Abstract
Malaria continues to cause alarming morbidity and mortality in more than 100 countries worldwide. Antigens in the various life cycle stages of malaria parasites are presented to the immune system during natural infection and it is widely recognized that after repeated malaria exposure, adults develop partially protective immunity. Specific antigens of natural immunity represent among the most important targets for the development of malaria vaccines. Immunity against the transmission stages of the malaria parasite represents an important approach to reduce malaria transmission and is believed to become an important tool for gradual elimination of malaria. Development of immunity against Plasmodium falciparum sexual stages was evaluated in primary school children aged 6–16 years in Makoni district of Zimbabwe, an area of low to modest malaria transmission. Malaria infection was screened by microscopy, rapid diagnostic tests and finally using nested PCR. Plasma samples were tested for antibodies against recombinant Pfs48/45 and Pfs47 by ELISA. Corresponding serum samples were used to test for P. falciparum transmission reducing activity in Anopheles stephensi and An. gambiae mosquitoes using the membrane feeding assay. The prevalence of malaria diagnosed by rapid diagnostic test kit (Paracheck)™ was 1.7 %. However, of the randomly tested blood samples, 66% were positive by nested PCR. ELISA revealed prevalence (64% positivity at 1:500 dilution, in randomly selected 66 plasma samples) of antibodies against recombinant Pfs48/45 (mean A405nm = 0.53, CI=0 .46 to 0.60) and Pfs47 (mean A405nm= 0.91, CI=0.80to 1.02); antigens specific to the sexual stages. The mosquito membrane feeding assay demonstrated measurable transmission reducing ability of the samples that were positive for Pfs48/45 antibodies by ELISA. Interestingly, 3 plasma samples revealed enhancement of infectivity of P. falciparum in An. stephensi mosquitoes. These studies revealed the presence of antibodies with transmission reducing immunity in school age children from a moderate transmission area of malaria, and provide further support to exploit target antigens such as Pfs48/45 for further development of a malaria transmission blocking vaccine.
1. Introduction
Despite significant reduction in the overall malaria cases and deaths, it still remains a major challenge in many parts of the world with >90% death reported in sub-Saharan Africa (WHO 2015). Children under the age of 5 years and pregnant women are at greatest risk of malaria mortality and morbidity. Recent gains in reducing malaria burden largely attributed to rapid diagnosis, use of insecticide treated bednet, indoor residual spraying and treatment using artemisinin combination therapy are constantly threatened by the development of insecticide resistance in the mosquito vector and parasites resistant to anti-malarial drugs. Vaccines targeting different life cycle stages of the parasite are likewise believed to offer additional tools as a long-term strategy to eliminate malaria. The rationale for many of these vaccine candidates being pursued is derived from partially protective stage specific immunity that develops during repeat exposure to malaria infection (Crompton et al., 2014). One such target stage includes gametocytes developing as intraerythrocytic parasites. Gametocytes are crucial for transmission of malaria from an infected person to mosquito vector. Upon ingestion, gametocytes undergo gametogenesis into male and female gametes which undergo fertilization and further sporogonic development (Dantzler et al., 2015; Nilsson et al., 2015; STONE et al., 2016).
Antigens in the gametocytes are also presented to the immune system of the host and studies have revealed age-related and transmission exposure related antibody responses against many sexual stage antigens (Bousema and Drakeley, 2011). This includes antibodies against Pfs48/45 and Pfs230, expressed within developing P. falciparum gametocytes and the expression persisting on the surface of extracellular male and female gametes as a membrane-bound complex (KUMAR, 1987; Kumar and Wizel, 1992). Transmission reducing immunity targeting sexual stage development of the parasite develops naturally during infection after exposure to gametocytes. Antibodies against Pfs48/45 and Pfs230 are associated with naturally occurring transmission reducing immunity, however their presence or titers do not accurately predict functionality measured by mosquito membrane feeding assays (Bousema and Drakeley, 2011). Studies have established that this form of immunity is primarily mediated by antibodies recognizing antigens uniquely expressed on male and female gametes (Carter et al., 2000; Sinden, 2010) and naturally occurring transmission reducing antibodies affect transmission success by preventing fertilization of gametes and further development of parasites in the mosquito midgut (Carter, 2001; Sinden, 2010). Antibodies directed against specific epitopes on Pfs230 and Pfs48/45 antigens when ingested along with gametocytes during transmission have been shown to negatively impact parasite development in the mosquito midgut and reduce transmission success (Quakyi et al., 1987; Rener et al., 1983). Antibodies to Pfs230 and Pfs48/45 prevent the fusion of the male and female gametes during sexual reproduction, consequently mosquitoes fail to produce oocysts and are ineffective for further transmission during the next blood meal, thereby stopping the parasite’s life cycle (Quakyi et al., 1987; Rener et al., 1983). This has led to the concept of transmission blocking immunity and Pfs48/45 and Pfs230 are being pursued as candidate vaccine antigens. Biologically, Pfs48/45 is critical for the fertility of the male gamete (van Dijk et al., 2001), and the Pfs47, a related molecule belonging to the 6-cysteine protein family is expressed in female gametocytes and gametes (Anthony et al., 2007; Molina-Cruz et al., 2013; van Schaijk et al., 2006).
In the present study we had the opportunity to investigate if transmission reducing immunity develops in children exposed to low to moderate level of malaria transmission. The study population came from Zimbabwe where P. falciparum is transmitted by Anopheles arabiensis, and no previous studies exist in the country on natural immunity to sexual stage antigens (Lukwa et al., 2014; Mabaso et al., 2004; Mharakurwa et al., 2012). To evaluate if immunity exists in naturally exposed individuals, school going children from a low to moderate area of malaria transmission were recruited to determine malaria positivity by rapid diagnostic tests and nested PCR, transmission reducing activity and the presence of antibodies against the recombinant proteins of Pfs48/45 and Pfs47 expressed in gametocytes and gametes.
2. Materials and Methods
2.1. Study area and population
The study was conducted at Bandanyenje primary school in Makoni district in the eastern Zimbabwe. Makoni district reported a malaria incidence rate of 46.2 in 1,000 in 2015 and ranked as 18/20 on the national malaria incidence top twenty chart (Government of Zimbabwe, Ministry of Health, 2011 data). It borders the Mutasa, Nyanga and Mutoko areas reporting four times higher incidence of malaria (160/1000). The study was part of a study on schistosomiasis and malaria co-infection in an endemic area east of Zimbabwe (Midzi et al., 2011). The study was registered and ethically approved by the country’s ethics board for biomedical research, the Medical Research Council of Zimbabwe (MRCZ/A/1710).
The study design comprised of a cross sectional survey of 150 Bandanyenje primary school-going children aged 6–16 during the malaria transmission season, between February and May 2013. Permission was granted from the community leaders, Provincial Medical Director, District Medical Officer and parents provided consent for participation of the children. The children were educated on the aims, risks and benefits of the study focusing on malaria and Schistosomiasis, and those who were willing to participate signed an assent form. Parents were also given assent forms to complete if they agreed to allow them to participate in the study and the participation was totally voluntary. The primary inclusion criterion for the study was based on willingness to provide required samples for the study goals. Children who visibly appeared malnourished and unfit or presenting with any unrelated illness were excluded from participation in the study. All the children provided a stool and urine specimen over three consecutive days for schistosomiasis and soil transmitted helminth detection. Blood was collected on the third day for malaria diagnosis (thick smear microscopy, rapid diagnostic test and PCR) and isolation of plasma for immunological investigations. Children positive based on urine and stool parasitology examination were treated with Praziquantel at 40 mg/ kg body weight for schistosomiasis and albendazole for soil transmitted helminth infection. The collected blood, plasma samples were kept frozen at −20°C in the field at a health facility then transported to the laboratory in Harare and kept at −80°C until transported as de-identified samples to Tulane University for analysis by nested PCR for P. falciparum, ELISA for antibodies against Pfs48/45, Pfs47 and total asexual lysates, and mosquito membrane feeding assays for assessment of transmission reducing activity.
2.2. Diagnosis of P. falciparum malaria
Immediately on collection, each individual blood sample was tested using rapid malaria diagnostic test (RDT) (Paracheck™). Thin and thick blood smears were stained in 10 % Giemsa stain, for 10–15 minutes, rinsed and air dried. The thick film was also examined under the microscope for detection of P. falciparum infection and were found to be negative. To confirm the lack of thick smear positivity recorded on the samples during the field examinations, the stained slides were also transported for quality assurance at a later date by an expert microscopist from the National Institute of Health Research in Harare. Results from both RDT and microscopy were used to provide nationally recommended treatment (Artemether + Lumefantrine) to malaria positive participants at the local health facility.
2.3. Nested PCR for the detection of P. falciparum
A Qiagen whole blood DNA extraction kit was used to extract DNA from the blood samples stored at −20°C. Briefly, 400 μl of blood sample was added to a tube containing 20 μl proteinase K (reconstituted as per the kit) and 200 μl of lysis buffer and DNA purified as per the kit protocol and measured using Nanondrop (Thermo Scientific). A nested PCR protocol was used for the detection of Plasmodium DNA (Mahajan et al., 2012). The primers used for pan Plasmodium PCR amplification were PaF (forward) 5′-GAACGAGATCTTAACCTGCTAA-3′, and PaR (reverse) 5′-TCAGCACAATCTGATGAATCAT-3′. Primers used for nested PCR specific for P. falciparum were PfnF 5′-ACATAGGTAACTATACATTTATTCAGT-3′ and PfnR 5′-AGCATCAAAGATACAAATATAAGCA-3′. All primers were synthesized by Qiagen and reconstituted in PCR grade water at appropriate concentration. First PCR reaction (25 μl) contained 1 μl of 10mM dNTPs, 1 μl of 50 mM MgCl2, PCR primers (PaF and PaR), 0.5 μl Taq polymerase, 5 μl DNA template, 2.5 μl of 10X PCR buffer and water. For the nested PCR reaction, 2 μl template (PCR product of first pan Plasmodium PCR) and primers PfnF and PfnR were used. First pan PCR cycling conditions consisted of denaturing at 94°C for 2 min, followed by 40 cycles of amplification (94°C for 30s, 57°C for 30s, and 72°C for 30s) and a final extension at 72°C for 7min. The nested PCR conditions included 3 minutes of denaturation at 94°C, followed by 30 cycles of amplification (94°C for 30s, 55°C for 30s, and 72°C for 30s) and a final extension at 72°C for 7min. The negative control contained all but PCR-grade water in place of template DNA and P. falciparum (3D7 strain) genomic DNA was used as a positive control. PCR amplified products were analyzed using 2% Agarose gel and staining with ethidium bromide and photographed using BioRad GelDoc XR+.
2.4. ELISA
Plasma samples were initially screened for antibodies against erythrocytic asexual stage antigens using P. falciparum (NF54) as described (Sangweme et al., 2010). Briefly, cultured parasites were treated with 0.15% saponin to free parasites from erythrocytes, lysed by sonication and centrifugation (10,000 rpm for 30 min at 4°C). Protein concentration in the supernatant stored at −80°C was measured using BCA method and used to determine antibodies against asexual stage antigens. To analyze plasma samples for antibodies to the gametocyte stage antigens, E. coli expressed recombinant Pfs48/45 (Chowdhury et al., 2009) and Pfs47 (Kumar Lab, unpublished) were used in ELISA. Full length codon harmonized sequences of Pfs48/45 and Pfs47 (without N-terminal signal and C-terminal anchor sequences) were cloned in the E. coli expression vector pET(K-) and purified as described (Chowdhury et al., 2009). Previous studies with purified recombinant Pfs48/45 have also established functionally effective potency of immune responses elicited in pre-clinical studies in mice and baboons (Chowdhury et al., 2009). Initially plasma dilutions (1:100 to 1:1000), antigen coating concentration (1–5 μg/ml) and secondary antibody (peroxidase conjugated anti-human IgG) dilutions (1:5000 to 1:20,000) were used to optimize the ELISA protocol. ELISA plates (Immulon 4HBX) were coated (100 μl/well) with 5 μg/ml (asexual stage antigens) or 1 μg/ml of antigens (Pfs48/45 or Pfs47) in 50 mM carbonate buffer (pH 9.6) overnight, washed three times with phosphate buffered saline containing 0.05% Tween 20 (PBST) and blocked with 5% non-fat milk in PBST. Plasma samples (100 μl/well) were added at 1:500 dilution in PBST and peroxidase-conjugated goat anti-human IgG was used at 1: 5000 dilution in blocking buffer. All incubations were done for 1 hour, at 37°C. The ABTS substrate (Kirkegaard Perry Labs) was added at 100 μl/ well and plates incubated in the dark for 20 minutes and the plates were read at 405 nm (Molecular Devices, USA). A pool of normal human sera (N=10) from North American donors who had never travelled to any malaria endemic countries was used as a negative control. Positive ELISA reactivity of test human plasma was defined if the absorbance at 405 nm was greater than the absorbance with normal human sera plus 2 standard deviation (SD) tested at the same dilution.
2.5. Membrane feeding assay (MFA)
The samples that were positive in the Pfs48/45 ELISA test were randomly picked and tested for transmission reducing activity by MFA in two different species of anopheline mosquitoes: An. gambiae and An. stephensi. Starved 25–30 female mosquitoes were tested with each plasma. Negative controls for MFAs included pooled normal human sera from North American donors who had never travelled to malaria endemic regions. A positive control included a pool of 20 human sera samples (de-identified sera samples for pooing from a previous study conducted in Zambia, Kumar et al, unpublished) that had previously shown ~90% transmission reducing activity in MFAs. P. falciparum (NF54) maintained in culture was used for MFA. Stage V mature gametocytes were diluted to 0.3% gametocytemia at 50% haematocrit using normal human sera and normal human red blood cells. Various sera were tested at 1:2 dilution and the mosquitoes were fed for 15 minutes using water jacketed glass feeders maintained at 37°C using a circulating water bath. Unfed mosquitoes were removed and blood-fed mosquitoes were maintained at 27°C and 70–80% relative humidity in ASL-2 insectaries. Eight to 10 days post blood feeding, mosquito midguts were dissected, stained with 0.1% mercurochrome and oocysts enumerated at 10X magnification. Percent reduction in the oocyst in the presence of test sera was calculated using oocyst counts with negative control sera as 100% infection. The percentage blocking was calculated as [(1-(X/Y)] ×100, where X is the mean oocysts number with test serum, and Y is mean oocysts number with normal human serum (negative) control. The positive control was used to check for consistency among various experiments.
3. Results
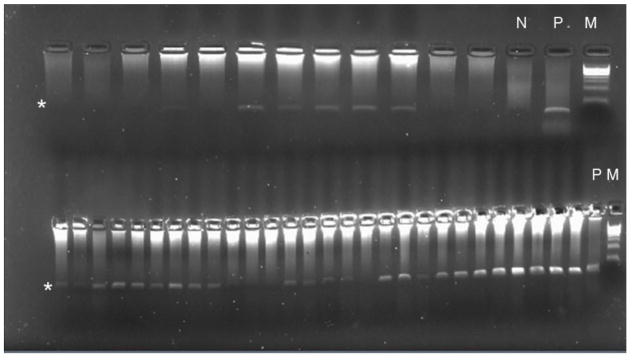
The study participants were school children enrolled in a schistosomiasis and malaria co-infection study. At the time of enrolment the participants were healthy with no reported signs or symptoms of active malaria infection. Microscopic examination of thick smears failed to detect any parasites, moreover when screened by RDT using Pf Paracheck™ revealed 1.7% (4/233) positivity for P. falciparum malaria. In order to confirm malaria diagnosis, the nested PCR protocol (Mahajan et al., 2012) was employed to amplify the extracted DNA from the participants. Based on nested PCR analysis a significantly higher prevalence (65/99 = 66%) of P. falciparum infection was detected (Figure 1).
Figure 1. Nested PCR detection of P. falciparum in the samples.
PCR band for positive sample is indicated by an asterisk. Lanes marked N and P show PCR results for negative and positive DNA samples. Lane marked M shows DNA standard ladder.
3.1. Analysis of antibody responses by ELISA
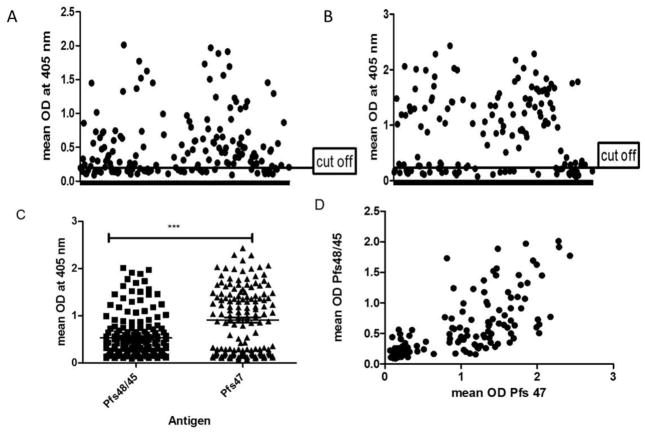
In view of rather higher PCR based P. falciparum prevalence, we next evaluated immune status in these study participants initially using the crude P. falciparum lysates in ELISA and 230/240 sera samples revealed the presence of positive antibody response (data not shown) suggesting population wide exposure to low level malaria. Further analysis focused on the evaluation of antibody response against specific antigens expressed in the gametocyte stage of the parasite. These antigens included recombinant Pfs48/45 and Pfs47 expressed in E. coli as 6xHis-tagged proteins. Figure 2A and 2B show ELISA reactivity of 141 sera samples to Pfs48/45 and Pfs47, respectively. The mean reactivity (absorbance at a single plasma dilution evaluated in ELISA) was higher for Pfs47 as compared to Pfs48/45 (Figure 2C), however these differences were not significantly different, p=0.061. When the ELISA data was analyzed for concordance between Pfs48/45 and Pfs47 ELISA positivity, a significant correlation was obtained (Pearson’s correlation coefficient of 0.696, p<0.001) (Figure 2D). The plates were checked for plate-to-plate variation by use of a positive control, which was consistent in all assays. We also clustered the population into age groups below ten years and those above ten years old, and the analysis showed no significant difference between the two age groups for Pfs48/45 (p= 0.5858, t=0.5640, df=163) and Pfs47 (p=0.4787, t=0.7103, df=145) (data not shown).
Figure 2. ELISA results for antibodies against Pfs48/45 and Pfs47.
Cross sectional antibody responses to Pfs48/45 (A) and Pfs47 (B). Population means for the two sexual stage antigens are shown in (C). Panel D shown correlation between ELISA values for Pfs48/45 and Pfs47.
3.2. Transmission-reducing activity
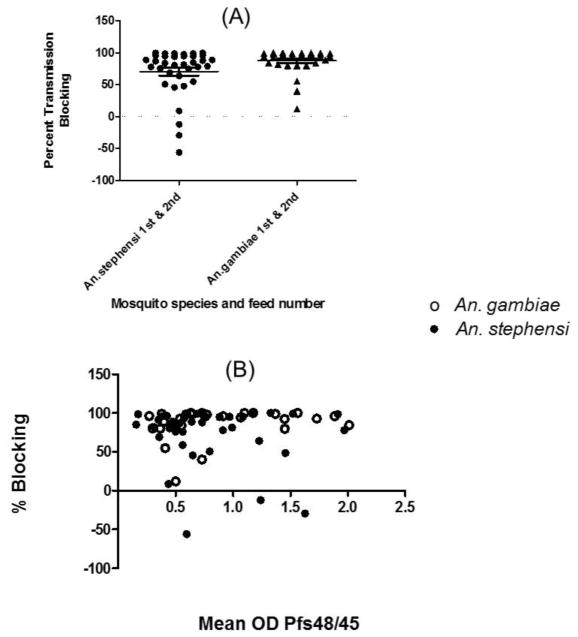
The strong reactivity to Pfs48/45 by antibodies in the plasma in this study population prompted us to further investigate if these plasma will exhibit transmission reducing activity. The plasma samples were randomly selected for evaluation by MFA using P. falciparum NF54 gametocytes and two different species of anopheline mosquitoes maintained in the laboratory. Figure 3A shows pooled results of plasma tested in two separate feeding assays with An. gambiae mosquitoes and An. stephensi mosquitoes. The transmission reducing activity varied from subject to subject, however plasma samples revealed strong transmission reducing activity in both the species of mosquitoes. In MFA three individual plasma samples revealed apparent enhancement of infection in An. stephensi and not in An. gambiae mosquitoes. We also analyzed the membrane feeding data for reproducibility between feeds, between species and for correlation with antibodies against Pfs48/45. The transmission reducing results within a species and between species were found to be highly reproducible. As shown in Figure 3B, there was a positive association between transmission reducing activity and Pfs48/45 ELISA values, however it was not statistically significant (Pearson R=0.004155, p=0.9734 for combined An. ggmbiae and An. stephensi MFA values versus anti-Pfs48/45 absorbance values).
Figure 3. Membrane Feeding Assay.
Percent transmission reduction in the presence of individual plasma samples tested in two different assays in An. gambiae and An. stephensi mosquitoes (A). Horizontal line shows average transmission reduction. Correlation analysis between combined transmission reducing activity in the two species and anti-Pfs48/45 ELISA values (B).
4. Discussion
This study was carried out in school age children living in a community with previously reported moderate transmission of malaria infection outbreaks. This was initially reaffirmed by low prevalence of malaria demonstrated by low RDT positive diagnosis of malaria. However, when analyzed by nested PCR analysis, it was astonishing to observe widespread prevalence of apparently asymptomatic malaria in this population. Generally, it is believed that such asymptomatic status is achieved in adults who have had repeat exposure to malaria resulting in induction of partially protective clinical immunity (Achtman et al., 2005; Baird, 1998; Langhorne et al., 2008; STONE et al., 2016). It was rather unexpected to find as much as 66% PCR positivity in the participants for two reasons. First the perception in the area is that the malaria is not a serious risk factor in this population (malaria incidence 46/1000, Ministry of Health, Zimbabwe). Second, all the study participants were school age children between 6–16 years of age, showing unlikely development of protective immunity which requires repeated exposures over a long time period (Baird, 1998; STONE et al., 2016). Clearly, similar PCR analysis needs to be established in other parts of Zimbabwe and other areas pursuing malaria elimination goal to ensure sufficient treatment coverage and complete absence of asymptomatic malaria (Bousema et al., 2016; Schneider et al., 2006; Shekalaghe et al., 2007a). While, we did not look for the presence of gametocytes by either microscopy or RT-PCR method, it is possible that widespread asymptomatic malaria infection may be sufficient to sustain persistent low level malaria transmission (Bousema and Drakeley, 2011; Bousema et al., 2010; Bousema et al., 2016; Jones et al., 2012; Mlambo et al., 2008; Ouédraogo et al., 2009; Schneider et al., 2004; Shekalaghe et al., 2007b). It should also be noted that the study participants came from an area undergoing schistosomiasis deworming using mass drug administration (Midzi et al., 2011). A previous study on co-infection with schistosomiasis had reported increased prevalence of gametocytes (Sangweme et al., 2010) which may also contribute to factors affecting malaria transmission on the one hand and the development of transmission reducing immunity on the other hand.
Although the results show that the younger population is at high risk of malaria, the presence of transmission reducing immunity in school going children under 16 years of age is a reflection of probable continuing repeat exposure to malaria infection. Reports of surges of malaria in recent years may also account for continuous low level exposure to malaria in the population (Mharakurwa et al., 2012). It is more than likely that these recent exposures may account for detected presence of antibodies not only against asexual stage antigens but also those expressed in the sexual stages responsible for malaria transmission. Of the two antigens evaluated in this study, Pfs48/45 and Pfs47, Pfs48/45 is a well-established target of malaria transmission blocking immunity (Chowdhury et al., 2009; Outchkourov et al., 2008). Monoclonal antibodies against Pfs48/45 as well as polyclonal antibodies elicited by immunization with recombinant Pfs48/45 have revealed strong transmission blocking activity (Chowdhury et al., 2009; Outchkourov et al., 2008; Rener et al., 1983). In the present study most sera that showed strong ELISA reactivity to Pfs48/45 also showed strong transmission reducing activity in MFA. Interestingly not all sera that were ELISA positive revealed transmission reducing activity, suggesting that antibodies in such sera are likely directed against non-functional epitopes. Indeed, targets of most potent blocking antibodies are reduction-sensitive conformational epitopes and antibodies recognizing different epitopes do not block transmission in MFAs (Carter et al., 1985; Carter et al., 1995; Outchkourov et al., 2007; Outchkourov et al., 2008). Intriguingly, three of the plasma samples tested in MFAs using An. stephensi mosquitoes apparently seem to enhance mosquito infectivity. This is not surprising; studies in Sri Lanka in Kataragama had previously revealed similarly enhanced malaria transmission in the presence of low levels of antibodies against P. vivax sexual stage specific antigens (GAMAGE-MENDIS et al., 1992). These results strongly support the need for additional studies on designing MFAs using different species of mosquitoes, especially region-specific local mosquito vector species for future programmatic needs. The samples that showed enhancement and low blocking were randomly picked from the Pfs48/45 ELISA positive samples. Although some of the plasma samples showed blocking to an extent that was commensurate with the anti-Pfs48/45 antibody determinations, others though with higher antibody reactivity were not as effective in blocking. Differences in the recognition of epitopes and in the presence of antibodies against other sexual stage specific target antigens may contribute to these differences (Graves et al., 1988; Skinner et al., 2015; STONE et al., 2016). Additional studies are needed to determination a detailed correlation between antibody titers, antigen specificity and transmission blocking activity. Other possibility may include polymorphism in the antigens affecting immunogenicity and functional effectiveness (Anthony et al., 2007; Drakeley et al., 1996; Escalante et al., 2002; Juliano et al., 2016).
Our results also emphasize the fact that immunity against malaria is complex and requires careful dissection of epitopes to develop effective antigen for eliciting functional antibody responses. The development of immunity against malaria, including transmission blocking immunity has been reported to be a slow process. While the findings of this study revealed the presence of partially effective immunity in apparently asymptomatic children between the ages 6–16, we rationalize that sustained transmission among such asymptomatic cases at the population level leading to repeated exposures might be an important factor in the acquisition of such natural immunity. More studies are needed in similar malarious areas to further understand the efficacy of this immunity during recurring transmission seasons.
Acknowledgments
We would like to thank the participants and community of Bandanyenje for their support. We also thank the NIHR team and the Biochemistry staff, University of Zimbabwe for assisting in fieldwork. This work received financial support from the Fogarty International Center Training Grant D43TW00158–06A2.
Footnotes
Author contribution
NHP, AV, TM, NM coordinated field sample collection; NHP carried out various assays; NK and GB designed and supervised immunological and PCR studies. NHP, TM, GB and NK analyzed the data. NHP performed all the assays. NHP, TM, JC, GB and NK contributed to writing and finalizing the manuscript for submission.
Conflict of interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- WHO. World Malaria Report 2015. World Health Organization; Geneva: 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en. [Google Scholar]
- Achtman A, Bull P, Stephens R, Langhorne J. Longevity of the immune response and memory to blood-stage malaria infection, Immunology and Immunopathogenesis of Malaria. Springer; 2005. pp. 71–102. [DOI] [PubMed] [Google Scholar]
- Anthony TG, Polley SD, Vogler AP, Conway DJ. Evidence of non-neutral polymorphism in Plasmodium falciparum gamete surface protein genes Pfs47 and Pfs48/45. Mol Biochem Parasitol. 2007:156. doi: 10.1016/j.molbiopara.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Baird JK. Age dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Annals of tropical medicine and parasitology. 1998;92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. Research Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malaria journal. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Stresman G, Baidjoe AY, Bradley J, Knight P, Stone W, Osoti V, Makori E, Owaga C, Odongo W, China P, Shagari S, Doumbo OK, Sauerwein RW, Kariuki S, Drakeley C, Stevenson J, Cox J. The Impact of Hotspot-Targeted Interventions on Malaria Transmission in Rachuonyo South District in the Western Kenyan Highlands: A Cluster-Randomized Controlled Trial. PLoS Med. 2016;13:e1001993. doi: 10.1371/journal.pmed.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. Transmission blocking malaria vaccines. Vaccine. 2001:19. doi: 10.1016/s0264-410x(00)00521-1. [DOI] [PubMed] [Google Scholar]
- Carter R, Bushell G, Saul A, Graves PM, Kidson C. Two apparently nonrepeated epitopes on gametes of Plasmodium falciparum are targets of transmission-blocking antibodies. Infect Immun. 1985;50:102–106. doi: 10.1128/iai.50.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Coulson A, Bhatti S, Taylor BJ, Elliott JF. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Molecular and Biochemical Parasitology. 1995;71:203–210. doi: 10.1016/0166-6851(94)00054-q. [DOI] [PubMed] [Google Scholar]
- Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. Malaria transmission-blocking vaccines?how can their development be supported? Nature Medicine. 2000;6:241. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- Chowdhury DR, Angov E, Kariuki T, Kumar N. A Potent Malaria Transmission Blocking Vaccine Based on Codon Harmonized Full Length Pfs48/45 Expressed in Escherichia coli. PLoS ONE. 2009;4:e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas C, Pierce SK. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annual review of immunology. 2014;32:157. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzler KW, Ravel DB, Brancucci NM, Marti M. Ensuring transmission through dynamic host environments: host–pathogen interactions in Plasmodium sexual development. Current opinion in microbiology. 2015;26:17–23. doi: 10.1016/j.mib.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley CJ, Duraisingh MT, Póvoa M, Conway DJ, Targett GAT, Baker DA. Geographical distribution of a variant epitope of Pfs48/45, a Plasmodium falciparum transmission-blocking vaccine candidate. Molecular and Biochemical Parasitology. 1996;81:253–257. doi: 10.1016/0166-6851(96)02718-1. [DOI] [PubMed] [Google Scholar]
- Escalante AA, Grebert HM, Chaiyaroj SC, Riggione F, Biswas S, Nahlen BL. Polymorphism in the gene encoding the Pfs48/45 antigen of Plasmodium falciparum. XI. Asembo Bay Cohort Project. Mol Biochem Parasitol. 2002:119. doi: 10.1016/s0166-6851(01)00386-3. [DOI] [PubMed] [Google Scholar]
- GAMAGE-MENDIS AC, RAJAKARUNA J, CARTER R, MENDIS KN. Transmission blocking immunity to human Plasmodium vivax malaria in an endemic population in Kataragama, Sri Lanka. Parasite immunology. 1992;14:385–396. doi: 10.1111/j.1365-3024.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Graves P, Carter R, Burkot T, Quakyi I, Kumar N. Antibodies to Plasmodium faciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988;10:209– 218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Jones S, Sutherland CJ, Hermsen C, Arens T, Teelen K, Hallett R, Corran P, van der Vegte-Bolmer M, Sauerwein R, Drakeley CJ. Filter paper collection of Plasmodium falciparum mRNA for detecting low-density gametocytes. Malaria journal. 2012;11:1–10. doi: 10.1186/1475-2875-11-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano JJ, Parobek CM, Brazeau NF, Ngasala B, Randrianarivelojosia M, Lon C, Mwandagalirwa K, Tshefu A, Dhar R, Das BK, Hoffman I, Martinson F, Mårtensson A, Saunders DL, Kumar N, Meshnick SR. Pooled Amplicon Deep Sequencing of Candidate Plasmodium falciparum Transmission-Blocking Vaccine Antigens. The American Journal of Tropical Medicine and Hygiene. 2016;94:143–146. doi: 10.4269/ajtmh.15-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite immunology. 1987;9:321–335. doi: 10.1111/j.1365-3024.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Kumar N, Wizel B. Further characterization of interactions between gamete surface antigens of Plasmodium falciparum. Molecular and biochemical parasitology. 1992;53:113–120. doi: 10.1016/0166-6851(92)90013-a. [DOI] [PubMed] [Google Scholar]
- Langhorne J, Ndungu F, Sponaas A, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725– 732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- Lukwa N, Sande S, Makuwaza A, Chiwade T, Netsa M, Asamoa K, Vazquez-Prokopec G, Reithinger R, Williams J. Nationwide assessment of insecticide susceptibility in Anopheles gambiae populations from Zimbabwe. Malaria journal. 2014;13:1. doi: 10.1186/1475-2875-13-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Tropical Medicine & International Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- Mahajan B, Zheng H, Pham PT, Sedegah MY, Majam VF, Akolkar N, Rios M, Ankrah I, Madjitey P, Amoah G. Polymerase chain reaction–based tests for pan-species and species-specific detection of human Plasmodium parasites. Transfusion. 2012;52:1949–1956. doi: 10.1111/j.1537-2995.2011.03541.x. [DOI] [PubMed] [Google Scholar]
- Mharakurwa S, Thuma PE, Norris DE, Mulenga M, Chalwe V, Chipeta J, Munyati S, Mutambu S, Mason PR. Malaria epidemiology and control in Southern Africa. Acta tropica. 2012;121:202–206. doi: 10.1016/j.actatropica.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzi N, Mtapuri-Zinyowera S, Mapingure MP, Paul NH, Sangweme D, Hlerema G, Mutsaka MJ, Tongogara F, Makware G, Chadukura V. Knowledge attitudes and practices of grade three primary schoolchildren in relation to schistosomiasis, soil transmitted helminthiasis and malaria in Zimbabwe. BMC infectious diseases. 2011;11:1. doi: 10.1186/1471-2334-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlambo G, Vasquez Y, LeBlanc R, Sullivan D, Kumar N. A Filter Paper Method for the Detection of Plasmodium falciparum Gametocytes by Reverse Transcription–Polymerase Chain Reaction. The American journal of tropical medicine and hygiene. 2008;78:114–116. [PubMed] [Google Scholar]
- Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, Ortega C, van Schaijk BC, Sauerwein RW, Taylor-Salmon E. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340:984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SK, Childs LM, Buckee C, Marti M. Targeting human transmission biology for malaria elimination. PLoS Pathog. 2015;11:e1004871. doi: 10.1371/journal.ppat.1004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouédraogo AL, Bousema T, Schneider P, De Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, Nébié I, Roeffen W, Verhave JP, Luty AJ. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PloS one. 2009;4:e8410. doi: 10.1371/journal.pone.0008410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outchkourov N, Vermunt A, Jansen J, Kaan A, Roeffen W, Teelen K. Epitope analysis of the malaria surface antigen pfs48/45 identifies a subdomain that elicits transmission blocking antibodies. J Biol Chem. 2007:282. doi: 10.1074/jbc.M700948200. [DOI] [PubMed] [Google Scholar]
- Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci USA. 2008:105. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- Rener J, Graves P, Carter R, Williams J, Burkot T. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med. 1983;158:976– 981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangweme DT, Midzi N, Zinyowera-Mutapuri S, Mduluza T, Diener-West M, Kumar N. Impact of schistosome infection on Plasmodium falciparum Malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PLoS Negl Trop Dis. 2010;4:e882. doi: 10.1371/journal.pntd.0000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Bousema T, Omar S, Gouagna L, Sawa P, Schallig H, Sauerwein R. (Sub) microscopic Plasmodium falciparum gametocytaemia in Kenyan children after treatment with sulphadoxine-pyrimethamine monotherapy or in combination with artesunate. International journal for parasitology. 2006;36:403–408. doi: 10.1016/j.ijpara.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Schneider P, Schoone G, Schallig H, Verhage D, Telgt D, Eling W, Sauerwein R. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Molecular and biochemical parasitology. 2004;137:35–41. doi: 10.1016/j.molbiopara.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Shekalaghe S, Drakeley C, Gosling R, Ndaro A, Van Meegeren M, Enevold A, Alifrangis M, Mosha F, Sauerwein R, Bousema T. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One. 2007a;2:e1023. doi: 10.1371/journal.pone.0001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekalaghe SA, Teun Bousema J, Kunei KK, Lushino P, Masokoto A, Wolters LR, Mwakalinga S, Mosha FW, Sauerwein RW, Drakeley CJ. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Tropical Medicine & International Health. 2007b;12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- Sinden R. A biologist’s perspective on malaria vaccine development. Human vaccines. 2010;6:3–11. doi: 10.4161/hv.6.1.9604. [DOI] [PubMed] [Google Scholar]
- Skinner J, Huang CY, Waisberg M, Felgner PL, Doumbo OK, Ongoiba A, Kayentao K, Traore B, Crompton PD, Williamson KC. Plasmodium falciparum gametocyte-specific antibody profiling reveals boosting through natural infection and identifies potential markers of gametocyte exposure. Infection and immunity. 2015;83:4229–4236. doi: 10.1128/IAI.00644-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE WJ, DANTZLER KW, NILSSON SK, DRAKELEY CJ, MARTI M, BOUSEMA T, RIJPMA SR. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology. 2016:1–12. doi: 10.1017/S0031182015001341. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JAM, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W. A Central Role for P48/45 in Malaria Parasite Male Gamete Fertility. Cell. 2001;104:153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- van Schaijk BC, van Dijk MR, van de Vegte-Bolmer M, van Gemert GJ, van Dooren MW, Eksi S, Roeffen WF, Janse CJ, Waters AP, Sauerwein RW. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Molecular and biochemical parasitology. 2006;149:216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]