Abstract
Introduction
We investigated relationships between treatment characteristics and long-term outcomes in patients with locally advanced thymoma or thymic carcinoma.
Methods
We retrospectively reviewed 146 patients treated in 1980–2011 at two tertiary cancer care centers, 110 with Masaoka-Koga stage III–IVa invasive thymoma and 36 with stage I–IVa thymic carcinoma. Survival probabilities were estimated with the Kaplan-Meier method. Risk factors related to survival were identified by univariate and multivariate competing risk analysis, with overall survival (OS) as the competing risk. Cox regression analysis was used to identify risk factors for OS.
Results
Median follow-up time for all patients was 64 months. At 5/10 years, rates of OS and freedom from recurrence (FFR) were 81/58% and 81/65%, respectively. Of patients who underwent surgery, trimodality treatment produced better survival compared to less aggressive treatment among patients with stage III disease (p=0.03). Among patients who underwent trimodality treatment, patients with stage III disease had better OS (p=0.03) and FFR (p<0.001) than those with stage IVA disease. On Cox regression analysis, decreased OS was associated with thymic carcinoma (hazard ratio [HR]=7.36, 95% CI=2.38–22.77, p=0.001), R2/unresectable disease (HR=8.45, 95% CI=1.44–49.42, p=0.02) and an Eastern Cooperative Oncology Group performance score of 1 (HR=8.14, 95% CI=1.55–42.75, p=0.01) or 2–3 (HR=29.60, 95% CI=4.0–218.98, p=0.001) versus 0.
Conclusion
Aggressive treatment with chemotherapy, surgical resection, and postoperative radiation therapy can produce long-term survival for patients with invasive thymic malignanices.
Keywords: thymoma, thymic carcinoma, multimodality/ trimodality therapy
INTRODUCTION
Thymic malignancies, the most common primary neoplasms of the anterior mediastinum, are relatively rare and sometimes have an indolent course, making the choice of treatment and analysis of outcomes challenging. The few prospective trials that have included patients with advanced-stage disease involved heterogeneous treatments.1 Retrospective and prospective analyses have demonstrated promising survival rates for patients undergoing multimodality therapy for advanced invasive thymoma2–5 or thymic carcinoma.6–8 However, investigations that include large numbers of patients from several institutions are sparse, particularly those that compare patients who received different treatments.
We sought here to assess prognostic factors for survival for patients undergoing treatment for thymoma or thymic carcinoma, and to compare outcomes for patients who completed trimodality therapy with those for patients who received other, less aggressive regimens. Specifically, our objectives were to (1) report long-term outcomes in patients with locally advanced thymoma or thymic carcinoma treated at two major tertiary oncologic referral centers, (2) assess differences in survival outcomes between trimodality therapy versus less aggressive (single or bimodality) treatments, and (3) identify factors predictive of outcome for patients who underwent trimodality treatment. We hypothesized that patients with advanced thymic malignancies could achieve long-term survival with aggressive treatment and that patients with thymic carcinoma of any stage would have worse outcomes than patients with locally advanced invasive thymoma, even when accounting for treatment approach.
MATERIALS AND METHODS
Eligibility
The institutional review and privacy boards at each institution approved this study, and patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act. Institutional records were searched for patients receiving definitive treatment for Masaoka-Koga stage III–IVA thymoma or stage I–IVA thymic carcinoma at either The University of Texas MD Anderson Cancer Center or Memorial Sloan-Kettering Cancer Center from 1980 through 2011. Patients presenting with recurrent disease or distant metastasis (DM) were excluded, as were patients who were only partially or incompletely treated at these institutions.
Follow-up
Follow-up data were collected through September 3, 2012 from institutional records and records from referring facilities. Follow-up visits took place and images were obtained according to institutional standards, and those set forth by the National Comprehensive Cancer Network when available.9 This generally consisted of a history and physical and repeat imaging (i.e. CT scan of the chest) approximately every 6 months to one year for the first 5 years, with more frequent or extensive follow-up occurring at the physician’s discretion. Patterns of failure were characterized in all patients who experienced disease recurrence; treatment failure within the bed of the thymus or intrathoracic recurrence not contiguous with the thymus (including the visceral and parietal pleura) was considered local-regional recurrence (LR) and all other sites of failure were considered DM.10 Local failure in patients with an initial R2 or unresectable status was diagnosed based on clear progression radiographically and/ or histologically after the completion of definitive therapy.
Statistical Analysis
Data analysis was done with Stata/MP 12.1 statistical software. Fisher’s exact tests were used to assess measures of association in frequency tables. Survival probabilities were estimated using Kaplan-Meier methods, and log-rank tests were used to assess the equality of the survivor function across groups. The equality of group medians was assessed with nonparametric tests for equality. A p value of 0.05 or less was considered to be statistically significant. Statistical tests were based on a two-sided significance level.
Survival times were calculated from the date of diagnosis to that of the first occurrence of the considered event (LR alone, DM alone, or any recurrence, local-regional or distant). Overall survival (OS) was defined as the time between diagnosis and death from any cause. Freedom-from-recurrence (FFR) was defined as the time between the date of diagnosis and the first evidence of recurrent disease (local-regional). These criteria are in accordance with the International Thymic Malignancy Interest Group’s (ITMIG) recommended standard outcome measures for patients who have undergone curative-intent treatment.10 Cox’s proportional hazards model was used for multivariate analysis to assess the effect of patient- or tumor-related characteristics and other possibly predictive factors on OS, and estimated hazards are reported with 95% confidence intervals (CIs). All factors found to have a p-value of 0.25 or less on univariate analysis were included in the multivariable analysis, with each factor eliminated in a step-wise manner until the most significant variables were identified. The Wald test was used to assess the role of covariates in the model. Competing-risks regression analysis was done according to the method of Fine and Gray,11 with a similar selection of predictive factors for multivariable analysis. Subhazard ratios are reported for FFR with death as the competing event.
RESULTS
We identified 146 patients who met our criteria, 110 with stage III–IVa invasive thymoma and 36 with stage I–IVa thymic carcinoma. Patient characteristics are listed in Table 1. The study population consisted of 82 (56%) men and 64 (44%) women, with a median age at diagnosis of 53 years (range, 13–89); 104 patients (71%) were white, and 64 patients (44%) had a baseline Eastern Cooperative Oncology Group performance score of 1. Eighteen patients (12%) were also diagnosed as having myasthenia gravis.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Institution | |
| MD Anderson | 83 (57) |
| Sloan-Kettering | 63 (43) |
| Age at diagnosis, years | |
| Median | 53.2 |
| Mean (range) | 52.7 (12.8–89.2) |
| Sex | |
| Male | 82 (56) |
| Female | 64 (44) |
| Ethnicity | |
| Caucasian | 104 (71) |
| African-American | 25 (17) |
| Hispanic | 7 (5) |
| Asian | 4 (3) |
| Other | 6 (4) |
| ECOG PS Score | |
| 0 | 32 (22) |
| 1 | 64 (44) |
| 2 | 14 (10) |
| 3 | 3 (2) |
| Not available | 33 (23) |
| Diagnosis | |
| Thymoma | 110 (75) |
| Thymic carcinoma | 36 (25) |
| Masaoka-Koga Disease Stage | |
| I† | 1 (<1) |
| IIA† | 0 (0) |
| IIB† | 4 (3) |
| III | 98 (67) |
| IVA | 43 (29) |
| Tumor Size (cm) | |
| Median | 8.0 |
| Mean (range) | 8.5 (1.3–18.5) |
| Myasthenia Gravis | |
| Yes | 18 (12) |
| No | 117 (80) |
| Unknown | 11 (8) |
Thymic carcinoma patients only.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status
Treatment Characteristics
Table 2 summarizes treatment characteristics for the study population. Eighty-seven patients (60%) received trimodality treatment (chemotherapy, surgery, and radiation therapy), 45 (31%) had bimodality treatment, and 14 (9%) underwent single-modality treatment. Reasons for not undergoing trimodality treatment include: poor performance status (6%), progression of disease (1%), unresectable disease (20%), patient declined or lost to follow-up (6%), physician discretion (28%), or reasons not specified in the patient’s records (39%). Of the 124 patients with surgically resectable disease, 124 had a thymectomy, 48 with an en-bloc lung resection, and 8 with extrapleural pneumonectomy; 14 patients had unresectable disease. All but one patient with unresectable disease received definitive radiation therapy, and all of these patients received chemotherapy. Thymectomy was done most often via median sternotomy; other approaches included thoracotomy, hemi-clamshell, clamshell, and video-assisted thoracotomy. Of those patients who underwent thymectomy, 60 had a complete resection. The median time to surgical resection after diagnosis was 3 months.
Table 2.
Treatment Characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Treatments received | |
| Trimodality | 87 (60) |
| Surgery and RT | 28 (19) |
| Surgery and chemo | 5 (3) |
| Chemo and RT | 12 (8) |
| Surgery only | 5 (3) |
| Chemo only | 2 (1) |
| RT only | 7 (5) |
| Type of surgery | |
| Thymectomy | 124 (85) |
| with lung resection | 48 (33) |
| with pneumonectomy | 8 (5) |
| Unresectable | 14 (10) |
| Resection Status | |
| R0 | 60 (41) |
| R1 | 56 (38) |
| R2 (and unresectable) | 25 (17) |
| Unknown | 5 (3) |
| Median Time to Surgery, months | 3.1 |
| Chemo Timing | |
| No chemo | 40 (27) |
| Induction | 60 (41) |
| Adjuvant | 20 (14) |
| Both before & after surgery | 26 (18) |
| Chemo Regimen | |
| CAP ± prednisone | 64 (44) |
| Other | 25 (17) |
| No chemo | 40 (27) |
| Unknown | 17 (12) |
| Radiation therapy modality | |
| Conventional 2D | 36 (25) |
| 3D conformal | 30 (21) |
| IMRT | 41 (28) |
| Proton therapy | 4 (3) |
| No radiotherapy | 11 (8) |
| Unknown | 24 (16) |
| Median dose, Gy | 54 |
| Median number of fractions | 30 |
| Median time to RT, months | 5.2 |
Abbreviations: RT, radiation therapy; chemo, chemotherapy; IMRT, intensity-modulated radiation therapy; CAP, cisplatin, doxorubicin, cyclophosphamide; R0, resection with clear margin; R1, resection with positive margins; R2, resection with gross tumor remaining; PET, positron emission tomography; SUV, standard uptake value.
Of the 106 patients given chemotherapy, 60 received it as preoperative induction therapy, 20 as postoperative adjuvant therapy, and 26 as both preoperative and post-operative. The most common regimen was cyclophosphamide, doxorubicin, and cisplatin (CAP), with or without prednisone (n=64). Other combinations included cisplatin with etoposide (n=9) and carboplatin with paclitaxel (n=5).
Of the 134 patients (92%) who received radiation therapy, 36 received 2-dimensional therapy, 30 received 3-dimensional conformal therapy, and 41 received intensity-modulated therapy, a pattern that reflected the technical advances in treatment planning and delivery over the period of study. Four patients underwent proton therapy, the first in 2009. The median radiation dose was 54 Gy (range, 54–66 Gy), delivered in 30 fractions (range, 10–50 fractions). The median interval between diagnosis and radiation therapy was 5 months.
Survival Outcomes According to Treatment Received
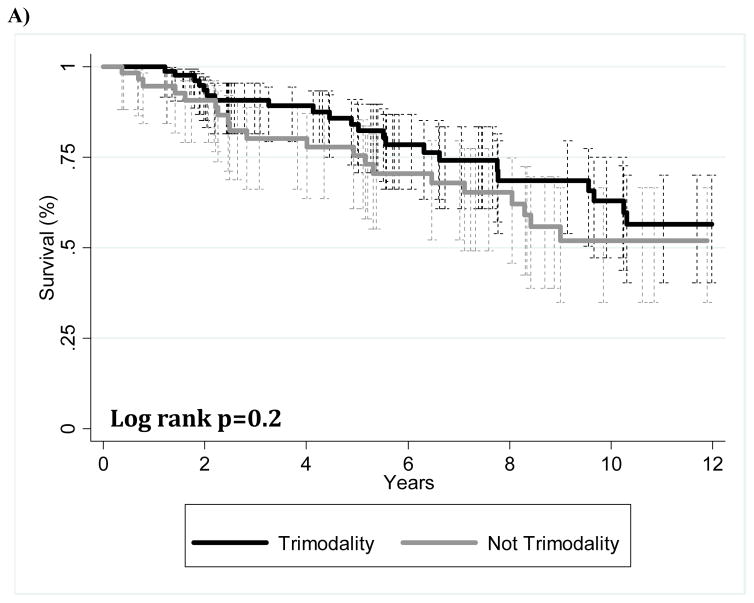
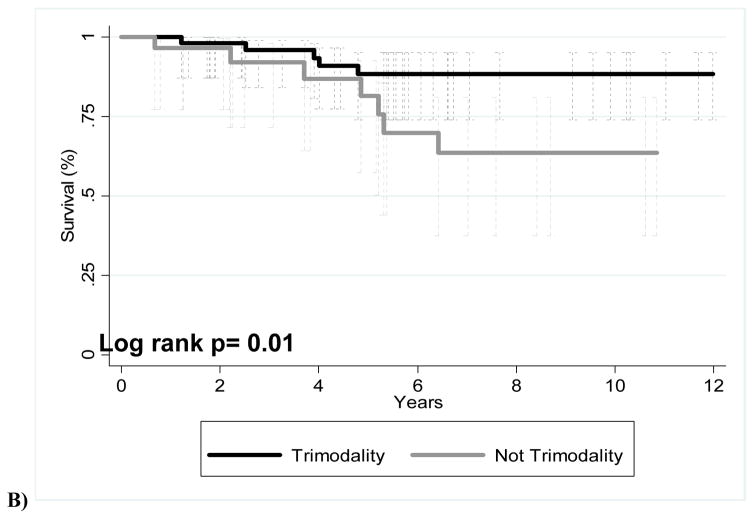
The median follow-up time was 63.8 months (range, 1.8–360.1 months) for all patients and 64.1 months (range, 1.8–360.1) for patients alive at the time of this analysis. Overall survival and freedom-from-recurrence at 5 and 10 years were as follows: OS 80.7%/ 58.3%, FFR 81.4%/64.6%. The 5-year OS and FFR for thymic carcinoma patients was 54.8% and 57.3%, respectively. Figure 1 shows Kaplan-Meier estimates of OS for those who received trimodality therapy (chemotherapy, surgery, and radiation) or non-trimodality therapy. When all cases were included in this comparison, no significant difference was found (Fig 1A) (p=0.2). Of the patients with stage III disease who received surgery as part of their disease management, trimodality treatment showed better OS than those with non-trimodality treatment (Fig. 1B).
FIGURE 1.
Kaplan-Meier overall survival curves for patients given trimodality or non-trimodality treatment. (A) All patients; (B) patients with Masaoka-Koga stage III disease who underwent surgery at part of their treatment.
Predictive Factors for Patients who Underwent Trimodality Treatment
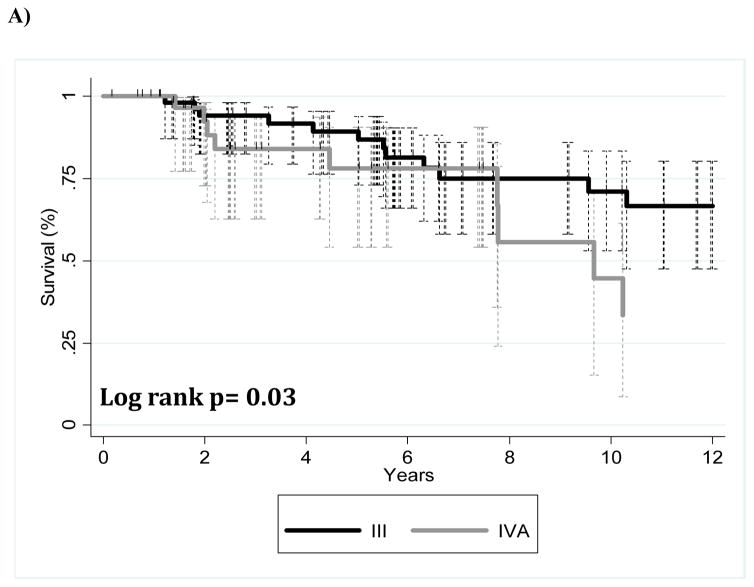
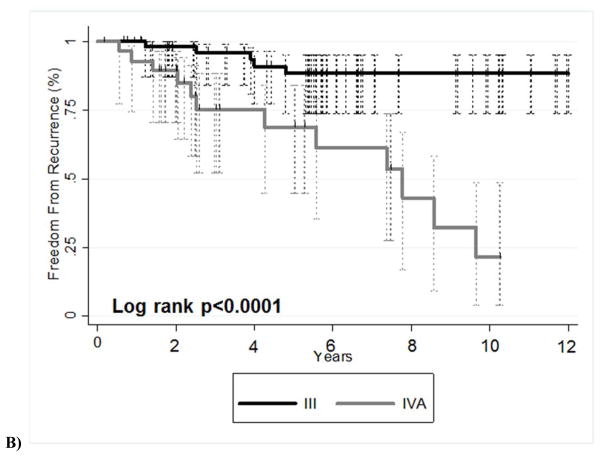
Patients who underwent chemotherapy, surgery, and radiation therapy were analyzed separately for the purposes of studying a more uniform patient population. Of this subgroup of patients who underwent trimodality treatment (n=87), those with stage III disease showed better OS (p=0.03) (Fig. 2A) and better FFR (p<0.001) (Fig. 2B) than did patients with stage IVA disease. Table 3 describes the competing risk analysis for FFR with death (OS) as the competing risk. Univariate analysis showed that patients with stage IVA disease or a higher ECOG performance score (and thus worse performance status) had worse disease outcomes. No multivariable models for FFR were found to be significant.
FIGURE 2.
Kaplan-Meier survival curves for patients who underwent trimodality therapy illustrating (A) overall survival and (B) freedom-from-recurrence for patients with stage III versus stage IVa disease.
Table 3.
Competing Risk Regression Analysis for Freedom from Recurrence among Patients Who Received Trimodality Therapy
|
|
|||
|---|---|---|---|
| FFR | |||
|
| |||
| Univariate | SHR† | 95% CI | p |
| Age, years | |||
| ≤60 | Ref. | ||
| >60 | 0.39 | 0.19–1.73 | 0.2 |
| Sex | |||
| Male | Ref. | ||
| Female | 1.30 | 0.57–2.98 | 0.5 |
| Masaoka-Koga Disease Stage | |||
| I–III | Ref. | ||
| IVa | 5.37 | 2.23–12.92 | <0.001 |
| Histology | |||
| Thymoma | Ref. | ||
| Thymic carcinoma | 1.54 | 0.51–4.62 | 0.4 |
| Resection Status | |||
| R0 | Ref. | ||
| R1 | 1.05 | 0.43–2.93 | 0.9 |
| R2/Unresectable | 1.40 | 0.20–9.81 | 0.7 |
| ECOG Performance Status Score | |||
| 0 | Ref. | ||
| 1 | 2.49 | 0.63–9.89 | 0.2 |
| 2–3 | 8.17 | 1.22–54.73 | 0.03 |
| Diagnosis year | |||
| Continuous | 1.07 | 0.99–1.15 | 0.08 |
Abbreviations: SHR, subhazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; SUVmax, maximum standardized uptake value
Subhazard ratio based on competing risk analysis.
Findings from the Cox regression analysis of OS for patients receiving trimodality treatment are shown in Table 4. On univariate analysis, age older than 60 years, an ECOG performance status score of 1 or higher, having R2 or unresectable disease, year of diagnosis, and having stage IV disease (with a trend towards significance) were linked with worse OS. On multivariate analysis for OS, having thymic carcinoma, R2 or unresectable disease, and an ECOG performance score of 1–3 were associated with worse OS. Note that neither age nor year of diagnosis was significantly associated with survival on multivariate analysis.
Table 4.
Cox Proportional Hazards Analysis of Overall Survival for Patients Who Received Trimodality Therapy
|
|
||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
|
| ||||||
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Age | ||||||
| ≤60 | Ref. | |||||
| >60 | 2.54 | 1.02–6.33 | 0.045 | -- | -- | -- |
| Sex | -- | -- | -- | |||
| Male | Ref. | |||||
| Female | 1.55 | 0.73–3.28 | 0.251 | -- | -- | -- |
| Masaoka-Koga Disease Stage | ||||||
| I–III | Ref. | |||||
| IVa | 2.20 | 0.99–4.89 | 0.05 | -- | -- | -- |
| Histology | ||||||
| Thymoma | Ref. | Ref. | ||||
| Thymic Carcinoma | 1.81 | 0.76–4.30 | 0.2 | 7.36 | 2.38–22.77 | 0.001 |
| Resection Status | ||||||
| R0 | Ref. | Ref. | ||||
| R1 | 1.48 | 0.66–3.34 | 0.3 | 1.24 | 0.48–3.22 | 0.7 |
| R2/Unresectable | 5.67 | 1.17–27.43 | 0.03 | 8.45 | 1.44–49.42 | 0.02 |
| ECOG Performance Status Score | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | 3.85 | 1.08–13.70 | 0.04 | 8.14 | 1.55–42.75 | 0.01 |
| 2–3 | 10.49 | 2.13–51.67 | 0.004 | 29.60 | 4.0–218.98 | 0.001 |
| Diagnosis year | ||||||
| Continuous | 1.08 | 1.00–1.17 | 0.048 | -- | -- | -- |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; SUVmax, maximum standardized uptake value
DISCUSSION
Here we present our findings from a large series of patients with locally advanced invasive thymoma or thymic carcinoma who had been treated at two tertiary cancer care centers with experience in treating these conditions. Our pertinent findings can be summarized as follows. First, although no difference in OS was found for all patients according to the aggressiveness of treatment received (trimodality versus single or bimodality), patients with stage III disease obtained the greatest benefit from trimodality therapy, including improved OS. Second, factors identified as having predictive value for patients who underwent trimodality therapy included less advanced disease stage, a histologic diagnosis of thymoma, complete surgical resection, and better performance status (indicated by lower ECOG score). These factors identified are consistent with the findings of the International Thymic Malignancy Interest Group, which include completeness of resection, stage, and histology (specifically thymic carcinoma), but not sex or the presence of myasthenia gravis, as being predictive of survival.12
Prior smaller studies of the role of trimodality therapy have concluded that this approach is promising for patients who can tolerate this aggressive form of therapy. For example, in a prospective analysis of 25 patients given trimodality therapy for advanced-stage disease, Venuta et al5 found that 8-year survival rates were 92% for those with stage III thymoma and 68% for those with limited stage IV disease. Another study of 16 patients with invasive thymoma (stage III and IVa) treated with trimodality therapy demonstrated a median survival time of 66 months and a 3-year survival rate of 70%.13 Finally, in a smaller study of 7 patients with stage IIIa (Verley and Hollmann staging) invasive thymoma treated with neoadjuvant chemotherapy followed by surgery and postoperative radiation therapy, the reported 2-year survival rate was 80%.14
While retrospective, the current larger analysis of outcomes at two tertiary cancer care centers serves to validate these smaller studies and shows that long-term survival can be achieved with trimodality therapy when patients are selected appropriately. It should be noted, however, that although the greatest benefit in our study was shown in patients with stage III disease, in this retrospective and hypothesis-generating setting, we do not consider these results definitive. Indeed, aggressive treatment can be considered to patients with stage IVa disease if tolerable and at the treating physician’s discretion. Further, our results show how adverse factors, such as histology or stage, affect the expected outcomes. These results will be useful for guiding the choice of treatment for patients with disease of specific subtypes to enhance the probability of long-term freedom from recurrence and OS.
Trimodality therapy, with consolidative chemotherapy, could also be considered for patients with initially unresectable disease. One prospective study of 22 patients with locally advanced unresectable malignant thymoma showed that the use of induction chemotherapy to optimize the resectability of thymoma followed by radiation and consolidation chemotherapy led to improved control of residual disease and high OS rates. Indeed, 21 of the 22 patients in that study underwent surgical exploration after induction chemotherapy and 16 (76%) had complete resections.15 Another reasonable approach is concurrent chemoradiation. In one trial of 26 patients with limited-stage unresectable thymoma treated with CAP therapy and standard radiation to a total dose of 54 Gy, the overall response rate was 69.9% and the 5-year OS rate was 52.5%, results that compare favorably to those from single-modality treatment.16 At MD Anderson and Memorial Sloan-Kettering, the most commonly used regimen consists of up-front chemotherapy, with chemoradiation reserved for patients in whom surgery is considered less feasible.
Our results are also consistent with prior reports of poorer survival rates for patients with thymic carcinoma versus those with invasive thymoma.17 In one large retrospective study of 1,320 patients with thymic epithelial tumors in Japan,18 the reported 5-year OS rate for patients with thymic carcinoma was 50.5%; however, clinical information on the patients in that study was somewhat limited. Consistent with our results, Huang et al. found differing patterns of relapse between patients with thymic carcinoma versus thymoma, with thymic carcinoma being associated with lower progression-free survival and higher rates of distant failures.19 If complete or incomplete resection can be achieved, long-term survival rates have been as high as 60%–70% for those who receive adjuvant treatment.6–8 A multimodality approach such as trimodality therapy or definitive concurrent chemoradiation in unresectable cases is recommended for thymic carcinoma regardless of disease stage.20 Additionally, only one-third of patients with thymic carcinoma are able to undergo a complete resection.17 More effective treatments for unresectable thymic carcinoma are thus needed, and several ongoing or recently completed clinical trials have assessed the effectiveness of adding targeted agents such as sunitinib.21 Ideally, trials such as these will bring forth regimens that will improve outcomes for this difficult-to-treat disease.
In addition to the constraints of any retrospective study, our study had several limitations. First, given the rarity of the diagnosis of invasive thymoma or thymic carcinoma, our analysis included patients who had received a variety of treatments, including chemotherapy, radiation, and surgery. It may be difficult differentiate between the effect of selection for a particular treatment and the effect of the treatment itself. We attempted to control for this limitation by analyzing patients in major subgroups to enhance the uniformity of the results and their applicability to other studies. We do acknowledge, however, that even though we controlled for performance status when comparing treatment regimens and identified reasons for not undergoing trimodality therapy, additional factors that cannot be reflected in this scoring system or documented in the medical record, such as a selection bias with regard to a patient’s perceived ability to tolerate this procedure, may have confounded our outcomes. Regardless of this inevitable limitation, we attempted to control for pertinent clinical and disease variables in multivariate analysis, which strengthens our conclusion. Another limitation of our study lies in the long study period and hence wide range of treatment dates, another manifestation of the rarity of thymic malignancies. Nevertheless, although we do acknowledge the evolution of radiation and surgical techniques over time, we did find that the year of treatment did not adversely affect outcomes when other factors were taken into consideration in multivariate analysis, and thus this finding should not change the results and recommendations from this analysis.
CONCLUSIONS
Patients with advanced invasive thymic malignancies can achieve long-term survival after aggressive treatment that includes chemotherapy, surgical resection, and postoperative radiation therapy. Factors are associated with the greatest benefit from this approach include low disease stage, a histologic diagnosis of thymoma versus thymic carcinoma, complete resection status, and good performance status. However, outcomes with this approach remain suboptimal in the setting of thymic carcinoma, despite treatment at two large tertiary cancer centers. Due to the high rate of distant metastasis, systemic imaging is essential in this subgroup, and the integration of systemic therapy is also of great importance. Studies examining novel agents in this scenario are needed.
Acknowledgments
Sources of support: Supported in part by NIH NCI Cancer Center Support (core) Grant CA016672 to MD Anderson.
The authors would like to thank Christine Wogan, M.S., for her work in reviewing and editing this manuscript.
Footnotes
Disclaimers: The authors declare no conflicts of interest regarding this study.
References
- 1.Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009;4:911–919. doi: 10.1097/jto.0b013e3181a4b8e0. [DOI] [PubMed] [Google Scholar]
- 2.Okereke IC, Kesler KA, Morad MH, et al. Prognostic indicators after surgery for thymoma. Ann Thorac Surg. 89:1071–1077. doi: 10.1016/j.athoracsur.2010.01.026. discussion 1077–1079. [DOI] [PubMed] [Google Scholar]
- 3.Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg. 2008;85:385–389. doi: 10.1016/j.athoracsur.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 4.Shin DM, Walsh GL, Komaki R, et al. A multidisciplinary approach to therapy for unresectable malignant thymoma. Ann Intern Med. 1998;129:100–104. doi: 10.7326/0003-4819-129-2-199807150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg. 1997;64:1585–1591. doi: 10.1016/s0003-4975(97)00629-2. discussion 1591–1582. [DOI] [PubMed] [Google Scholar]
- 6.Hsu HC, Huang EY, Wang CJ, et al. Postoperative radiotherapy in thymic carcinoma: treatment results and prognostic factors. Int J Radiat Oncol Biol Phys. 2002;52:801–805. doi: 10.1016/s0360-3016(01)02656-6. [DOI] [PubMed] [Google Scholar]
- 7.Magois E, Guigay J, Blancard PS, et al. Multimodal treatment of thymic carcinoma: Report of nine cases. Lung Cancer. 2008;59:126–132. doi: 10.1016/j.lungcan.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa K, Toita T, Uno T, et al. Treatment and prognosis of thymic carcinoma: a retrospective analysis of 40 cases. Cancer. 2002;94:3115–3119. doi: 10.1002/cncr.10588. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger DS, Akerley W, Bepler G, et al. Thymic malignancies. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8:1302–1315. doi: 10.6004/jnccn.2010.0096. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol. 2010;5:2017–2023. doi: 10.1097/JTO.0b013e3181f13682. [DOI] [PubMed] [Google Scholar]
- 11.Finea JP, Grayb RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 12.Detterbeck F, Youssef S, Ruffini E, et al. A review of prognostic factors in thymic malignancies. J Thorac Oncol. 2011;6:S1698–1704. doi: 10.1097/JTO.0b013e31821e7b12. [DOI] [PubMed] [Google Scholar]
- 13.Rea F, Sartori F, Loy M, et al. Chemotherapy and operation for invasive thymoma. The Journal of thoracic and cardiovascular surgery. 1993;106:543–549. [PubMed] [Google Scholar]
- 14.Macchiarini P, Chella A, Ducci F, et al. Neoadjuvant chemotherapy, surgery, and postoperative radiation therapy for invasive thymoma. Cancer. 1991;68:706–713. doi: 10.1002/1097-0142(19910815)68:4<706::aid-cncr2820680407>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer. 2004;44:369–379. doi: 10.1016/j.lungcan.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Loehrer PJ, Sr, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:3093–3099. doi: 10.1200/JCO.1997.15.9.3093. [DOI] [PubMed] [Google Scholar]
- 17.Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg. 2004;77:1860–1869. doi: 10.1016/j.athoracsur.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878–884. doi: 10.1016/s0003-4975(03)00555-1. discussion 884–875. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. The Journal of thoracic and cardiovascular surgery. 2009;138:26–31. doi: 10.1016/j.jtcvs.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaggiari L, Casiraghi M, Guarize J. Multidisciplinary treatment of malignant thymoma. Curr Opin Oncol. 24:117–122. doi: 10.1097/CCO.0b013e32834ea6bb. [DOI] [PubMed] [Google Scholar]
- 21.Institute NC. [Accessed January 25 2013];Sunitinib for Advanced Thymus Cancer Following Earlier Treatment. 2013 Feb 9; Available at http://clinicaltrials.gov/ct2/show/record/NCT01621568.