Abstract
Acute Zika virus infection usually presents with a self-limiting triad of fever, rash and arthritis. There is limited information on severe or lethal cases. We report three cases of lethal acute Zika infection, confirmed with polymerase chain reaction, in adult patients with some co-morbidities. The patients showed rapid clinical deterioration with hemorrhagic and septic shock, and exaggerated acute and innate inflammatory responses with pronounced coagulopathy, and died soon after admission to the hospital. It remains unclear whether the fatal outcomes were due to acute Zika virus infection alone or to the combination with exacerbated underlying prior disease or co-infection. Nonetheless, the severity of these cases implies that increased awareness for atypical presentations of Zika virus infection, and careful clinical assessment of patients with symptoms of Zika, is warranted during current and future outbreaks.
Keywords: Zika, ZIKV, Sepsis, Hemorrhagic fever
Introduction
Zika virus (ZIKV) is a vector-borne virus (family Flaviviridae, genus Flavivirus) transmitted by Aedes mosquitos [1]. In 2015 the virus rapidly caused an epidemic in many South and Central American and Caribbean countries [2]. Two geographically distinct lineages (i.e., the African and Asian lineage) of ZIKV exist [1]. The ZIKV strain in Suriname resembles the Asian lineage and the first clinical cases appeared around September 2015 [3].
ZIKV infection usually presents with a mild self-limiting triad of fever, maculopapular rash, and polyarthralgia, sometimes accompanied by bilateral conjunctivitis [1], [2]. ZIKV infection has been associated with congenital brain malformations and microcephaly among newborns and neurological complications (e.g., Guillain-Barre syndrome) in adults .[4], [5] The exact impact of these complications is now under further investigation, but may indicate increased virulent potential of ZIKV in the Americas. Lethal potential of acute ZIKV infection in adults has been described in a recent report from Colombia [6]. In these cases, underlying prior disease may have contributed to the lethal outcome and host-responses such as severe thrombocytopenia and septic shock were consistently observed. A confirmed severe non-lethal case from Suriname also presented with thrombocytopenia and subcutaneous bleeding [7]. Additionally, a case of lethal ZIKV infection in an adolescent girl with sickle cell anemia suggests an association of a fatal outcome with aberrant vascular responses leading to exacerbation of vaso-occlusive events [8].
Here, we provide a detailed clinical and diagnostic report of three adults presenting with septic shock and multi-organ failure in which ZIKV was the only identified pathogen. With our report we intend to emphasize the need for increased awareness of atypical and severe presentations of acute ZIKV infection during the current and future outbreaks.
Methods
Real-time PCR
For all RT-PCR analyses, viral RNA was extracted manually from 150 μL of an EDTA-plasma specimen by using the E.Z.N.A. Viral RNA Kit (OMEGA Bio-Tek, Norcross, GA, USA), according to the manufacturer’s instructions. CHIKV RT-PCR was performed according to Pastorino et al. with a CHIKV specific primer set targeting a region of the envelope-protein 1 gene of CHIKV (F-CHIK: 5′-AAGCTYCGC GTCCTTTACCAAAG-3′ and R-CHIK: 5′-CCAAATTGTCCYGGTCTTCCT-3′).[9] DENV PCR was performed according to Lanciotti et al. with a primer set specific for the DENV-envelope gene (DEN-F: 5′-TTAGAGG AGACCCCTCCC-3′ DEN-R: 5′-TCTCCTCTAACCTCTAGTCC-3′).[10] For ZIKV PCR, a ZIKV-positive control was obtained from the Arboviral Diseases Branch of the Center for Disease Control and Prevention (Fort Collins, Colorado, USA). RT-PCR for ZIKV was performed according to the protocol of Lanciotti et al., with the following modification: SYBR Green I Dye (Thermo Scientific) was used for detection [11]. For this method, high sensitivity was already shown in studies for detection of dengue and chikungunya viruses [12]. A set of ZIKV specific primers were used: ZIKV 835: 5′ TTGGTCATGATACTGCTGATTGC-3′; ZIKV 911c 5′CCTTCCACAAAGTCCCTATTGC-3′; ZIKV 1086: 5′ CCGCTGCCCAAC ACAAG-3′; ZIKV 1162c: 5′ CCACTAACGTTCTTTTGCAGACAT-3′. Amplification of viral RNA was performed with a LightCycler 480 (LC480) Real-Time PCR system (Roche) and viral load (Fig. 1B) was determined with LC480 software. Nucleotide sequence of the ZIKV envelope was obtained and a phylogenetic tree was constructed with CLC Main Workbench 6 Software by neighbor joining of ZIKV envelope gene sequences of earlier Surinam cases to trace the origin of the ZIKV isolate of our first case (Fig. 1C, branch 21068, 2015, Suriname).[3] For Case 2 a 22 multiplex PCR (Pathofinder BV, Maastricht, The Netherlands) for viral and bacterial pathogens was performed on an oro-pharyngeal swab kept in universal transport medium (Copan diagnostics, Italy).
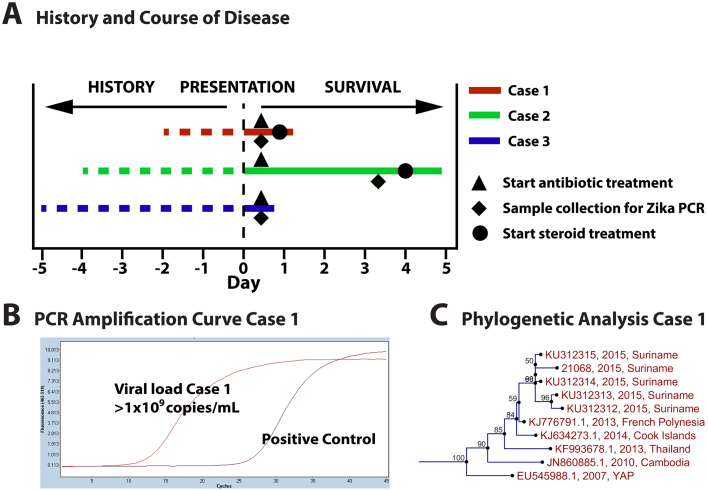
Fig. 1.
A. Schematic representation of history and course of disease for all described cases. B. RT-PCR amplification result for ZIKV performed in patient serum of Case 1. C. Phylogenetic analysis of Case 1 constructed from nucleic-acid sequence data from former Surinamese cases by neighbor-joining algorithm.
Serologic testing
Serology for chikungunya was performed with an Anti-CHIKV IgM and IgG ELISA (EuroImmun, Lubeck, Germany) and for dengue with the RapidSignal IgG/IgM quicktest (Orgenics, Yavne, Israel), both according to the manufacturers’ instructions. Presence of acute or prior HIV infection was performed with the Architect HIV antigen/antibody assay (Abbott). Leptospirosis IgM detection was performed with and Leptocheck (Zephyr Biomedicals, Verna, India).
Ethics statement
We received approval from our institutional ethics committee and obtained informed consent from the patients’ family members.
Case reports
Case 1
A 61-year-old Surinamese male with known history of hypertension, treated with atenolol, presented at the emergency room (ER) with a 2-day history of vomiting, watery diarrhea, and arthritis (Fig. 1A). Physical examination revealed tachypnea with hypoxia, tachycardia with hypotension and acrocyanosis of the left upper extremity. During examination the patient appeared unresponsive. Bedside glucose level was low and rapidly corrected with intravenous administration of glucose. Laboratory results on admission showed severe leukocytosis with neutrophilia, high C-reactive protein (CRP), severe metabolic acidosis, severe renal failure with electrolyte disorders, elevated liver panel, and elevated lactate dehydrogenase (LDH) (Table 1). Ciprofloxacin treatment was started after culture samples were taken.
Table 1.
Results of laboratory testing on admission and microbiological analysis.
| Case 1 | Case 2 | Case 3 | Reference Values | |
|---|---|---|---|---|
| Hemoglobin (mmol/L) | 8.2 | 10.1 | 8.4 | 8.7–11.2 |
| Hematocrit (L/L) | 0.36 | 0.45 | 0.36 | 0.37–0.47 |
| White blood cell count (% polymorphonuclear) (x109/L) |
39.8 (75.8) | 19.2 (88.5)[1] | 16.0 (85.7) | 4.5–11.0 |
| Platelets (x109/L) | 108 | 154 | 52 | 130–400 |
| C-reactive protein (mg/dL) | 26.3 | 39.1 | 37.8 | 0–0.5 |
| Glucose (mg/dL) | 37.8 | 281.1 | 129.7 | 72.1–117.1 |
| Creatinine (mg/dL) | 7.2 | 1.6 | 2.4 | 0.7–1.2 |
| Blood urea nitrogen (mg/dL) | 60.2 | 20.1 | 47.6 | 8.4–19.6 |
| Aspartate aminotransferase (IU/L) | 656 | 68 | 205 | 0–30 |
| Alanine aminotransferase (IU/L) | 176 | 37 | 66 | 0–41 |
| Total Bilirubin (direct) (mg/dL) | NA | 0.9 | 6.1 (4.1) | 0–1.0 |
| Creatinine Kinase (IU/L) | 21,272 [1], [2] | 5180[2] | 2624 | 38–174 |
| Creatinine Kinase MB (IU/L) | 750.6 [1], [2] | 172.2[2] | NA | 0–24 |
| Albumin (g/L) | 16.5 [1] | 19.4 [1] | NA | 32–56 |
| Lactate Dehydrogenase (IU/L) | 1770 | 376 | 377 | 98–192 |
| Activated Partial Thromboplastin Time (s) | 97.8 [1] | 83.1 [2] | NA | 24–33 |
| Prothrombin Time (s) | 25.8 [1] | 15.7 [2] | NA | 10–14 |
| International Normalized Ratio | 1.68 [1] | 0.94 [2] | NA | <5 |
| ZIKV PCR | Pos. | Pos. | Pos. | |
| CHIKV IgM/IgG/PCR | W. pos./Neg./Neg. | Neg./Neg./Neg. | Neg./Neg./Neg. | |
| DENV IgM/IgG/PCR | Neg./Neg./Neg. | Neg./Neg./Neg. | Neg./Neg./Neg. | |
| HIV IgM/IgG | Neg./Neg. | Neg./Neg. | Neg./Neg. | |
| Leptospirosis IgM | Neg./Neg. | Neg./Neg. | Neg./Neg. | |
| Zika viral load (copies/mL) | >1 × 109 | 8.4 × 103 | 1.4 × 103 |
The patient was admitted to the ICU where his condition deteriorated requiring mechanical ventilation. Severe hemodynamic instability urged treatment with high-dose inotropics and large volumes of intravenous fluids. The patient developed hypothermia, multiple organ failure, and persisting lactate acidosis despite bicarbonate infusion and steroids. Anuric renal failure urged continuous veno-venous hemofiltration. Over the course of admission the patient exhibited clinical coagulation disorders by elongated prothrombin time (PT) and activated partial thromboplastin time (APTT) with mucosal bleeding, petechiae and ecchymosis and was treated with fresh frozen plasma (FFP) and vitamin K. Creatinine kinase (CK) and creatinine kinase-MB (CK-MB) were elevated (Table 1). Electrocardiography (ECG) showed no sign of myocardial infarction. Thirty-one hour after admission the patient died due to irreversible bradycardia followed by cardiac arrest. Bacterial cultures from blood, sputum, urine and stool were negative. Leptospirosis IgM and HIV serology were negative. Serology was negative for dengue IgM and IgG, but weakly positive for chikungunya IgM. Specific reverse transcription real-time polymerase chain reaction (RT-PCR) was negative for dengue and chikungunya, but positive for ZIKV. Viral load was high with 1 × 109 copies/mL (Fig. 1B).
Case 2
A 64-year-old male from the Netherlands with a history of type 2 diabetes mellitus (DM), treated with metformin, and epilepsy, treated with carbamazepine, presented at ER with a 4-day history of fever, productive cough, vomiting and diarrhea (Fig. 1A). He also complained of overall weakness and had collapsed twice prior to presentation. Physical examination revealed obesity, fever and tachypnea, irregular heart rhythm, but normal blood pressure. Initial laboratory results showed leukocytosis, thrombocytopenia and elevated CRP, creatinine, blood glucose, and CK levels (Table 1). He was admitted to the ward, where empiric antimicrobial treatment with amoxicillin/clavulanic acid and gentamicin for suspected community-acquired pneumonia or sepsis was started after blood cultures were taken. Within twelve hours, the patient developed respiratory and circulatory failure, and neurological deterioration. He was intubated and transferred to the ICU for mechanical ventilation and hemodynamic support with fluids and inotropics. Laboratory results showed coagulopathy (Table 1).
His condition seemed to stabilize for one day, but then quickly deteriorated further into what was interpreted as septic shock with progressive multi-organ failure, anasarca, petechiae and ecchymosis, despite high-dose inotropics, prednisone, and switch of antimicrobials to meropenem. CK and CK-MB levels increased (Table 1), yet there were no signs of myocardial infarction on ECG. Repeated chest X-rays showed no pulmonary infiltration. CT scan of the brain and abdominal ultrasound were normal. He remained dependent on mechanical ventilation and circulatory support with high-dose inotropics. After three days in the ICU, the patient died. Serology for leptospirosis IgM and HIV was negative. Spinal fluid analysis showed no evidence for (meningococcal) meningitis and bacterial cultures from blood, sputum, urine, stool and cerebrospinal fluid were negative. Multiplex PCR for respiratory microbial pathogens, including Influenza A (including H1N1) and B, was negative. Serology and RT-PCR were negative for dengue and chikungunya, but RT-PCR was positive for ZIKV, with a viral load of 8400 copies/mL.
Case 3
A 59-year-old Surinamese male with prior history of chronic obstructive pulmonary disease presented with a 5-day history of fever and chills, generalized arthralgia, nausea, vomiting, and diarrhea (Fig. 1A). Physical examination revealed fever, tachycardia, hypotension, and signs of dehydration. Intravenous fluids initially improved circulatory parameters, and because leptospirosis or gram negative septicemia was suspected, empirical therapy with intravenous amoxicillin/clavulanic acid was started after blood cultures were drawn. While on this treatment for less than twelve hours, the patient’s condition worsened and he developed a cardiorespiratory arrest. After cardiopulmonary resuscitation, breathing and sinus rhythm were restored, but his pupils were dilated and not responsive to light. He died shortly after. Laboratory results revealed marked leukocytosis with neutrophilia, thrombocytopenia, metabolic acidosis, highly elevated CRP, conjugated hyperbilirubinemia, and raised CK, urea and creatinine levels (Table 1). Bacterial blood and urine culture and leptospirosis IgM and HIV serology were negative. Serology and RT-PCR were negative for dengue and chikungunya, but RT-PCR was positive for ZIKV. Viral load was 1400 copies/mL.
Discussion
In this paper, we describe three fatal cases of ZIKV infection presenting with severe and fatal course of disease. Only three other cases of lethal ZIKV infection in adults have been described in a report from Colombia [6]. Strikingly, two patients had a similar septic presentation and rapid disease progression. Additionally, mild (e.g., hypertension, diabetes mellitus) and severe (i.e., leukemia) co-morbidity was also present in these patients, indicating that such co-morbidities may predispose to a lethal outcome. Reports from Guadeloupe and Martinique and Venezuela on severe and lethal cases of chikungunya describe septic presentations in similar age groups with similar co-morbidities [13], [14] Additionally, in all three cases, potential acute myocardial infarction, evidenced by high CK (in all three) and CK-MB (in case 1 and 2) levels, may have been excacerbated by acute ZIKV infection, for which these patients may have been prone due to their pre-existing conditions. However, ECG did not show evidence of myocardial infarction. Another option could be direct involvement of ZIKV causing acute myocarditis, as has been described in acute dengue infection [15]. Unfortunately, given the absence of further evidence for such a relationship and absent possibility for post-mortem analysis, it remains speculative whether ZIKV infection has caused an irreversible downward spiral of pre-existing disease or was directly responsible for the fatal outcome in these patients.
An important limitation to completely confirm a causal connection between ZIKV infection and the lethal outcome in these patients is the possibility of co-infection. However, we were unable to identify other common pathogens, present in Suriname, in these patients. Serum tested weakly positive for chikungunya IgM in case 1, yet RT-PCR was negative. All patients tested negative for bacterial infections or HIV. Leptospirosis was assessed with a quicktest with limited negative predictive value for acute phase leptospirosis [16]. Further investigation into this would be warranted, since it was previously shown that leptospirosis can present as a febrile illness similar to dengue, and can co-exist in febrile patients with dengue [17], [18]. Malaria was not tested, but there had been no travel to endemic regions in Suriname [19]. It was recently suggested that prior infection with dengue virus may cause antibody-dependent enhancement (also seen in heterotypic secondary dengue infection) of ZIKV infection leading to increased virus replication in the host with potential downstream effects on virulence [20]. However, serology did not reveal evidence for prior dengue infection in our patients. With respect to the ZIKV itself, mutations could have increased its virulence, yet partial sequencing of ZIKV from case 1 revealed an envelope sequence similar to former Surinamese cases (Fig. 1C) [3]. Limited availability of material of these patients made further investigation into co-infections or ZIKV mutations impossible.
Two patients presented with thrombocytopenia and two with coagulopathy, all with clinical sequelae of both such as pronounced bleeding, petechiae and ecchymosis. These host-responses were observed in earlier confirmed cases from Colombia and Suriname and may imply hemorrhagic potential of acute ZIKV infection, reminiscent of dengue hemorrhagic fever (DHF) [6], [7]. Generally, DHF is associated with high viral loads in the host. Indeed, earlier studies on dengue virus have shown that high viral loads, due to high replication rates and delayed viral clearance, accompany the transition from mild dengue fever to severe and potentially lethal DHF [17]. Likewise, in the case of chikungunya virus infection, there is evidence for a positive association between high viral load and severity of disease [21]. It is striking that our first patient also exhibited a very high viral load (Fig. 1B), who also exhibited the most pronounced clinical hemorrhagic symptoms. Interestingly, the patient presented with a short history of symptoms and died very quickly after presentation. It is tempting to speculate that the high ZIKV viraemia in this patient played a role in his rapid disease progression. Potential reasons for absence of high viral load in the other patients may have been different kinetics and later presentation after onset of symptoms (Fig. 1A).
How Zika virus is directly linked to severe disease remains to be determined, yet a possibility could be an exaggerated immune response of the host. Laboratory results show evidence for activation of acute and innate immune responses (Table 1), similarly seen in severe, mostly secondary, cases of dengue fever, and in cases of chikungunya [13], [14], [22]. Indeed, especially dengue is known to cause an intravascular ‘cytokine storm’ associated with increased vascular permeability, which may result in potentially lethal dengue shock syndrome [23]. At this point, only one study has reported slight differences in cytokine levels between acute and reconvalescent phases of ZIKV infection in a small cohort of patients [24]. It has also been shown that dengue virus non-structural protein 1 can target endothelial cells directly during acute phase infection and cause disruption of endothelial barrier function and vascular leak [25]. A similar mechanism could be involved in ZIKV infection. Of note, we are currently evaluating host responses in patients with mild and severe disease. During prospective inclusion of patients for this objective we continued to observe severe and lethal cases of acute ZIKV infection with high viral loads in similar patients (unpublished data).
In conclusion, considering the severity of our cases, we would like to emphasize the need for clinical awareness and vigilance for atypical presentations of ZIKV infection. Indeed, our study shows that acute ZIKV infection, whether accompanied by underlying prior diseases or co-infection, may lead to rapid disease progression. Additionally, hemorrhagic potential of the virus may be, along with high viraemia, involved in severe and potentially lethal outcomes.
Conflict of interest
The authors report no conflict of interest.
Acknowledgments
We acknowledge Mr. Henk Brunings for performing all RT-PCR, and Dr. Mirdad Kazanji, Dr. Dominique Roussett, and Antoine Enfissi of Institute Louis Pasteur in Cayenne, French Guyana, for sequencing, phylogenetic analysis and fruitful discussion, and the Arboviral Diseases Branch of the Center for Disease Control and Prevention in Fort Collins, Colorado, USA, for providing Zika virus RNA.
References
- 1.Petersen L.R., Jamieson D.J., Powers A.M., Honein M.A. Zika Virus. N Engl J Med. 2016;374(16):1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 2.Fauci A.S., Morens D.M. Zika virus in the americas − yet another arbovirus threat. N Engl J Med. 2016;374(7):601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 3.Enfissi A., Codrington J., Roosblad J., Kazanji M., Rousset D. Zika virus genome from the Americas. Lancet. 2016;387(10015):227–228. doi: 10.1016/S0140-6736(16)00003-9. [DOI] [PubMed] [Google Scholar]
- 4.Brasil P., Pereira J.P., Jr., Raja Gabaglia C., Damasceno L., Wakimoto M., Ribeiro Nogueira R.M. Zika virus infection in pregnant women in rio de janeiro − preliminary report. N Engl J Med. 2016;(March) doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao-Lormeau V.M., Blake A., Mons S., Lastère S., Roche C., Vanhomwegen J. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarmiento-Ospina A., Vásquez-Serna H., Jimenez-Canizales C.E., Villamil-Gómez W.E., Rodriguez-Morales A.J. Zika virus associated deaths in Colombia. Lancet. 2016;16(April) doi: 10.1016/S1473-3099(16)30006-8. [DOI] [PubMed] [Google Scholar]
- 7.Karimi O., Goorhuis A., Schinkel J., Codrington J., Vreden S.G., Vermaat J.S. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet. 2016;387(10022):939–940. doi: 10.1016/S0140-6736(16)00502-X. [DOI] [PubMed] [Google Scholar]
- 8.Arzuza-Ortega L., Polo A., Pérez-Tatis G., López-García H., Parra E., Pardo-Herrera L.C. Fatal sickle cell disease and Zika virus infection in girl from Colombia. Emerg Infect Dis. 2016;22(5):925–927. doi: 10.3201/eid2205.151934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastorino B., Bessaud M., Grandadam M., Murri S., Tolou H.J., Peyrefitte C.N. Development of a TaqMan RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J Virol Methods. 2005;124(1–2):65–71. doi: 10.1016/j.jviromet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Lanciotti R.S., Calisher C.H., Gubler D.J., Chang G.J., Vorndam A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30(3):545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanciotti R.S., Kosoy O.L., Laven J.J., Velez J.O., Lambert A.J., Johnson A.J. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H., Parimelalagan M., Lai Y.L., Lee K.S., Koay E.S., Hapuarachchi H.C. Development and evaluation of a SYBR green-based real-time multiplex RT-PCR assay for simultaneous detection and serotyping of dengue and chikungunya viruses. J Mol Diagn. 2015;17(6):722–728. doi: 10.1016/j.jmoldx.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres J.R., Leopoldo Códova G., Castro J.S., Rodríguez L., Saravia V., Arvelaez J. Chikungunya fever: atypical and lethal cases in the Western hemisphere. A Venezuelan experience. IDCases. 2015;2(1):6–10. doi: 10.1016/j.idcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosby L., Perreau C., Madeux B., Cossic J., Armand C., Herrmann-Storke C. Severe manifestations of chikungunya virus in critically ill patients during the 2013–2014 Caribbean outbreak. Int J Infect Dis. 2016;48:78–80. doi: 10.1016/j.ijid.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Miranda C.H., Borges Mde C., Schmidt A., Pazin-Filho A., Rossi M.A., Ramos S.G. A case presentation of a fatal dengue myocarditis showing evidence for dengue virus-induced lesion. Eur Heart J Acute Cardiovasc Care. 2013;2(2):127–130. doi: 10.1177/2048872613475889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen A.L., Dowell S.F., Nisalak A., Mammen M.P., Jr., Petkanchanapong W., Fisk T.L. Rapid diagnostic tests for dengue and leptospirosis: antibody detection is insensitive at presentation. Trop Med Int Health. 2007;12(1):47–51. doi: 10.1111/j.1365-3156.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- 17.Varma M.D., Vengalil S., Vallabhajosyula S., Krishnakumar P.C., Vidyasagar S. Leptospirosis and dengue fever: a predictive model for early differentiation based on clinical and biochemical parameters. Trop Doct. 2014;44(2):100–102. doi: 10.1177/0049475513515212. [DOI] [PubMed] [Google Scholar]
- 18.Lindo J., Brown P.D., Vickers I., Brown M., Jackson S.T., Lewis-Fuller E. Leptospirosis and malaria as causes of febrile illness during a dengue epidemic in Jamaica. Pathog Glob Health. 2013;107(6):329–334. doi: 10.1179/2047773213Y.0000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiwat H., Mitro S., Samjhawan A., Sardjoe P., Soekhoe T., Takken W. Collapse of Anopheles darlingi populations in Suriname after introduction of insecticide-treated nets (ITNs); malaria down to near elimination level. Am J Trop Med Hyg. 2012;86(4):649–655. doi: 10.4269/ajtmh.2012.11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejnirattisai W., Supasa P., Wongwiwat W., Rouvinski A., Barba-Spaeth G., Duangchinda T. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;23(June) doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilarde A.O., Turchi M.D., Siqueira J.B., Jr., Feres V.C., Rocha B., Levi J.E. Dengue and dengue hemorrhagic fever among adults: clinical outcomes related to viremia, serotypes, and antibody response. J Infect Dis. 2008;197(6):817–824. doi: 10.1086/528805. [DOI] [PubMed] [Google Scholar]
- 22.Deeba F., Islam A., Kazim S.N., Naqvi I.H., Broor S., Ahmed A. Chikungunya virus: recent advances in epidemiology, host pathogen interaction and vaccine strategies. Pathog Dis. 2016;74(Dcember (3)) doi: 10.1093/femspd/ftv119. Epub. [DOI] [PubMed] [Google Scholar]
- 23.Makhluf H., Shresta S. Innate antiviral immunity against dengue virus. Crit Rev Immunol. 2015;35(3):253–260. doi: 10.1615/critrevimmunol.2015014251. [DOI] [PubMed] [Google Scholar]
- 24.Tappe D., Pérez-Girón J.V., Zammarchi L., Rissland J., Ferreira D.F., Jaenisch T. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2016;205(3):269–273. doi: 10.1007/s00430-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beatty R.P., Puerta-Guardo H., Killingbeck S., Glasner D., Harris E. Dengue virus non-structural protein 1 triggers endothelial permeability and vascular leak that can be inhibited by anti-NS1 antibodies. Sci Transl Med. 2015;7(304) doi: 10.1126/scitranslmed.aaa3787. 304ra141. [DOI] [PubMed] [Google Scholar]