The transcription factor (TF) HNF4α is known to be expressed in stomach, but its function was unknown. We show here that inducing deletion of HNF4α caused increased proliferation and collapse of chief cell endoplasmic reticulum (ER) and secretory architecture. We show HNF4α binds and upregulates the known ER-regulating TF XBP1. As we have previously shown XBP1 governs expression of the TF MIST1, we now show chief cell maturation depends on a HNF4α→XBP1→MIST1 transcriptional sequence.
Keywords: mucous neck cell, scaling factor, secretory cells
Abstract
We have previously shown that the sequential transcription factors Xbp1→Mist1 (Bhlha15) govern the ultrastructural maturation of the secretory apparatus in enzyme-secreting zymogenic chief cells (ZCs) in the gastric unit. Here we sought to identify transcriptional regulators upstream of X-box binding protein 1 (XBP1) and MIST1. We used immunohistochemistry to characterize Hnf4αflox/flox adult mouse stomachs after tamoxifen-induced deletion of Hnf4α. We used qRT-PCR, Western blotting, and chromatin immunoprecipitation to define the molecular interaction between hepatocyte nuclear factor 4 alpha (HNF4α) and Xbp1 in mouse stomach and human gastric cells. We show that HNF4α protein is expressed in pit (foveolar) cells, mucous neck cells, and zymogenic chief cells (ZCs) of the corpus gastric unit. Loss of HNF4α in adult mouse stomach led to reduced ZC size and ER content, phenocopying previously characterized effects of Xbp1 deletion. However, HNF4αΔ/Δ stomachs also exhibited additional phenotypes including increased proliferation in the isthmal stem cell zone and altered mucous neck cell migration, indicating a role of HNF4α in progenitor cells as well as in ZCs. HNF4α directly occupies the Xbp1 promoter locus in mouse stomach, and forced HNF4α expression increased abundance of XBP1 mRNA in human gastric cancer cells. Finally, as expected, loss of HNF4α caused decreased Xbp1 and Mist1 expression in mouse stomachs. We show that HNF4α regulates homeostatic proliferation in the gastric epithelium and is both necessary and sufficient for the upstream regulation of the Xbp1→Mist1 axis in maintenance of ZC secretory architecture.
NEW & NOTEWORTHY
The transcription factor (TF) HNF4α is known to be expressed in stomach, but its function was unknown. We show here that inducing deletion of HNF4α caused increased proliferation and collapse of chief cell endoplasmic reticulum (ER) and secretory architecture. We show HNF4α binds and upregulates the known ER-regulating TF XBP1. As we have previously shown XBP1 governs expression of the TF MIST1, we now show chief cell maturation depends on a HNF4α→XBP1→MIST1 transcriptional sequence.
while gastric cancer is the third leading cause of cancer mortality worldwide (15), the events that drive the progression from a healthy gastric epithelium to precancerous metaplasia, and finally to the development of adenocarcinoma, are not well understood. To understand the progression to disease, we must understand the molecular pathways that govern the normal differentiation of cell lineages. Physiologically, it is known that the mammalian gastric epithelium is an organized network of cells which function to secrete mucus, acid, and digestive enzymes into the gastric lumen. These cells are located in repeating glandular invaginations called gastric units. Based on anatomy and cell function, each unit can be divided into four distinct sections: the pit zone opens into the gastric lumen and contains mucus-secreting pit (also known as foveolar) cells; the isthmus region, deeper to the pit, houses stem cells and early progenitors; deeper still, the neck zone contains both acid-secreting parietal cells (PCs) and mucus-secreting neck cells; and the base zone contains mostly digestive-enzyme secreting zymogenic cells (ZCs) (Fig. 1A). The differentiation of each lineage has been characterized by detailed morphological studies and analysis of gene expression patterns, which are conserved in each gastric unit (27–31).
Fig. 1.
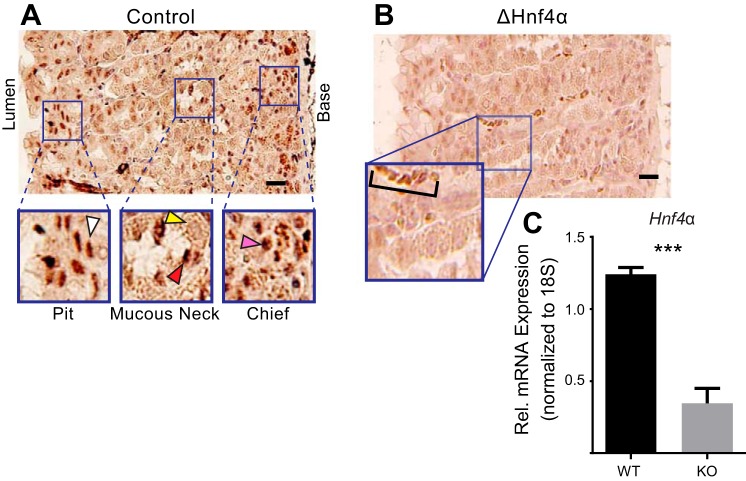
HNF4α is expressed in the gastric epithelium and can be deleted by Cre recombinase from the adult stomach. A: immunohistochemical stain of HNF4α shows it is expressed in the nuclei of isthmal progenitor and pit cells (white arrowhead), neck cells (red arrowhead), and zymogenic chief cells (pink arrowhead), but not in parietal cells (yellow arrowhead) of the mouse gastric corpus. B: immunohistochemical stain of HNF4α in mice 3 wk postinduction of cre recombinase to delete the floxed Hnf4α allele shows its expression is largely lost vs. control staining in A. Note the background positivity of red blood cells in the inset (bracket) but lack of detectable epithelial cell label (images are representative of n = 4 biological replicates). C: HNF4α mRNA levels in control vs. deleted stomachs. Columns represent means ± SD of three biological replicates; ***P < 0.001. Scale bars = 50 μm.
The gastric unit is a particularly well-suited system to study transcriptional regulators of cell differentiation and morphology, because development of each cell type is tightly controlled along a well-defined, spatiotemporal gradient and because renewal and differentiation occur throughout life. Although our knowledge of molecular pathways regulating this differentiation overall is scant, we do know some details of maturation in the ZC lineage. Mature ZCs arise from a mucus-rich precursor in the neck of the unit (30, 37, 46). As ZCs differentiate from their progenitor neck cells and migrate toward the base, abundance of the precursor (so-called “unspliced” form) of the mRNA for the transcription factor XBP1 increases (23). Increased XBP1 protein subsequently 1) establishes an elaborate rER network in ZCs to supply secretory cargo via protein synthesis and 2) directly transcriptionally activates the bHLH transcription factor MIST1 (BHLHA15), which establishes the apical secretory apparatus in the ZC (6, 23, 46).
The nuclear hormone receptor, hepatocyte nuclear factor 4α (HNF4α), is a key developmental regulator of differentiation in multiple endodermally derived organs. Loss of Hnf4α results in embryonic lethality in mouse models, as it is required for proper gastrulation (10). Tissue-specific knockouts of the Hnf4α gene have established its importance in the development and maintenance of secretory lineages in multiple tissues. HNF4α is required for both the development of the intestine (4, 16) and maintenance of its secretory cell-homeostasis, architecture, and function (8). Interestingly, human patients with inflammatory bowel (Crohn's) disease have low levels of HNF4α expression, and loss of HNF4α has been used to model colitis in mice (2, 11). Similarly, tissue-specific knockouts have shown that HNF4α is a crucial component in the initial embryonic development and maintenance of the adult liver (5, 21, 45). In the adult pancreatic beta-cells, HNF4α is required for proper secretion of insulin in response to glucose (19, 40). The fundamental role of HNF4α in these tissues and human disease has led to studies defining its structure (9), preferred binding sequences and partners (14, 39), expression variants (13), and its regulatory interplay with other related transcription factors (44), Despite the high level of Hnf4α expression in the gastric epithelium (12, 33, 49) and its pivotal role in the maintenance of closely related secretory tissue, e.g., the intestine and pancreas (12), the function of HNF4α has never been explored in the stomach. Here we show that HNF4α is required for normal differentiation in the gastric epithelium. HNF4α functions in part by inducing and maintaining Xbp1 expression (and, in turn, MIST1) expression in ZCs.
METHODS
Cell lines and transient transfection.
AGS cells (from ATCC, Manassas, VA) were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 0.9% glutamine, 0.4% HEPES, 1% Na pyruvate, 2.5% glucose, and 100 ng/ml each of penicillin and streptomycin. For overexpression of myc-tagged HNF4α2 or HNF4α8, coding regions (plasmids obtained from Addgene, human constructs IDs 31100 and 31094) were subcloned into a pcDNA3.1 expression vector, and 5 μg of each plasmid or the pmaxGFP(lonza) control plasmid was transiently transfected using TransIT-2020 (Mirus, Madison, WI) according to the manufacturer's protocol.
Western blot.
Cells for Western blot analysis were lysed in RIPA buffer. Proteins were quantified by DC protein assay (Bio-Rad) and then separated on NuPAGE Bis-Tris gels (Invitrogen), transferred onto Amersham Hybond ECL nitrocellulose (GE Healthcare) membranes, and detected by Immobilon chemiluminescence (Millipore). Primary antibodies used were rabbit anti- spliced-XBP1 (BioLegend), mouse anti-c-myc (Dshb), goat anti-HNF4α specific to P1 α1-α3 isoforms (Novus biological), and rabbit anti-α- and β-tubulin (Cell Signaling). Secondary antibodies were horseradish peroxidase-conjugated donkey anti-rabbit, anti-goat, and anti-mouse Ig (Santa Cruz Biotechnology). Quantifications of immunoblots were performed by scanning 16-bit images into ImageJ. Band intensities for XBP1 and α/β tubulin were selected and calculated by using the “Gels: Plot lanes” measurement tool. Standardized values were calculated determining the ratio of XBP1 signal to α/β tubulin signal.
RNA isolation and quantitative RT-PCR.
RNA was isolated using RNeasy (Qiagen) per the manufacturer's protocol. RNA was treated with DNase I (Invitrogen) and then reverse-transcribed using the SuperScript III (Invitrogen) standard protocol (most cDNA syntheses started with 1 μg of total RNA). Quantification of cDNA was performed by qRT-PCR using a Stratagene (La Jolla, CA) MX3000P detection system. Absolute QPCR SYBR green mix (Thermo Scientific) fluorescence was used to quantify relative amplicon amounts of Hnf4a, Xbp1, Mist1, and the normalizer, 18s.
Chromatin immunoprecipitation.
Chromatin immunoprecipitation (ChIP) was performed as described previously (26). Briefly 25 mg of stomach was fixed in 1.5% formalin in PBS at room temperature for 15 min before the cross-linking reaction was stopped with 0.125 M glycine. Tissue was disaggregated using a MediMachine (Becton Dickinson). Ten microliters of anti-HNF4α (rabbit, Cell Signaling) or whole rabbit serum (preimmune control) together with protein A/G plus agarose (Simple Chip Kit, Cell Signaling) were added to the homogenized tissue for immunoprecipitation. Quantitative real-time PCR (qRT-PCR) was performed to assess the quantity of genomic sequences immunoprecipitated by either preimmune control or HNF4α antiserum, as well as a 1:10 dilution of the cell extract prior to immunoprecipitation (input). Two predicted HNF4α binding sites (41) were probed in addition to an intronic control region with no predicted HNF4α binding sites nearby.
Mouse studies.
All experiments involving animals were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. Floxed Hnf4α, CAGGCreERTM transgenic mice were generated by crossing Hnf4αfloxed/floxed mice (a gift from Frank Gonzalez, NIH) (21) with CAGGCreERTM;Hnf4αfloxed/+ (20) mice to allow systemic, tamoxifen-inducible knockout of HNF4α. Six- to eight-week-old CAGGCreERTM;Hnf4αfloxed/floxed mice and CAGGCreERTM;Hnf4αfloxed/+ littermate controls were injected intraperitoneally with tamoxifen (1 mg/20 g body wt, 5 consecutive days) to induce Cre-mediated Hnf4α deletion (24). Mice were euthanized 4 wk after first tamoxifen injection. Mice are routinely monitored for toxicity due to tamoxifen, and none was noted at this relatively low dose (23–25).
Immunohistology and quantification.
Stomachs were prepared and stained as described previously (46). Stomachs were inflated with 1.5 ml 10% formalin fixative and suspended in fixative for 4 h at room temperature. Tissue was rinsed with 70% EtOH multiple times, the forestomach discarded, and the remaining stomach cut into 4 transverse rings from proximal corpus to antrum. Rings were arranged in 2% agar in tissue cassettes such that lesser and greater curvatures of each transverse ring were aligned; tissue was then paraffin processed. Sections (5 μm) were deparaffinized and rehydrated, and antigen retrieval was performed by boiling in Trilogy Buffer (Cell Marquee). Slides were blocked in 1% BSA, 0.3% Triton X-100 in PBS, then incubated in primary followed by secondary antibodies [or for Immunofluorescent staining fluorescently labeled lectin Griffonia simplicifolia-II (neck cell-specific GS-II; 1:1,000; Invitrogen)]. Finally, slides were incubated for 5 min in 1 μg/ml bisbenzimide (Invitrogen) prior to mounting in 1:1 PBS-glycerol. Primary antibodies used for immunostaining were goat anti-Calregulin (1:200, Sigma), goat anti-human gastric intrinsic factor (GIF) (1:2,000; gift of Dr. David Alpers, Washington University), rabbit anti-HNF4α (1:100 Cell Signaling), goat anti-BrdU (1:2,000; gift of Dr. Jeff Gordon, Washington University), rabbit anti-Ki67 (AbCam), and sheep anti-PGC (1:10,000; Abcam).
For morphological analysis, stomach rings cut into paraffin sections were hematoxylin and eosin (H&E) stained and were analyzed from lesser to greater curvature and from proximal to distal. Whole slides were scanned with the Nanozoom microscope, and every fifth unit was measured across each slide using Nanozoom Digital Pathology software (Hamamatsu). Area of ZCs with both apical and basal contacts was measured from random units chosen throughout the corpus of the stomach using ImageJ to determine area. Aberrant localization/migration of mucous neck cells into the base of units was confirmed by GS-II/GIF immunofluorescent labeling (mucous neck cells are GS-II+/GIF−). Scoring of mucous neck cell localization was then performed using PAS-stained sections. Samples were randomized, and the scorer was blinded to ensure unbiased quantification.
Graphing and statistics.
Experiments were performed with at least three biological replicates independently. Values represent means ± SD as indicated. All statistics and graphs were determined using GraphPad Prism and depicted in final graphical form in Adobe Illustrator. Statistical analysis was by one- or two-tailed Student's t-test, depending on the hypothesis prior to commencing the experiment, or by ANOVA with Dunnet's comparison where indicated.
RESULTS
HNF4α is expressed in the isthmus, pit, neck, and zymogenic cells in the gastric corpus.
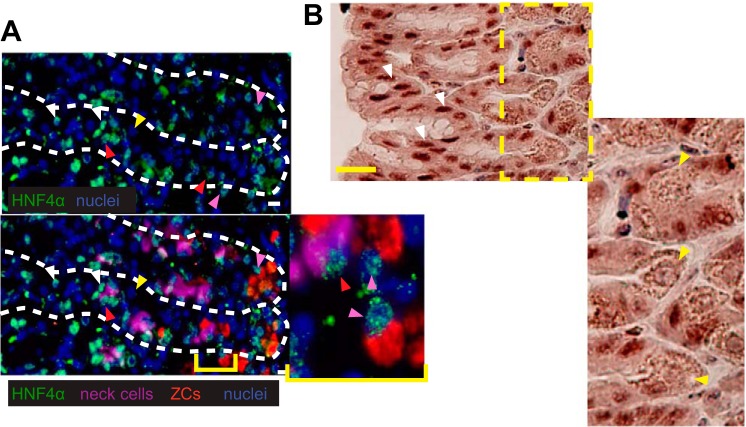
Like most endodermally derived tissues, the stomach also expresses HNF4α (12). However, its specific cellular localization in mouse stomach has not been shown. We found that HNF4α was expressed at high levels in pit cells and progenitor cells in the isthmus. Mucous neck cells also express HNF4α as do the cells that derive from them, ZCs (Figs. 1A and 2A). HNF4α was not detected in parietal cells, which are cells that pump hydrogen ions into the gastric lumen via plasma membrane transporters (Fig. 2B). Thus the cells and their progenitors that actively secrete abundant cargo from cytoplasmic vesicles (mucus, digestive enzymes) express HNF4α. This pattern of expression is consistent with previous work describing the role of HNF4α as both a repressor of progenitor proliferation and a promoter of differentiation of cell architecture (8, 21).
Fig. 2.
Immunofluorescent staining of HNF4α in the gastric epithelium. Images of HNF4α (green) expression in the nucleus (blue) of isthmal progenitor and pit cells (white arrowheads), neck cells (red arrowheads, GSII = purple), and zymogenic cells (pink arrowheads, GIF = red), but again not in parietal cells (yellow arrowheads). Top panel: HNF4α and nuclei; bottom panel: merge with all 4 colors. B: immunohistochemical stain for HNF4α (brown) in region of gastric pit shows strong pit cell nuclear staining (white arrowheads) and only background staining in parietal cells (yellow arrowheads). Scale bars represent 20 μm.
Loss of HNF4α in the stomach causes increased epithelial cell proliferation in the isthmus of the gastric unit.
We sought to determine the function of HNF4α in the gastric epithelium by deleting its expression in adult mice. We generated a mouse model of acute loss of HNF4α by crossing the tamoxifen inducible CAGCreERT mice, previously shown by our group to be an efficient driver of Cre recombinase activity in gastric epithelium (23), to mice containing a Hnf4αflox/flox allele (21). This model allowed us to induce loss of HNF4α via injection of doses of tamoxifen (1 mg/20 g mouse) that are well under the gastric toxicity level (Fig. 1, B and C) (23–25). This approach, generating mice we will refer to hereafter as Hnf4αΔ/Δ, reduced HNF4α expression in the stomach by ∼70% and caused loss of immunohistochemical staining for HNF4α in most gastric units (Fig. 1, B and C), although all mice retained some regions that preserved HNF4α as well (not shown). The CAG promoter is the most efficient of the global, inducible Cre recombinase pedigrees we have examined in the lab, and higher doses of tamoxifen cause gastric toxicity (23–25). Hence, we performed the remaining experiments on Hnf4αΔ/Δ mice generated using this protocol. We note that the quantification of histological changes (described below) induced by loss of HNF4α are likely underestimates of the actual effects because we included in these analyses all regions of the stomach, some of which may have not had HNF4α deleted due to mosaicism of gene deletion.
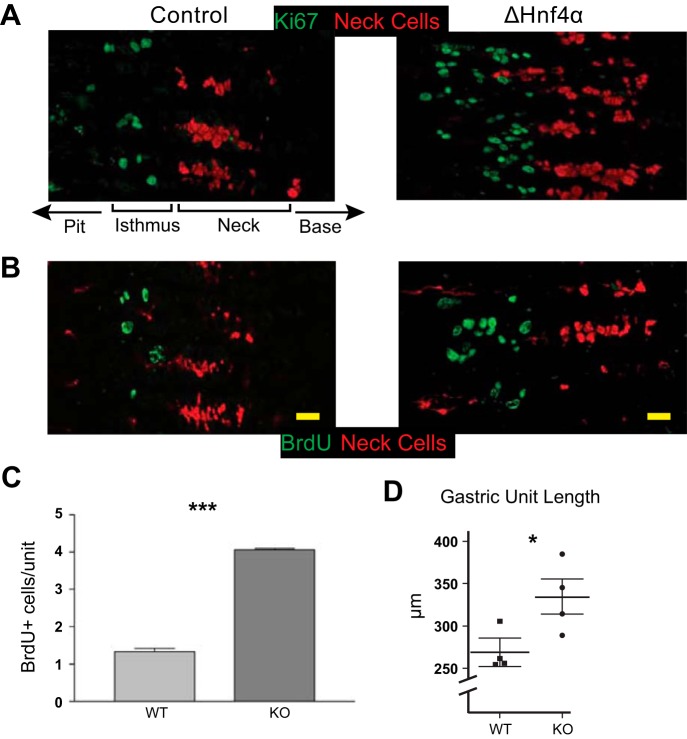
Loss of HNF4α led to a ∼4-fold increase in proliferation compared with CAGCreERT ;Hnf4αfloxed/+ mice treated with equivalent Tamoxifen (hereafter referred to as “control”), measured by Ki-67 expression and BrdU staining (Fig. 3, A–C). This increased proliferation correlated with increased gastric unit length (Fig. 3D). The increased proliferation was confined to the isthmal region of the unit where proliferation from the stem cell and progenitor cells normally takes place, and did not occur in the base of the unit where proliferation is often observed in response to stress or damage (24, 32, 36, 43).
Fig. 3.
Loss of HNF4α leads to increased proliferation in the gastric epithelium. A: immunofluorescent staining with Ki67 (green) shows increased proliferation in ΔHNF4α vs. control mouse gastric corpus (mucous neck cells labeled red with GS-II lectin). Note orientation of gastric units with gastric lumen to left and base to right; A and B focus only on isthmus and neck zones. B and C: staining and quantification of BrdU (green) incorporation in control and ΔHNF4α epithelium. Columns = means ± SE, n = 3 biological replicates; ***P < 0.001. D: increased proliferation correlated with longer units. n = 4 mice; mean ± SE; *P < 0.05. Scale bars = 20 μm.
HNF4α is required for normal ZC development.
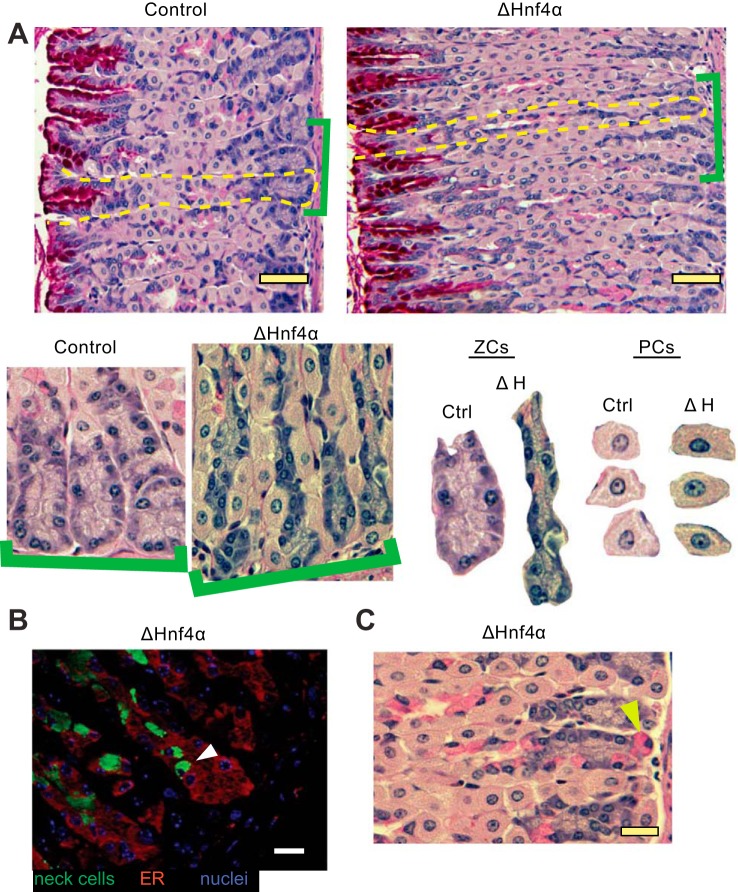
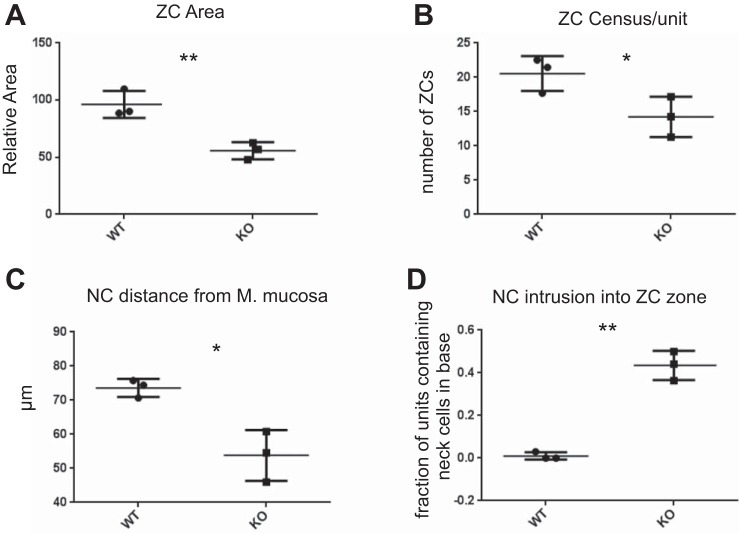
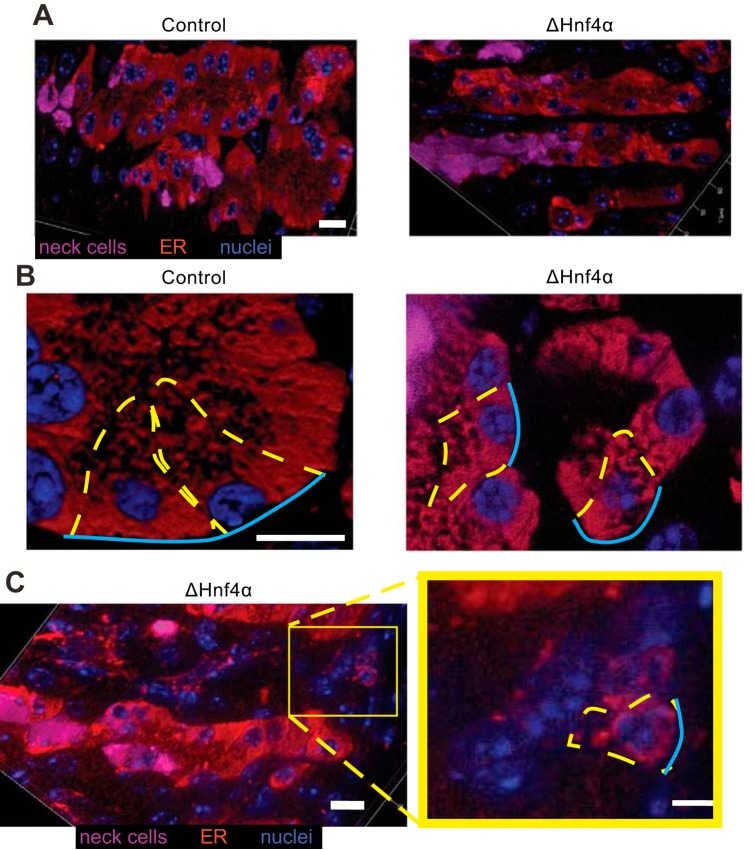
The other striking phenotype of loss of HNF4α was a dramatic change in ZC morphology. The base/ZC region was markedly narrowed in the Hnf4αΔ/Δ mouse gastric corpus (Fig. 4, A and C). Not only were the ZCs in ΔHNF4α mouse stomachs half the size of control ZCs on average (Fig. 5A), but the overall number of ZCs was ∼25% lower in the Hnf4αΔ/Δ mice (Fig. 5B). Previous work by our lab and others has defined HNF4α as a crucial regulator of multiple factors required for the development and maintenance of vast ER networks that secretory cells require to synthesize and secrete large biosynthetic loads (41, 47). To determine if HNF4α is required to maintain the ER in the gastric epithelium, we examined the effect of loss of HNF4α by immunofluorescent staining for the ER-localized protein, Calregulin. We found the ER volume was substantially reduced in size upon loss of HNF4α (Fig. 4B and Fig. 6, A and B). Some basal epithelial cells were so reduced in ER content that they were nearly not recognizable as ZCs (Fig. 6C, inset).
Fig. 4.
Loss of HNF4α disrupts the normal differentiation of the mucous neck cell/zymogenic cell lineage. A: H&E + periodic acid Schiff (to detect mucins) of mouse gastric epithelium in control and ΔHNF4α mice. Brackets highlight higher magnification views, below, of bases of gastric units. Isolated bases (labeled “ZCs”) and random PCs from the images are shown side by side at the same magnification. Note that ZCs are smaller in ΔHNF4α mice, whereas PCs do not show morphological changes. Scale bars = 50 μm. B: immunofluorescent stain of the ER marker Calregulin (red) and the mucous neck cell marker GSII (green) in ΔHNF4α mice. Note that mucous neck cells show aberrant localization to the base of the unit, within the ZC zone (white arrowhead), also visible by PAS staining for mucus in C (e.g., yellow arrowhead). Scale bars = 20 μm.
Fig. 5.
Quantification of histological data from n = 3 biological replicates. A: ZC size (measured as pixels per cell), normalized to control (WT). B: number of ZCs per unit. C: distance between the mucous neck cell (NC) nearest the base of the gastric unit (i.e., the muscularis mucosa basement membrane) and the base of the unit. D: the mean fraction of units per mouse containing mucous neck cells in the base regions where ZCs reside. *P < 0.05, **P < 0.01 by one-tailed t-test, unequal variance; data expressed as mean from each mouse + range of all 3 mice.
Fig. 6.
Zymogenic cell abnormalities in the absence of HNF4α are caused by loss of HNF4α targets regulating ER and the cell cycle. Immunofluorescent stain of the ER marker Calregulin (red) and the mucous neck cell marker GSII (magenta) in control and ΔHNF4α mice (nuclei: blue). Note less elaborate ER network in HNF4α mice (left and right panels at same magnification). A: optical sections. B: higher magnification Z-stack 3-dimensional projection of 20 0.5-μm optical sections in different mice. C: optical section of ΔHNF4α showing occasional bases harboring small ZCs containing markedly scant ER. Representative ZCs outlined in dashed yellow with blue lines showing basement membrane surface. All scale bars = 10 μm.
Loss of HNF4α disrupts mucous neck→zymogenic cell differentiation.
To become a functioning digestive-enzyme-secreting cell, the ZC differentiates and migrates first from a proliferative progenitor cell in the isthmus, then into a mucous neck cell before finally reaching the base of the unit and assuming its mature phenotype. Because of the role of HNF4α in other tissues as a repressor of both proliferation and a regulator of cell architecture, we further examined the phenotype of ZC differentiation. We observed cells expressing precursor neck cell markers ectopically in the bases of the gastric unit in ΔHNF4α mice (Fig. 4, B and C). Mucous neck cells are almost never observed in the base of control gastric units (30). Both the average distance of neck cells from the basement membrane overlying the muscularis mucosa deep to the bottom of the unit (Fig. 5C) and the percentage of ZC-regions that contained a neck cell (Fig. 5D) were significantly different upon loss of HNF4α, with over one-third of ΔHNF4α ZC regions exhibiting neck cell intrusion. Note that these cells expressed only mucous neck cell markers and not ZC markers; thus they were not the metaplastic cells that arise during certain types of damage to the gastric unit (6, 17, 18, 32, 34, 42). Taken together, the data indicate that HNF4α is required for the orderly progression of mucous neck cells into ZCs, although whether differentiation of neck cells to ZCs is impaired or whether mucous neck cells simply migrate too far basally without differentiating is not clear.
HNF4α is required to maintain Xbp1 expression in the mouse stomach.
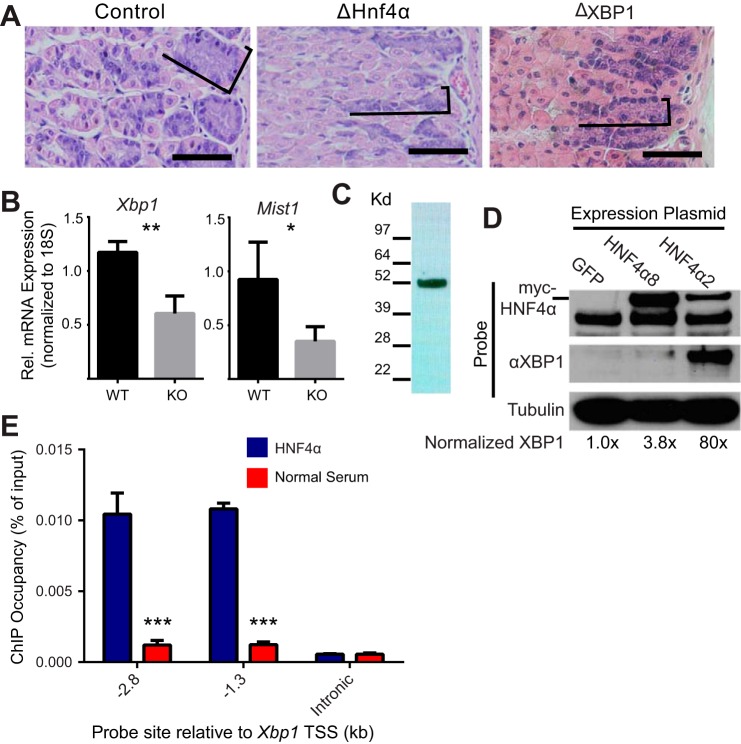
Our lab has previously shown that one of the most important transcriptional regulators of the development and maintenance of the ER, XBP1, is required for normal ZC ultrastructural maturation (23). Interestingly, mouse gastric epithelium lacking HNF4α strongly resembles that of epithelium lacking XBP1 in both overall ZC morphology, i.e., reduced ZC size (Fig. 4A), and with loss of ER in ZCs, as assessed by staining with Calregulin (Fig. 4B and Fig. 6, A–C). Figure 7A demonstrates a side-by-side comparison of ΔHNF4α with ΔXBP1 ZCs. Because of these results, and recent work showing HNF4α is important for expression of XBP1 in pancreatic beta-cells (41), we sought to determine whether HNF4α drives expression of XBP1 to maintain ZC differentiation. We isolated RNA from the gastric corpus of Hnf4αΔ/Δ mice and found that loss of HNF4α significantly decreased both the abundance of Xbp1 and the abundance of a downstream target of XBP1, Mist1, by ∼50% (Fig. 7B) (1, 23).
Fig. 7.
HNF4α is required to maintain Xbp1 expression in the mouse stomach. A: H&E staining of mouse gastric epithelium in control, ΔHNF4α and ΔXBP1 mice. As in ΔHNF4α stomachs (Fig. 4A), ZCs are noticeably smaller (brackets outline ZC zone) upon loss of XBP1 (scale bar = 100 μm). B: measurement of mRNA with quantitative-RT-PCR in control and ΔHNF4α mouse gastric corpus shows abundance of Xbp1, and its downstream target Mist1, decrease significantly after deletion of Hnf4α. Means ± SD, n = 3 biological replicates; *P < 0.05, **P < 0.01. C: Western blot of protein isolated from normal mouse stomach and probed with an antibody specific to the P1 splice variant of HNF4α. D: Western blot of AGS gastric cell line transiently transfected with expression plasmids encoding two splice variants of HNF4α or a GFP control shows XBP1 expression is enhanced by overexpression of HNF4α. E: chromatin immunoprecipitation of mouse gastric corpus shows that HNF4α occupies two sites in the Xbp1 promoter compared with immunoprecipitation with normal rabbit serum but not an intronic control region (i.e., with no HNF4α preferred binding sequences predicted). Bars = means ± SD of n = 3 biological replicates, significance was determined via ANOVA with Dunnet's post hoc comparison; ***P < 0.001.
In human stomach, it has been reported that splice variants of Hnf4α using the so-called P2 promoter predominate over the P1 promoter (49), although the P1 promoter is expressed more prominently in intestinal metaplasia and gastric cancer (33). A similar predominance of P1 in adult rat stomach has been reported (12); however, we used isoform-specific primers and were able to amplify both isoforms in adult mouse stomach, although the P1 promoter transcript did amplify at an earlier cycle suggesting it was more abundant (not shown). We detect both the protein translated from the P2 promoter transcript as expected (not shown) as well as, unexpectedly, a strong P1 promoter protein band on Western blot (Fig. 7C). We transiently transfected gastric cancer-derived AGS cells with a plasmid containing either of two common splice variants of Hnf4α. XBP1 expression, measured by Western blot, increased about 4-fold in cells transfected with the P2 variant (HNF4α8) and nearly 80-fold in transfection with the P1 variant (HNF4α2) (Fig. 7D). Thus HNF4α is sufficient to augment XBP1 expression. It is not clear why the two isoforms produce differing responses, although the increased expression of P1 variants in gastric cancer may make the cells more responsive to this variant. To determine if regulation of Xbp1 by HNF4α was a direct effect or a downstream effect, we performed chromatin immunoprecipitation of the Xbp1 promoter. We previously had searched the Xbp1 locus for HNF4α binding sites and found two putative sites in the XBP1 promoter region, 2.8 and 1.3 kb upstream of the mouse Xbp1 promoter (41). We measured HNF4α occupancy at each of these sites in mouse stomach, and at a downstream internal control site, and found significant occupancy of only the two promoter sites compared with a normal serum control in mouse stomach tissue (Fig. 7E). In sum, the data show that HNF4α is a direct transcriptional enhancer of XBP1 in the mouse gastric epithelium and may thus explain why ZC ER and size is reduced and suggesting that the clusters of markedly small cells in the base are ZCs that have lost most of their cell volume, as we previously saw when we deleted XBP1 in ZCs (23).
DISCUSSION
We show for the first time that HNF4α is expressed in the gastric epithelium in mouse pit and isthmal progenitor zones, and in mucous neck and zymogenic cells. It is required for normal differentiation in the gastric unit. We also present data that HNF4α is a direct transcriptional regulator of Xbp1 expression in the mouse stomach, suggesting that enhancing/maintaining transcription of Xbp1 is one of the mechanisms through which HNF4α regulates ZC development. To our knowledge, this is the first time the role of HNF4α in gastric cellular differentiation has been described.
Loss of HNF4α not only disrupts the differentiation of ZCs but also causes increased epithelial cell proliferation. This proliferation is confined to the isthmal region, which houses the normal stem and progenitor cell populations. That isthmus-confined proliferation is in contrast to other models in the stomach in which parietal cells die and ZCs undergo metaplastic changes to reenter the cell cycle (36). For example, we, and others, have shown that cells in the base of the gastric unit become proliferative in response to agents such as high-dose (5 mg/20 g mouse weight) tamoxifen, DMP-777, or Helicobacter pylori infection (3, 17, 32, 37).
There are few studies identifying a transcriptional regulator responsible for gastric epithelial development. Our data characterizing the transcriptional enhancement of Xbp1 expression by HNF4α 1) further build on the only well-defined signaling cascade that orchestrates the emergence of ZC secretory capacity XBP1→MIST1 and 2) exemplify the emerging concept that tight regulation of Xbp1 expression is pivotal to its role as a developmental coordinator of secretory cell architectural maturation that may be independent of cell fate choice (7, 22, 23, 48, 50).
We now propose that the full transcription factor sequence dictating ZC differentiation is HNF4α→XBP1→MIST1. Thus HNF4α is at the top of the transcription factor hierarchy in stomach (present study) and other endodermally-derived tissues like pancreas and liver (41). That is consistent with HNF4α being a more organ-specific transcription factor, involved in repressing proliferation in adult endodermal organs but also critical for endodermal early development. On the other hand, we and others have shown that the XBP1→MIST1 sequence also characterizes the maturation of diverse secretory cells in non-endodermally-derived secretory tissues as well, like salivary glands and antibody-secreting plasma cells (1, 7, 22, 35). We have proposed that XBP1 and MIST1 are transcription factors that function as “scaling factors” to coordinate the expansion of secretory apparatus in cells of diverse organs that do not share a common embryonic germ layer derivation (38, 41, 48). Loss of either XBP1 or MIST1 in adult cells usually leads only to “downscaling” of the elaborate, mature secretory architecture but not to changes in cell identity or differentiation patterns (7, 22, 23, 48, 50). Thus, if HNF4α is typical of the factors upstream of XBP1 in all tissues (including non-endodermally derived ones), then transcriptional activators of XBP1 in non-endodermal tissues may also be, like HNF4α, part of the normal embryonic and homeostatic, tissue-specific differentiation pattern in those tissues. In other words, non-endodermally derived cell lineages, such as parotid acinar cells and plasma cells, that depend on the XBP1→MIST1 sequence likely have some other more tissue-specific transcription factor or factors in the HNF4α role (35). In agreement with HNF4α's having a more tissue-specific, cell fate-regulating role (and not a scaling factor role like XBP1 and MIST1) are our findings that its loss causes not just downscaling of secretory apparatus in ZCs but also mis-migration/differentiation of mucous neck cells and increased proliferation of progenitor cells.
Future experiments identifying the transcription factors responsible for the transcriptional upregulation of XBP1 in dedicated secretory cells outside of the endoderm would be in order. We also suggest that identifying such factors may lead to a better understanding of diseases involving aberrant secretory activity (e.g., plasma cell dyscrasias). In the specific case of endodermally derived organs that normally express HNF4α, we speculate that some diseases in these tissues might be caused or worsened by mutation or deficiency in the HNF4α→XBP1 axis, e.g., liver-dyslipidemia in hepatocytes, inflammatory bowel disease caused by aberrant Paneth/goblet cell secretion, and diabetes caused by β-cell-secretory dysfunction (21, 41, 45).
GRANTS
J. C. Mills is funded by the NIH National Institute of Diabetes and Digestive and Kidney Diseases (R01s DK094989 and DK105129) and an award from the Siteman Cancer Center Investment Program. The Washington University DDRCC AITAC is funded by P30 DK052574-12.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.D.M. and J.C.M. conception and design of research; B.D.M., S.S.K., W.J.H., and J.C.M. performed experiments; B.D.M. and J.C.M. analyzed data; B.D.M. and J.C.M. interpreted results of experiments; B.D.M. and J.C.M. prepared figures; B.D.M. and J.C.M. drafted manuscript; B.D.M. and J.C.M. edited and revised manuscript; B.D.M., S.S.K., W.J.H., and J.C.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Washington University Digestive Disease Research Core Center Advanced Imaging and Tissue Analysis Core (DDRCC AITAC).
Present address of W. J. Huh: Depts. of Medicine and of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, TN.
REFERENCES
- 1.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Ahn SH, Shah YM, Inoue J, Morimura K, Kim I, Yim S, Lambert G, Kurotani R, Nagashima K, Gonzalez FJ, Inoue Y. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis 14: 908–920, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anti M, Armuzzi A, Gasbarrini A, Gasbarrini G. Importance of changes in epithelial cell turnover during Helicobacter pylori infection in gastric carcinogenesis. Gut 43, Suppl 1: S27–S32, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babeu JP, Darsigny M, Lussier CR, Boudreau F. Hepatocyte nuclear factor 4alpha contributes to an intestinal epithelial phenotype in vitro and plays a partial role in mouse intestinal epithelium differentiation. Am J Physiol Gastrointest Liver Physiol 297: G124–G134, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Battle MA, Konopka G, Parviz F, Gaggl AL, Yang C, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci USA 103: 8419–8424, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol 325: 211–224, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capoccia BJ, Lennerz JK, Bredemeyer AJ, Klco JM, Frater JL, Mills JC. Transcription factor MIST1 in terminal differentiation of mouse and human plasma cells. Physiol Genomics 43: 174–186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattin AL, Le Beyec J, Barreau F, Saint-Just S, Houllier A, Gonzalez FJ, Robine S, Pincon-Raymond M, Cardot P, Lacasa M, Ribeiro A. Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol Cell Biol 29: 6294–6308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra V, Huang P, Potluri N, Wu D, Kim Y, Rastinejad F. Multidomain integration in the structure of the HNF-4alpha nuclear receptor complex. Nature 495: 394–398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE Jr. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev 8: 2466–2477, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Darsigny M, Babeu JP, Dupuis AA, Furth EE, Seidman EG, Levy E, Verdu EF, Gendron FP, Boudreau F. Loss of hepatocyte-nuclear-factor-4alpha affects colonic ion transport and causes chronic inflammation resembling inflammatory bowel disease in mice. PloS one 4: e7609, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean S, Tang JI, Seckl JR, Nyirenda MJ. Developmental and tissue-specific regulation of hepatocyte nuclear factor 4-alpha (HNF4-alpha) isoforms in rodents. Gene Express 14: 337–344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewes T, Senkel S, Holewa B, Ryffel GU. Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol Cell Biol 16: 925–931, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang B, Mane-Padros D, Bolotin E, Jiang T, Sladek FM. Identification of a binding motif specific to HNF4 by comparative analysis of multiple nuclear receptors. Nucleic Acids Res 40: 5343–5356, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359–E386, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Garrison WD, Battle MA, Yang C, Kaestner KH, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4alpha is essential for embryonic development of the mouse colon. Gastroenterology 130: 1207–1220, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res 317: 2759–2764, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 138: 2207–2210, 2210 e2201, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest 115: 1006–1015, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21: 1393–1403, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess DA, Humphrey SE, Ishibashi J, Damsz B, Lee AH, Glimcher LH, Konieczny SF. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology 141: 1463–1472, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology 139: 2038–2049, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142: 21–24 e27, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am J Physiol Gastrointest Liver Physiol 299: G368–G380, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Im H, Grass JA, Johnson KD, Boyer ME, Wu J, Bresnick EH. Measurement of protein-DNA interactions in vivo by chromatin immunoprecipitation. Methods Mol Biol 284: 129–146, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 236: 314–332, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236: 259–279, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 236: 280–296, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec 236: 333–340, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, Noto J, Peek RM Jr, Stenson WF, Mills JC. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem 288: 16085–16097, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima K, Kishimoto T, Nagai Y, Tanizawa T, Nakatani Y, Miyazaki M, Ishikura H. The expression of hepatocyte nuclear factor-4alpha, a developmental regulator of visceral endoderm, correlates with the intestinal phenotype of gastric adenocarcinomas. Pathology 38: 548–554, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol 177: 1514–1533, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzler MA, Venkatesh SG, Lakshmanan J, Carenbauer AL, Perez SM, Andres SA, Appana S, Brock GN, Wittliff JL, Darling DS. A systems biology approach identifies a regulatory network in parotid acinar cell terminal differentiation. PloS one 10: e0125153, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills JC, Sansom OJ. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 8: re8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology 140: 412–424, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills JC, Taghert PH. Scaling factors: transcription factors regulating subcellular domains. Bioessays 34: 10–16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misawa K, Horiba T, Arimura N, Hirano Y, Inoue J, Emoto N, Shimano H, Shimizu M, Sato R. Sterol regulatory element-binding protein-2 interacts with hepatocyte nuclear factor-4 to enhance sterol isomerase gene expression in hepatocytes. J Biol Chem 278: 36176–36182, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Miura A, Yamagata K, Kakei M, Hatakeyama H, Takahashi N, Fukui K, Nammo T, Yoneda K, Inoue Y, Sladek FM, Magnuson MA, Kasai H, Miyagawa J, Gonzalez FJ, Shimomura I. Hepatocyte nuclear factor-4alpha is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. J Biol Chem 281: 5246–5257, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Moore BD, Jin RU, Lo H, Jung M, Wang H, Battle MA, Wollheim CB, Urano F, Mills JC. Transcriptional regulation of X-Box-binding protein one (XBP1) by hepatocyte nuclear factor 4alpha (HNF4Alpha) is vital to beta-cell function. J Biol Chem 291: 6146–6157, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore BD, Jin RU, Osaki L, Romero-Gallo J, Noto J, Peek RM Jr, Mills JC. Identification of alanyl aminopeptidase (CD13) as a surface marker for isolation of mature gastric zymogenic chief cells. Am J Physiol Gastrointest Liver Physiol 309: G955–G964, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, Konieczny SF, and Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037 e2029, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science 303: 1378–1381, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parviz F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS, Duncan SA. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet 34: 292–296, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211–222, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Sato Y, Hatta M, Karim MF, Sawa T, Wei FY, Sato S, Magnuson MA, Gonzalez FJ, Tomizawa K, Akaike T, Yoshizawa T, Yamagata K. Anks4b, a novel target of HNF4alpha protein, interacts with GRP78 protein and regulates endoplasmic reticulum stress-induced apoptosis in pancreatic beta-cells. J Biol Chem 287: 23236–23245, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21: 81–93, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka T, Jiang S, Hotta H, Takano K, Iwanari H, Sumi K, Daigo K, Ohashi R, Sugai M, Ikegame C, Umezu H, Hirayama Y, Midorikawa Y, Hippo Y, Watanabe A, Uchiyama Y, Hasegawa G, Reid P, Aburatani H, Hamakubo T, Sakai J, Naito M, Kodama T. Dysregulated expression of P1 and P2 promoter-driven hepatocyte nuclear factor-4alpha in the pathogenesis of human cancer. J Pathol 208: 662–672, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Todd DJ, McHeyzer-Williams LJ, Kowal C, Lee AH, Volpe BT, Diamond B, McHeyzer-Williams MG, Glimcher LH. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med 206: 2151–2159, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]