Dempster EL, Lee THA, Schalkwyk LC, Mill J, Fernandes C. Maternal separation is associated with strain-specific responses to stress and epigenetic alterations to Nr3c1, Avp, and Nr4a1 in mouse. Brain Behav. 2012;2(4):455–467. doi: 10.1002/brb3.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14(6):777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauke B, Deckert J, Reif A, Pauli P, Zwanzger P, Baumann C, et al. Serotonin transporter gene and childhood trauma—A G*E effect on anxiety sensitivity. Depress Anxiety. 2011;28(12):1048–1057. doi: 10.1002/da.20840. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Koehl M, Lemaire V, Vallee M, Abrous N, Piazza PV, Mayo W, et al. Long term neurodevelopmental and behavioral effects of perinatal life events in rats. Neurotox Res. 2001;3(1):65–83. doi: 10.1007/BF03033231. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Silverman WK, Lai B, Jaccard J. Hurricane-related exposure experiences and stressors, other life events, and social support: concurrent and prospective impact on children’s persistent posttraumatic stress symptoms. J Consult Clin Psychol. 2010;78(6):794–805. doi: 10.1037/a0020775. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne LL, Hepworth JT, Pawlak R, Chiafery M. Parental coping and activities during pediatric critical care. Am J Crit Care. 1992;1(2):76–80. [PubMed] [Google Scholar]
- Langley AK, Falk A, Peris T, Wiley JF, Kendall PC, Ginsburg G, et al. The child anxiety impact scale: examining parent- and child-reported impairment in child anxiety disorders. J Clin Child Adolesc Psychol. 2013 doi: 10.1080/15374416.2013.817311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last CG, Hersen M, Kazdin AE, Francis G, Grubb HJ. Psychiatric illness in the mothers of anxious children. Am J Psychiatry. 1987;144(12):1580–1583. doi: 10.1176/ajp.144.12.1580. [DOI] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, et al. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: evidence from a high-risk community sample of young adults. Int J Neuropsychopharmacol. 2009;12(6):737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- Lebowitz ER. Parent-based treatment for childhood and adolescent OCD. J Obsessive-Compuls Relat Disord. 2013;2(4):425–431. doi: 10.1016/j.jocrd.2013.08.004. [DOI] [Google Scholar]
- Lebowitz ER, Panza KE, Su J, Bloch MH. Family accommodation in obsessive–compulsive disorder. Expert Rev Neurother. 2012;12(2):229–238. doi: 10.1586/ern.11.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz ER, Woolston J, Bar-Haim Y, Calvocoressi L, Dauser C, Warnick E, et al. Family accommodation in pediatric anxiety disorders. Depress Anxiety. 2013;30(1):47–54. doi: 10.1002/da.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz ER, Omer H, Hermes H, Scahill L. Parent training for childhood anxiety disorders: the SPACE program. Cogn Behav Pract. 2014a;21(4):456–469. doi: 10.1016/j.cbpra.2013.10.004. [DOI] [Google Scholar]
- Lebowitz ER, Scharfstein LA, Jones J. Comparing family accommodation in pediatric obsessive-compulsive disorder, anxiety disorders, and nonanxious children. Depress Anxiety. 2014b;31(12):1018–1025. doi: 10.1002/da.22251. [DOI] [PubMed] [Google Scholar]
- Lebowitz ER, Shic F, Campbell D, MacLeod J, Silverman WK. Avoidance moderates the association between mothers’ and children’s fears: findings from a novel motion-tracking behavioral assessment. Depress Anxiety. 2015;32(2):91–98. doi: 10.1002/da.22333. [DOI] [PubMed] [Google Scholar]
- Lebowitz ER, Leckman JF, Feldman R, Zagoory-Sharon O, McDonald N, Silverman WK. Salivary oxytocin in clinically anxious youth: associations with separation anxiety and family accommodation. Psychoneuroendocrinology. 2016;65:35–43. doi: 10.1016/j.psyneuen.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30(10):1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- Lepine JP. The epidemiology of anxiety disorders: prevalence and societal costs. J Clin Psychiatry. 2002;63(Suppl 14):4–8. [PubMed] [Google Scholar]
- Levy DM. Maternal over-protection and rejection. J Nerv Ment Dis. 1931;73(73):65–77. [Google Scholar]
- Li X, Sundquist J, Sundquist K. Age-specific familial risks of anxiety. A nation-wide epidemiological study from Sweden. Eur Arch Psychiatry Clin Neurosci. 2008;258(7):441–445. doi: 10.1007/s00406-008-0817-8. [DOI] [PubMed] [Google Scholar]
- Lieb R, Wittchen HU, Hofler M, Fuetsch M, Stein MB, Merikangas KR. Parental psychopathology, parenting styles, and the risk of social phobia in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2000;57(9):859–866. doi: 10.1001/archpsyc.57.9.859. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Oxytocin’s role in anxiety: a critical appraisal. Brain Res. 2014;1580:22–56. doi: 10.1016/j.brainres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Mah BL, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Smith R. oxytocin promotes protective behavior in depressed mothers: a pilot study with the enthusiastic stranger paradigm. Depress Anxiety. 2014 doi: 10.1002/da.22245. [DOI] [PubMed] [Google Scholar]
- Manassis K, Lee TC, Bennett K, Zhao XY, Mendlowitz S, Duda S, et al. Types of parental involvement in CBT with anxious youth: a preliminary meta-analysis. J Consult Clin Psychol. 2014;82(6):1163–1172. doi: 10.1037/a0036969. [DOI] [PubMed] [Google Scholar]
- Maron E, Hettema JM, Shlik J. Advances in molecular genetics of panic disorder. Mol Psychiatry. 2010;15(7):681–701. doi: 10.1038/mp.2009.145. [DOI] [PubMed] [Google Scholar]
- Martin RP, Noyes J, Wisenbaker J, Huttunen MO. Prediction of early childhood negative emotionality and inhibition from maternal distress during pregnancy. Merrill-Palmer Q. 1999;45(3):370–391. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mendlowicz MV, Stein MB. Quality of life in individuals with anxiety disorders. Am J Psychiatry. 2000;157(5):669–682. doi: 10.1176/appi.ajp.157.5.669. [DOI] [PubMed] [Google Scholar]
- Merckelbach H, de Rutter C, Van den Hout MA, Hoekstra R. Conditioning experiences and phobias. Behav Res Ther. 1989;27(6):657–662. doi: 10.1016/0005-7967(89)90149-6. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Cook M. Immunization against the observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol. 1986;95(4):307–318. doi: 10.1037//0021-843x.95.4.307. [DOI] [PubMed] [Google Scholar]
- Moore PS, Whaley SE, Sigman M. Interactions between mothers and children: impacts of maternal and child anxiety. J Abnorm Psychol. 2004;113(3):471–476. doi: 10.1037/0021-843x.113.3.471. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu YH, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress (vol 12, pp 1559, 2009) Nat Neurosci. 2010;13(5):649–649. doi: 10.1038/nn0510-649e. [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Meesters C. The role of parental fearfulness and modeling in children’s fear. Behav Res Ther. 1996;34(3):265–268. doi: 10.1016/0005-7967(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Murray L, Cooper P, Creswell C, Schofield E, Sack C. The effects of maternal social phobia on mother-infant interactions and infant social responsiveness. J Child Psychol Psychiatry. 2007;48(1):45–52. doi: 10.1111/j.1469-7610.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Norman KR, Silverman WK, Lebowitz ER. Family accommodation of child and adolescent anxiety: mechanisms, assessment, and treatment. J Child Adolesc Psychiatr Nurs. 2015;28(3):131–140. doi: 10.1111/jcap.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141(2):559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychol Sci. 2004;15(12):822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10(9):1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Ost L-G. Ways of acquiring phobias and outcome of behavioral treatments. Behav Res Ther. 1985;23(6):683–689. doi: 10.1016/0005-7967(85)90066-x. [DOI] [PubMed] [Google Scholar]
- Parker G. Parental representations of patients with anxiety neurosis. Acta Psychiatr Scand. 1981;63(1):33–36. doi: 10.1111/j.1600-0447.1981.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- Pena CJ, Neugut YD, Champagne FA. Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology. 2013;154(11):4340–4351. doi: 10.1210/en.2013-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponirakis A, Susman EJ, Stifter CA. Negative emotionality and cortisol during adolescent pregnancy and its effects on infant health and autonomic nervous system reactivity. Dev Psychobiol. 1998;33(2):163–174. doi: 10.1002/(sici)1098-2302(199809)33:2<163:aid-dev7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Poulton R, Menzies RG. Non-associative fear acquisition: a review of the evidence from retrospective and longitudinal research. Behav Res Ther. 2002;40(2):127–149. doi: 10.1016/s0005-7967(01)00045-6. [DOI] [PubMed] [Google Scholar]
- Poulton R, Milne BJ, Craske MG, Menzies RG. A longitudinal study of the etiology of separation anxiety. Behav Res Ther. 2001;39(12):1395–1410. doi: 10.1016/s0005-7967(00)00105-4. [DOI] [PubMed] [Google Scholar]
- Provencal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci. 2012;32(44):15626–15642. doi: 10.1523/JNEUROSCI.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S, Costello CG. The aetiology and treatment of children’s phobias: a review. Am J Psychiatry. 1961;118:97–105. doi: 10.1176/ajp.118.2.97. [DOI] [PubMed] [Google Scholar]
- Radke S, Roelofs K, de Bruijn ER. Acting on anger: social anxiety modulates approach-avoidance tendencies after oxytocin administration. Psychol Sci. 2013;24(8):1573–1578. doi: 10.1177/0956797612472682. [DOI] [PubMed] [Google Scholar]
- Rapee RM. Potential role of childrearing practices in the development of anxiety and depression. Clin Psychol Rev. 1997;17(1):47–67. doi: 10.1016/s0272-7358(96)00040-2. [DOI] [PubMed] [Google Scholar]
- Reynolds S, Wilson C, Austin J, Hooper L. Effects of psychotherapy for anxiety in children and adolescents: a meta-analytic review. Clin Psychol Rev. 2012;32(4):251–262. doi: 10.1016/j.cpr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Rieger M, Pirke KM, Buske-Kirschbaum A, Wurmser H, Papousek M, Hellhammer DH. Influence of stress during pregnancy on HPA activity and neonatal behavior. Ann N Y Acad Sci. 2004;1032:228–230. doi: 10.1196/annals.1314.026. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry. 2011;70(3):291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MM, van Ijzendoorn MH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur Neuropsychopharmacol. 2013;23(10):1288–1295. doi: 10.1016/j.euroneuro.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Kong L, Sim LW, Sanmugam S, Broekman BF, Chen H, et al. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl Psychiatry. 2015;5:e668. doi: 10.1038/tp.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32(4):407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schiele MA, Reinhard J, Reif A, Domschke K, Romanos M, Deckert J, Pauli P. Developmental aspects of fear: comparing the acquisition and generalization of conditioned fear in children and adults. Dev Psychobiol. 2016;58(4):471–481. doi: 10.1002/dev.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada-Sugimoto M, Otowa T, Hettema JM. Genetics of anxiety disorders: genetic epidemiological and molecular studies in humans. Psychiatry Clin Neurosci. 2015;69(7):388–401. doi: 10.1111/pcn.12291. [DOI] [PubMed] [Google Scholar]
- Silove D. Perceived parental characteristics and reports of early parental deprivation in agoraphobic patients. Aust N Z J Psychiatry. 1986;20(3):365–369. doi: 10.3109/00048678609158884. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Cerny JA, Nelles WB, Burke AE. Behavior problems in children of parents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 1988;27(6):779–784. doi: 10.1097/00004583-198811000-00020. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Pina AA, Viswesvaran C. Evidence-based psychosocial treatments for phobic and anxiety disorders in children and adolescents. J Clin Child Adolesc Psychol. 2008;37(1):105–130. doi: 10.1080/15374410701817907. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Kurtines WM, Jaccard J, Pina AA. Directionality of change in youth anxiety treatment involving parents: an initial examination. J Consult Clin Psychol. 2009;77(3):474–485. doi: 10.1037/a001576119485589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueland L, Kendall PC, Steinberg L. Anxiety in children: perceived family environments and observed family interaction. J Clin Child Psychol. 1996;25(2):225–237. doi: 10.1207/s15374424jccp2502_12. [DOI] [Google Scholar]
- Sjostrom K, Valentin L, Thelin T, Marsal K. Maternal anxiety in late pregnancy and fetal hemodynamics. Eur J Obstet Gynecol Reprod Biol. 1997;74(2):149–155. doi: 10.1016/s0301-2115(97)00100-0. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76(4):281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce JF, Emde RN, Campos JJ, Klinnert MD. Maternal emotional signaling: its effect on the visual cliff behavior of 1-year-olds. Dev Psychol. 1985;21(1):195–200. doi: 10.1037/0012-1649.21.1.195. [DOI] [Google Scholar]
- Sotnikov SV, Markt PO, Malik V, Chekmareva NY, Naik RR, Sah A, et al. Bidirectional rescue of extreme genetic predispositions to anxiety: impact of CRH receptor 1 as epigenetic plasticity gene in the amygdala. Transl Psychiatry. 2014;4 doi: 10.1038/tp.2013.127. ARTN e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33(2):312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Steinhausen H-C, Foldager L, Perto G, Munk-Jorgensen P. Family aggregation of mental disorders in the nationwide Danish three generation study. Eur Arch Psychiatry Clin Neurosci. 2009;259(5):270–277. doi: 10.1007/s00406-008-0865-0. [DOI] [PubMed] [Google Scholar]
- Storch EA, Salloum A, Johnco C, Dane BF, Crawford EA, King MA, et al. Phenomenology and clinical correlates of family accommodation in pediatric anxiety disorders. J Anxiety Disord. 2015;35:75–81. doi: 10.1016/j.janxdis.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol Med. 2015;21(2):134–144. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res. 1992;574(1–2):131–137. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ. 1999;318(7177):153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36(1):144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Hollands J, Kerns CE, Pincus DB, Comer JS. Parental accommodation of child anxiety and related symptoms: range, impact, and correlates. J Anxiety Disord. 2014;28(8):765–773. doi: 10.1016/j.janxdis.2014.09.00725261837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV, Lyons MJ. Identification of the phenotype in psychiatric genetics. Eur Arch Psychiatry Clin Neurosci. 1993;243(3–4):131–142. doi: 10.1007/BF02190719. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR. The influence of maternal emotions during pregnancy on fetal and neonatal behavior. J Prenat Perinat Psychol Health. 1990;5(2):119–130. [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75(4):1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29(2):237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Vaughan KB, Lanzetta JT. Vicarious instigation and conditioning of facial expressive and autonomic responses to a model’s expressive display of pain. J Pers Soc Psychol. 1980;38(6):909–923. doi: 10.1037/0022-3514.38.6.909. [DOI] [PubMed] [Google Scholar]
- Viaux-Savelon S, Dommergues M, Rosenblum O, Bodeau N, Aidane E, Philippon O, et al. Prenatal ultrasound screening: false positive soft markers may alter maternal representations and mother-infant interaction. PLoS One. 2012;7(1):e30935. doi: 10.1371/journal.pone.0030935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AR. Prenatal influences on character. Nature. 1893;48(1243):389–390. [Google Scholar]
- Waszczuk MA, Zavos HM, Gregory AM, Eley TC. The phenotypic and genetic structure of depression and anxiety disorder symptoms in childhood, adolescence, and young adulthood. JAMA Psychiatry. 2014;71(8):905–916. doi: 10.1001/jamapsychiatry.2014.655. [DOI] [PubMed] [Google Scholar]
- Watt MC, Stewart SH, Cox BJ. A retrospective study of the learning history origins of anxiety sensitivity. Behav Res Ther. 1998;36(5):505–525. doi: 10.1016/s0005-7967(97)10029-8. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weems CF, Silverman WK. An integrative model of control: implications for understanding emotion regulation and dysregulation in childhood anxiety. J Affect Disord. 2006;91(2–3):113–124. doi: 10.1016/j.jad.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Weems CF, Berman SL, Silverman WK, Rodriguez ET. The relation between anxiety sensitivity and attachment style in adolescence and early adulthood. J Psychopathol Behav Assess. 2002;24(3):159–168. doi: 10.1023/a:1016058600416. [DOI] [Google Scholar]
- Weems CF, Silverman WK, Rapee RM, Pina AA. The role of control in childhood anxiety disorders. Cogn Ther Res. 2003;27(5):557–568. doi: 10.1023/a:1026307121386. [DOI] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38(5):694–701. doi: 10.1016/j.psyneuen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Leckman JF, Merikangas KR, Gammon GD, Prusoff BA. Depression and anxiety disorders in parents and children. Results from the Yale family study. Arch Gen Psychiatry. 1984;41(9):845–852. doi: 10.1001/archpsyc.1984.01790200027004. [DOI] [PubMed] [Google Scholar]
- Whaley SE, Pinto A, Sigman M. Characterizing interactions between anxious mothers and their children. J Consult Clin Psychol. 1999;67(6):826–836. doi: 10.1037//0022-006x.67.6.826. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Vazdarjanova A, Terry AV., Jr Exposure to variable prenatal stress in rats: effects on anxiety-related behaviors, innate and contextual fear, and fear extinction. Behav Brain Res. 2013;238:279–288. doi: 10.1016/j.bbr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, McLeod BD, Sigman M, Hwang WC, Chu BC. Parenting and childhood anxiety: theory, empirical findings, and future directions. J Child Psychol Psychiatry. 2003;44(1):134–151. doi: 10.1111/1469-7610.00106. [DOI] [PubMed] [Google Scholar]
- Wu YH, Patchev AV, Daniel G, Almeida OFX, Spengler D. Early-life stress reduces DNA methylation of the Pomc gene in male mice. Endocrinology. 2014;155(5):1751–1762. doi: 10.1210/en.2013-1868. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Brydges NM, Wood ER, Drake AJ, Hall J. Prenatal glucocorticoid exposure in rats: programming effects on stress reactivity and cognition in adult offspring. Stress. 2015;18(3):353–361. doi: 10.3109/10253890.2015.1055725. [DOI] [PubMed] [Google Scholar]
- Ziegler C, Dannlowski U, Brauer D, Stevens S, Laeger I, Wittmann H, et al. Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology. 2015;40(6):1528–1538. doi: 10.1038/npp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C, Richter J, Mahr M, Gajewska A, Schiele MA, Gehrmann A, et al. MAOA gene hypomethylation in panic disorder-reversibility of an epigenetic risk pattern by psychotherapy. Transl Psychiatry. 2016;6:e773. doi: 10.1038/tp.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]