Abstract
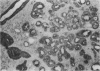
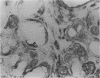
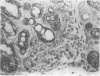
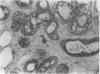
AIM: To determine whether transforming growth factor-beta 1 (TGF-beta 1) has a pathogenetic role in disease of the salivary glands. METHODS: An indirect immunohistochemical technique was used to analyse TGF-beta 1 expression in six specimens of normal salivary gland and 23 surgical specimens. RESULTS: TGF-beta 1 was strongly expressed in the ductal epithelial cells of normal salivary gland tissues (six of six cases) and in inflammatory conditions (eight of 11 cases). In contrast, TGF-beta 1 was not detectable in ductal epithelial cells expressing HLA-DR around infiltrating CD4+ CD45RO+ activated T cells, in the salivary gland tissue of patients with Sjögren's syndrome. CONCLUSION: Because TGF-beta 1 has an essential role in the mucosal immunity of salivary glands, abnormal expression of this cytokine must be regarded as a candidate in the pathogenesis of Sjögren's syndrome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- Chin Y. H., Cai J. P., Xu X. M. Transforming growth factor-beta 1 and IL-4 regulate the adhesiveness of Peyer's patch high endothelial venule cells for lymphocytes. J Immunol. 1992 Feb 15;148(4):1106–1112. [PubMed] [Google Scholar]
- Czarniecki C. W., Chiu H. H., Wong G. H., McCabe S. M., Palladino M. A. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988 Jun 15;140(12):4217–4223. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Bumol T., Fantozzi R., Bone R., Schreiber R. Expression of histocompatibility antigen HLA-DR by salivary gland epithelial cells in Sjögren's syndrome. Arthritis Rheum. 1986 Sep;29(9):1105–1111. doi: 10.1002/art.1780290908. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Kang H. I., Ando D., Abrams J., Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren's syndrome. J Immunol. 1994 Jun 1;152(11):5532–5539. [PubMed] [Google Scholar]
- Fox R. I., Saito I. Criteria for diagnosis of Sjögren's syndrome. Rheum Dis Clin North Am. 1994 May;20(2):391–407. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. R., Mcniece I. K., Sill K. T., Ellingsworth L. R., Quesenberry P. J., Sing G. K., Ruscetti F. W. Transforming growth factor beta directly regulates primitive murine hematopoietic cell proliferation. Blood. 1990 Feb 1;75(3):596–602. [PubMed] [Google Scholar]
- Kilshaw P. J., Murant S. J. Expression and regulation of beta 7(beta p) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol. 1991 Oct;21(10):2591–2597. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Hedfors E., Klareskog L., Forsum U. Epithelial HLA-DR expression and T lymphocyte subsets in salivary glands in Sjögren's syndrome. Clin Exp Immunol. 1985 Sep;61(3):475–482. [PMC free article] [PubMed] [Google Scholar]
- Mann D. L., Moutsopoulos H. M. HLA DR alloantigens in different subsets of patients with Sjögren's syndrome and in family members. Ann Rheum Dis. 1983 Oct;42(5):533–536. doi: 10.1136/ard.42.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., O'Shea J. J., Smyth M. J., Falk L. A., Kennedy I. C., Longo D. L., Ruscetti F. W. Mechanistic studies of transforming growth factor-beta inhibition of IL-2-dependent activation of CD3- large granular lymphocyte functions. Regulation of IL-2R beta (p75) signal transduction. J Immunol. 1991 Jun 1;146(11):3791–3798. [PubMed] [Google Scholar]
- Parker C. M., Cepek K. L., Russell G. J., Shaw S. K., Posnett D. N., Schwarting R., Brenner M. B. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1924–1928. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow H. J. BSC-1 growth inhibitor/type beta transforming growth factor is a strong inhibitor of thymocyte proliferation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5531–5533. doi: 10.1073/pnas.83.15.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Servenius B., Compton T., Fox R. I. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989 Jun 1;169(6):2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Terauchi K., Shimuta M., Nishiimura S., Yoshino K., Takeuchi T., Tsubota K., Miyasaka N. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren's syndrome. J Clin Lab Anal. 1993;7(3):180–187. doi: 10.1002/jcla.1860070309. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. TGF-beta: problems and prospects. Cell Regul. 1990 Nov;1(12):875–882. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi M., Saito I., Yamamoto K., Miyasaka N. Spontaneous production of Epstein-Barr virus by B lymphoblastoid cell lines obtained from patients with Sjögren's syndrome. Possible involvement of a novel strain of Epstein-Barr virus in disease pathogenesis. Arthritis Rheum. 1993 Jun;36(6):827–835. doi: 10.1002/art.1780360614. [DOI] [PubMed] [Google Scholar]
- Tsubota K. The importance of the Schirmer test with nasal stimulation. Am J Ophthalmol. 1991 Jan 15;111(1):106–108. doi: 10.1016/s0002-9394(14)76908-9. [DOI] [PubMed] [Google Scholar]