Abstract
Microglial engulfment is a basic function to clean up dead and injured cells and invaders, such as bacteria. This study was designed to assess the effects of isoflurane on the microglial engulfment induced by lipopolysaccharide (LPS) plus interferon-γ (IFN-γ) and the involvement of p38 mitogen-activated protein kinase (MAPK) in these effects. C8-B4 microglial cells were exposed to 1, 2, and 3% isoflurane at 2 h after the initiation of LPS (100 ng/μl) and IFN-γ (1 ng/μl) stimulation. Fluorescent immunostaining was performed to assess the percentage of cells with engulfment of fluorescent microspheres after stimulation for 24 h. P38 and phosphorylated p38 were determined by Western blotting. Isoflurane concentration-dependently decreased microglial engulfment stimulated by LPS and IFN-γ. LPS and IFN-γ increased the phosphorylated p38 in microglial cells. This up-regulation was decreased by isoflurane. SB203580, a p38 MAPK inhibitor, abolished the LPS and IFN-γ-induced increase of engulfment activity; whereas anisomycin, a p38 MAPK activator, partly reversed the isoflurane-decreased microglial engulfment activity. These results suggest that isoflurane reduces LPS and IFN-γ-induced microglial engulfment and that these effects may be mediated by inhibiting p38 MAPK.
Keywords: engulfment, isoflurane, microglia, p38 MAPK
Introduction
Microglia are the major immune cells with phagocytic activity in the central nervous system (CNS) [1]. They respond to various endogenous and exogenous stimuli, such as bacterial products, virus, prion and β-amyloid, to maintain tissue homeostasis [1]. Microglial activation can be beneficial or detrimental depending on the stimuli and the environment [2]. The functional consequences of microglial phagocytosis remain largely unexplored. Phagocytosis is beneficial when it eliminates dead cells and induces an anti-inflammatory response. Rapid phagocytic removal of dead or dying cells prevents the release of proinflammatory intracellular components and contributes to the resolution of inflammation [3]. On the other hand, microglial phagocytosis can exacerbate the loss of viable cells during neuroinflammation [4] and can also activate the respiratory burst to produce toxic reactive oxygen species [5].
Volatile anesthetics have been known to reduce neuroinflammation induced by lipopolysaccharide (LPS) and interferon (IFN)-γ [6, 7]. However, there is very little information regarding whether volatile anesthetics affect microglial phagocytosis or engulfment. Hence, this study was designed to assess the effects of isoflurane on the microglial engulfment induced by LPS plus IFN -γ in C8-B4 mouse microglial cells. Since the engulfment of microglia has been shown to be mediated by p38 mitogen-activated protein kinase (MAPK) [8, 9], we also assessed the role of p38 MAPK in the isoflurane effects on microglial engulfment.
Materials and methods
Materials
C8-B4 cells (CRL-2540™), a microglial clone isolated from 8-day old mouse cerebellum, were purchased from the American Type Culture Collection (Manassas, VA). Heat inactivated fetal bovine serum (FBS) and fluorescent microspheres (FluoSpheres®) were purchased from Invitrogen Corporation (Carlsbad, CA). Isoflurane was purchased from Abbott Laboratories (North Chicago, IL). Rabbit monoclonal anti-ionized calcium binding adaptor molecule 1 (Iba1) antibody was purchased from Wako Chemical USA, Inc. (Richmond, VA). Chamber slides, Hoechst solution and Pierce™ BCA Protein Assay Kit were from Thermo Scientific (Waltham, MA). Precast gels for Western blots were purchased from Bio-Rad Laboratories, Inc. (Hercules, CA). Lipopolysaccharide (Escherichia coli 0111:B4) and other general chemicals (recombinant rat IFN-γ produced from E. coli, anisomycin and SB203580) except for those described below were obtained from Sigma-Aldrich (St. Louis, MO).
Cell culture
C8-B4 microglial cells were cultured as described previously [6]. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4 mM L-glutamine, 4500 mg/l glucose, 1 mM sodium pyruvate, 1500 mg/l sodium bicarbonate, and supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin and 100 pg/ml streptomycin in a humidified atmosphere of 95% air–5% CO2 at 37°C. The medium was changed every 2 or 3 days. The cells were plated at a density of 3 - 4 ×104 cells/well on 8-well chamber slides for fluorescent staining and at a density of 2.5 - 3 × 105 cells/mL in 6-well plates for protein assay and Western blotting experiments. Microglial cells were cultured for 24 h before LPS and IFN-γ stimulation.
Isoflurane exposure and application of chemicals
The first experiment was designed to determine the concentrations of LPS and IFN-γ used in the subsequent experiments. Cells were incubated with various concentrations of LPS and IFN- γ for 24 h and the cells were then harvested. Preliminary work showed that 100 ng/μl LPS plus 1 ng/μl IFN- γ significantly increased the engulfment of fluorescent microspheres and this combination was chosen for the subsequent experiment.
Isoflurane was applied for 1 h at 2 h after the administration of LPS plus IFN- γ to the cells to assess the post-treatment effect of isoflurane. This isoflurane exposure of the cells was performed in an airtight chamber as we described previously [6, 7]. The chamber slides were gassed with 95% air–5% CO2 through an isoflurane vaporizer set at 0, 1, 2, or 3 % for 1 h at 37 °C. The isoflurane concentrations in the gases from the outlet of the chambers were monitored with a DatexTM infrared analyzer (Capnomac, Helsinki, Finland) and reached the target concentrations at 5 min after the onset of gassing. The chamber was sealed and the incubation was for 55 min at 37°C (the total isoflurane exposure time is 1 h). At the end of the incubation time, the isoflurane concentrations in the gases from the outlet of the chamber were confirmed to be at the target concentrations. The cells in chamber slides were then transferred from the air tight chamber to their normal culture conditions for 22 h at 37°C. Isoflurane at 2 % was used for other experiments after the isoflurane concentration-response experiment.
The p38 MAPK activator anisomycin at 0.1 μM or inhibitor SB203580 at 20 μM was applied just before the isoflurane administration to determine the involvement of p38 MAPK in the isoflurane effects on microglial engulfment.
Analysis of engulfment
Engulfment was assessed using 1.0 μm Nile red polystyrene microspheres (FluoSpheres ® Fluorescent Microspheres F-8819) at a concentration of 10,000 particles/ml culture media. These microspheres were added and incubated for 30 min (37°C, 5% CO2) at 24 h after LPS plus IFN-γ stimulation. Bovine serum albumin (BSA) was used to facilitate engulfment as an opsonizing agent.
Cell staining and fixation
To reveal the fluorescent microspheres, C8-B4 cells were washed with phosphate buffered saline (PBS) for 3 times (5 min/wash) in dark. Cells were then fixed with 100% MeOH for 10 min at −20°C, permeabilized with 0.1% Triton X-100 diluted in PBS for 10 min and blocked with 1% BSA for 30 min at room temperature. Cells were incubated with rabbit monoclonal anti-Iba1 antibody at 4°C overnight in dark. Incubation with secondary antibody for 1 h was performed at room temperature. Cells were washed and nuclei were counterstained with Hoechst 33342 for 5 min at room temperature. The gasket and rubber seal were removed from the chamber slides that were covered with 2 - 3 drops of Vectashied mounting media (Vector Laboratories) and coverslip. The coverslip was sealed with clear nail polish and allowed to dry for 20 min in the dark.
Cell count and engulfment assay
Cell images were obtained with a fluorescent microscope (Olympus DP70 Digital Microscope Camera System) after staining and fixation. Each chamber of slide was divided into 6 compartments to obtain the images. Cells with or without fluorescent microspheres were counted in each compartment. The percentage of microglia engulfing one or more microspheres was calculated (microglial cells with fluorescent microspheres /total microglial cells x100).
Western blotting
Western blotting was performed as described before [6]. Cells for Western blotting were plated at a density of 2.5 - 3 × 105 cells/mL in 6-well plates. After being cultured in the plates for 24 h, they were subjected to the treatment with LPS, IFN-γ and isoflurane as described above. Cells were lysed and homogenized in 25 mM Tris–HCl, pH 7.4, containing 1 mM EDTA, 1 mM EGTA, 0.1% (vol/vol) α-mercaptoethanol, 1 μM phenylmethylsulfonyl fluoride, 2 μM leupeptin, and 1 μM pepstatin A. The homogenates were centrifuged at 14,000 X g for 10 min at 4°C. The supernatant was used for protein assay with a bicinchoninic acid protein assay kit (Pierce). The protein was loaded at 20 μg protein per lane on a sodium dodecyl sulfate (SDS)-polyacrylamide gel, and then transferred to a polyvinylidene difluoride membrane (Millipore). After incubation with an anti-phospho-p38 antibody(1:1000; Cell Signaling Technology) and an anti-p38 antibody (1:500; Cell Signaling Technology), the protein bands were visualized by using Western blotting detection reagents (GE Healthcare, Piscataway, NJ). The protein bands were densitometrically analyzed by an ImageQuant 5.0 densitometer (Amersham Biosciences, Piscataway, NJ). The results of cells treated with various conditions were then normalized by the data of control cells.
Data analysis
Each experimental condition was repeated more than 5 times (n for each condition is described in the figure legends), each with a different batch of cells. All statistical analyses were performed with SPSS software. Statistical analyses were performed by one-way analysis of variance followed by the t-test. Data are expressed as mean ± S.D. A P value < 0.05 was considered to indicate statistical significance.
Results
Effects of isoflurane on microglial engulfment
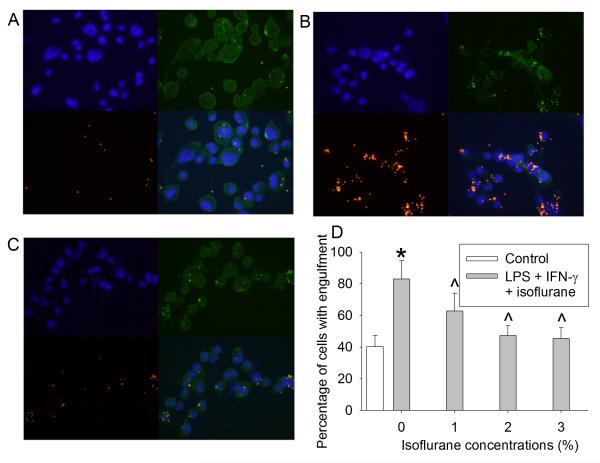
The administration of LPS and IFN-γ to C8-B4 microglial cells increased the percentage of microglia with engulfment compared with control (40 ± 7 % for control group vs. 82 ± 11 % for LPS and IFN-γ group; P < 0.001, Fig. 1). Isoflurane applied 2 h after the initiation of LPS and IFN-γ stimulation concentration-dependently decreased microglial engulfment (Fig. 1).
Fig. 1. Effects of isoflurane on microglial engulfment.
(A) image of control cells; (B) images of cells incubated with 100 ng/μl LPS plus 1 ng/μl IFN-γ; (C) images of cells exposed to 2% isoflurane applied 2 h after the initiation of LPS and IFN-γ stimulation. Blue: Hoechst 33342 staining, green: Iba-1 staining, red: fluorescent microspheres. (D) concentration-response of isoflurane effects on microglial engulfment. Data are expressed as the mean ± S.D. (n = 6). * P < 0.05 compared with control, ^ P < 0.05 compared with LPS plus IFN-γ alone group.
Role of p38 MAPK in the isoflurane effects
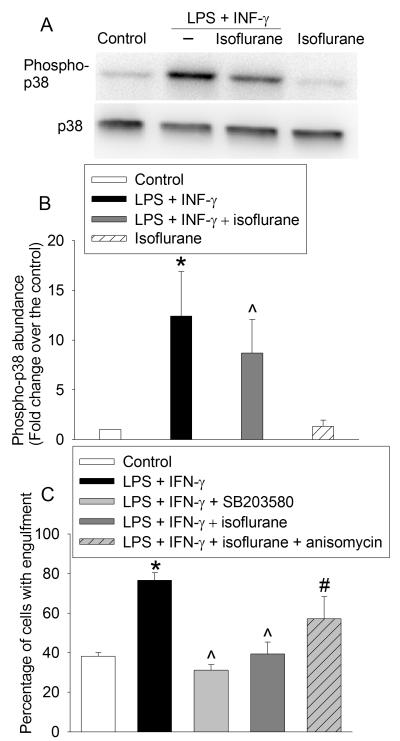
Western blotting revealed that the phosphorylated p38 in the microglial cells was up-regulated in LPS plus IFN-γ group compared with control group (4 ± 0 % for control group vs. 35 ± 6 % for LPS and IFN-γ group, P < 0.001, Fig. 2). This up-regulation was decreased by 2% isoflurane (25 ± 7 % for LPS plus IFN-γ plus isoflurane group, P = 0.028, Fig. 2). SB203580 at 20 μM abolished the LPS and IFN-γ-induced increase of the percentage of cells with engulfment (77 ± 4 % for LPS and IFN-γ group vs. 38 ± 2 % for LPS and IFN-γ plus SB 203580 group, P < 0.001, Fig. 2). In addition, anisomycin at 0.1 μM partly reversed the isoflurane-induced decrease of microglial engulfment (40 ± 6 % for LPS plus IFN-γ plus 2% isoflurane group vs. 57 ± 11 % for LPS plus IFN-γ plus 2% isoflurane plus anisomycin group, P < 0.018, Fig. 2).
Fig. 2. Role of p38 mitogen activated protein kinase (MAPK) in the effects of isoflurane on microglial engulfment.
(A) representative of Western blotting images; (B) quantitative results of Western blotting; (C) effects of p38 MAPK inhibition and activation on microglial engulfment. Data are expressed as the mean ± S.D. (n = 5 for panel B and = 6 for panel C). * P < 0.05 compared with control, ^ P < 0.05 compared with LPS plus IFN-γ alone group, # P < 0.05 compared with LPS plus IFN-γ plus isoflurane group.
Discussion
Our study showed that LPS plus IFN-γ increased microglial engulfment. The combination of LPS and IFN-γ was shown to activate microglial cells to engulf cell debris [9]. LPS is a major component of the outer membrane of gram-negative bacteria and has been frequently used to induce a strong immune response of microglia [9]. IFN-γ is a cytokine that acts as an activator of microglia and enhances the LPS effect. The combination of LPS and IFN-γ has been a potent stimulator of microglia [6, 10, 11]. The results of the present study with LPS plus IFN-γ were consistent with the previous in vitro and in vivo investigations of phagocytosis. For example, Tanaka et al. showed that treatment with LPS or IFN-β was necessary for the primary microglial cultures to become phagocytic [9] and systemic injection of LPS accelerated phagocytic activity during Wallerian degeneration in the injured central nervous system [12].
Isoflurane is a volatile anesthetic that is often used for neurosurgical patients. In the present study, isoflurane post-treatment at clinically relevant concentrations decreased LPS and IFN-γ-induced enhanced engulfment of microglia. Previous studies showed that pre-conditioning and post-conditioning with isoflurane decreased microglial activation and injury induced by LPS & IFN-γ through inducible nitric oxide synthase–nitric oxide–glutamate pathway [6, 7] but there are no data on the effects of isoflurane on the microglial engulfment. We observed a concentration-dependent effect of isoflurane on microglial engulfment.
After injuries to the central nervous system, microglial cells are activated and migrate within a few hours toward the lesion site to eliminate the debris [13]. However, the mechanism underlying phagocytosis remains to be explored. P38 MAPK is a group of important intracellular signaling molecules that are responsive to cellular stress and inflammatory cytokines [14]. LPS has been known to activate p38 MAPK by binding to Toll-like receptors that are a class of pattern-recognition receptors in the innate immune system to induce inflammatory responses [14]. Recent data have shown that p38 MAPK is involved in LPS-induced microglial phagocytosis of axonal debris [9] and fluorescent material-conjugated Escherichia coli particles [15]. Tanaka et al. established an in vitro assay system to estimate phagocytosis of axon debris and found that p38 MAPK was activated in microglial cells when they were co-cultured with degenerated axons [9]. In addition, engulfment of axon debris was blocked by the p38 MAPK inhibitor SB203580, indicating that p38 MAPK is required for phagocytic activity [9]. Therefore, p38 MAPK was chosen for the elucidation of the mechanism for the effects of isoflurane on the microglial engulfment in this study.
Our study showed that the phosphorylated p38 MAPK was up-regulated by LPS and IFN-γ and this up-regulation was decreased by the isoflurane post-treatment. Thus, activation of p38 MAPK may be involved in the isoflurane effect on microglial engulfment. Consistent with this idea, LPS & IFN-γ-stimulated microglia engulfment were significantly blocked by a p38 MAPK inhibitor (SB203580) and isoflurane effect was partially reversed by a p38 MAPK activator (anisomycin).
Dead and dying neurons are quickly removed through phagocytosis by the microglia during brain inflammation but phagocytosis may induce neuronal injury [16], which is relevant to a variety of brain pathologies including central nervous system infections, stroke, Alzheimer’s disease, and Parkinson’s disease [16]. Our data indicate that isoflurane post-treatment after microglial activation can reduce engulfment. Attenuated phagocytosis might be beneficial and neuroprotective during brain trauma, ischemia, and neuroinflammation. However, microglial engulfment and phagocytosis is a complex process. Further studies are needed to fully understand the implication of isoflurane effects on microglial engulfment.
Our study has limitations. First, microglial engulfment was assessed in vitro. One must be cautious in directly extrapolating these results to in vivo conditions. Second, microglial cells were incubated with fluorescent microspheres for 30 min. The promptness of microglia to engulf fluorescent microspheres is one of the important parameters to consider when analyzing the dynamics of phagocytosis. A previous investigation showed that the time to completely eliminate an apoptotic cell by microglia was 25 – 95 min in a co-culture system in vitro [17]. Our preliminary experiments showed that the attachment of fluorescent microspheres to the slide and cells was difficult to wash away if the incubation time was more than 40 min. Therefore, 30 min was chosen as the incubation time.
Conclusion
our study showed that isoflurane post-treatment after microglial activation with LPS and IFN-γ can reduce microglial engulfment. This effect may involve inhibition of p38 MAPK.
Acknowledgment
This study was supported by a grant (R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, MD and the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, VA
Abbreviations
- CNS
central nervous system
- FBS
fetal bovine serum
- Iba1
ionized calcium binding adaptor molecule 1
- IFN-γ
interferon-γ
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
Footnotes
Conflict of interest: No.
References
- 1.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 2.Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, Schwartz A, Smirnov I, Pollack A, Jung S, Schwartz M. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006a;116(4):905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010;17(3):381–397. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 4.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64(1):110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JA, Li L, Zuo Z. Delayed treatment with isoflurane attenuates lipopolysaccharide and interferon gamma-induced activation and injury of mouse microglial cells. Anesthesiology. 2009;111(3):566–573. doi: 10.1097/ALN.0b013e3181af5b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Kim JA, Zuo Z. Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma. Neurosci. 2008;154(3):1002–1008. doi: 10.1016/j.neuroscience.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama T, Kobayashi H, Okamura T, Yamasaki-Katayama Y, Kibayashi T, Kimura H, Ohsawa K, Kohsaka S, Minami M. Accumulating microglia phagocytose injured neurons in hippocampal slice cultures: involvement of p38 MAP kinase. PLoS One. 2012;7(7):e40813. doi: 10.1371/journal.pone.0040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka T, Ueno M, Yamashita T. Engulfment of axon debris by microglia requires p38 MAPK activity. J Biol Chem. 2009;284(32):21626–21636. doi: 10.1074/jbc.M109.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong HJ, Lin D, Li L, Zuo Z. Delayed treatment with lidocaine reduces mouse microglial cell injury and cytokine production after stimulation with lipopolysaccharide and interferon gamma. Anesth Analg. 2012;114(4):856–861. doi: 10.1213/ANE.0b013e3182460ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Choi JJ, Park BK, Yoon SJ, Choi JE, Jin M. Pheophytin a and chlorophyll a suppress neuroinflammatory responses in lipopolysaccharide and interferon-gamma-stimulated BV2 microglia. Life Sci. 2014;103(2):59–67. doi: 10.1016/j.lfs.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Vallieres N, Berard JL, David S, Lacroix S. Systemic injections of lipopolysaccharide accelerates myelin phagocytosis during Wallerian degeneration in the injured mouse spinal cord. Glia. 2006;53(1):103–113. doi: 10.1002/glia.20266. [DOI] [PubMed] [Google Scholar]
- 13.Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15(4):209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- 14.Doyle SE, O'Connell RM, Miranda GA, Vaidya SA, Chow EK, Liu PT, Suzuki S, Suzuki N, Modlin RL, Yeh WC, Lane TF, Cheng G. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199(1):81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun HN, Kim SU, Lee MS, Kim SK, Kim JM, Yim M, Yu DY, Lee DS. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent activation of phosphoinositide 3-kinase and p38 mitogen-activated protein kinase signal pathways is required for lipopolysaccharide-induced microglial phagocytosis. Biol Pharm Bull. 2008;31(9):1711–1715. doi: 10.1248/bpb.31.1711. [DOI] [PubMed] [Google Scholar]
- 16.Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186(8):4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- 17.Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10(14):857–860. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]