Abstract
It has been considered whether during whole body exercise the increase in cardiac output is large enough to support skeletal muscle blood flow. This review addresses four lines of evidence for a flow limitation to skeletal muscles during whole body exercise. First, even though during exercise the blood flow achieved by the arms is lower than that achieved by the legs (∼160 vs. ∼385 ml·min−1·100 g−1), the muscle mass that can be perfused with such flow is limited by the capacity to increase cardiac output (42 l/min, highest recorded value). Secondly, activation of the exercise pressor reflex during fatiguing work with one muscle group limits flow to other muscle groups. Another line of evidence comes from evaluation of regional blood flow during exercise where there is a discrepancy between flow to a muscle group when it is working exclusively and when it works together with other muscles. Finally, regulation of peripheral resistance by sympathetic vasoconstriction in active muscles by the arterial baroreflex is critical for blood pressure regulation during exercise. Together, these findings indicate that during whole body exercise muscle blood flow is subordinate to the control of blood pressure.
Keywords: arm exercise, baroreflex, blood pressure, muscle blood flow, cerebral blood flow
recruitment of muscle capillaries during exercise and an oxygen diffusion model to account for transport from the capillaries to the tissue was published in the Journal of Physiology by August Krogh (94, 95, 96) and formed the basis for the 1920 Physiology or Medicine Nobel award. Yet, diffusion of gas takes place throughout the arterial system, as demonstrated by Sejrsen (184) with the use of 133Xenon, and for skeletal muscles it is realized that diffusion from the arterioles is important (38, 143, 144, 189, 190). Nevertheless, Krogh's findings and evaluations set the framework for determination of muscle blood flow during exercise. Based on ideas originated by DeJager (35), Krogh (97) also presented a circulation model that describes the important role of downregulated splanchnic blood flow during exercise for the maintenance of blood pressure. Krogh's model was verified by the observation that occlusion of the dog's aorta above the superior mesenteric artery redirects blood volume from the capacious splanchnic region and increases cardiac output. On the other hand, cardiac output is reduced when the occlusion is below that level (15). However, in supine humans (64), as probably in most animals (e.g., 21), cardiac output is not limited by preload to the heart and thereby is sufficient to satisfy peripheral circulatory needs, and hence, redistribution of cardiac output during exercise may not be required, as shown in dogs (68).
Yet, the situation is much different in upright humans where the central blood volume is reduced (e.g., 108, 109) and accordingly sympathetic activity elevated (153). Thus the ability to constrict high compliant vascular regions to support cardiac end-diastolic volume and consequently cardiac output is important not only for upright posture but also during exercise as demonstrated with the reduction of splanchnic (162) and kidney blood flow (57). That is the case although during exercise vasoconstriction is less relevant for the superior mesenteric artery flow (41, 141). Accordingly, the elevated cardiac output during exercise is supported by increased central blood volume (58), despite enlargement of leg (140) and cutaneous blood volume with increasing body temperature (164). These observations lead to the understanding that the blood volume available to the heart depends on the fraction of cardiac output directed to noncompliant vs. compliant regions, i.e., working muscles vs. viscera and the skin (33, 136).
The reduction in abdominal blood flow during exercise is established by an enormous increase in sympathetic vasomotor outflow, as indicated by the inverse relationship between splanchic blood flow and heart rate (29, 163) and thereby, at least indirectly, indicates sympathetic control of splanchnic blood volume. The identification of norepinephrine as the sympathetic neurotransmitter by von Euler (44), the ability to record muscle and skin sympathetic nerve activity in humans (205, 206), and perfection of the norepinephrine spillover technique by Esler et al. (43) provided insight into the activity of the sympathetic nervous system and thus allowed for appreciation of the sympathetic system's contribution to cardiovascular control (27).
Sympathetic activation during exercise is mediated primarily by a reflex mechanism (“the exercise pressor reflex”) that arises from stimulation of both metabolically and mechanically (4) sensitive thinly myelinated and unmyelinated nerve endings within contracting skeletal muscles (88), possibly as a result of muscle ischemia (7), and the combination of increased ATP, protons, prostaglandins (116), and maybe lactate (103). Also, during intense exercise the motor-neural drive elicits parallel activation of sympathetic pathways, named “central command” (114). Furthermore, the reduced central blood volume associated with upright posture enhances sympathetic activity (225), while the muscle pump during upright exercise increases the central blood volume and reduces sympathetic activity (153). Such modulation of sympathetic vasomotor activity indicates a role for the cardiopulmonary baroreceptors in resetting the arterial baroreflex during exercise (45, 219). Yet, the sympathetic system cannot explain, or would be considered to hinder the increase in blood flow to active muscles, but their flow is secured by metabolite-induced attenuation of sympathetic vasoconstriction, named “functional sympatholysis” (61, 154, 155), and yet muscle oxygenation is not maintained during exercise (80), as confirmed in studies using near infrared spectroscopy (e.g., 26).
It is recognized that functional sympatholysis promotes vasodilation in active muscles and thus is critical for establishing the metabolically required blood flow; nevertheless, interaction between muscle blood flow and sympathetic vasoconstriction introduces a paradox: restriction of blood flow to active muscles during whole body exercise to maintain arterial pressure could be overridden by their demand for flow. This apparent paradox may reflect that sympathetically mediated restriction of blood flow is most effective in feed arteries and primary arterioles, whereas vasodilation prevails downstream in distal arterioles (203). Feed arteries are external to the muscle and therefore not exposed to vasoactive metabolites that mediate functional sympatholysis within the active muscles (209). Thus at the level of the feed arteries sympathetic vasoconstriction could limit muscle blood flow and thereby support arterial pressure.
Yet, it has remained controversial whether during whole body exercise the increase in cardiac output is large enough to support skeletal muscle flow. In animals (rats, 10; dogs, 121; and horses, 139), muscle blood flow during exercise has been determined to be ∼400 ml·min−1·100 g−1. However, until recently such values have not been established in humans due to both the method used to determine flow and the experimental set-up.
Cardiovascular regulation during whole body exercise has been addressed in the classical reviews by Rowell (164) and Clausen (28) and recently by Laughlin et al. (100) and Joyner and Casey (81). The present review provides four lines of evidence for a flow limitation to skeletal muscles during whole body exercise in humans. Most studies on the role of cardiac output for muscle blood flow during exercise are concerned with leg blood flow (156, 173), while this review adds the implication of cardiac output capacity for establishing arm blood flow. Furthermore, it is considered whether cardiac output influences, besides skin blood flow (31), cerebral blood flow (CBF) during whole body exercise (181). It is argued that during exercise regional blood flow, including perfusion of active muscles, is subordinate to the control of blood pressure.
Skeletal Muscle Blood Flow
The first line of evidence for a flow limitation to working skeletal muscles during whole body exercise comes from the measurement of muscle blood flow. Early use of plethysmography reported calf blood flow during rhythmic plantar flexion up to ∼30 ml·min−1·100 g−1 (14). However, it was recognized that flow was probably hindered by the intensity of the muscular contractions that eliminates flow when the contraction intensity exceeds ∼30% of maximal voluntary strength (18, 50). Thus, taking postexercise hyperemia as an index of flow during the relaxation phase between muscle contractions, the immediate postexercise value of ∼80 ml·min−1·100 g−1 might be more representative for calf blood flow during plantar flexion (14), while after running calf blood flow of ∼40 ml·min−1·100 g−1 has been reported (17).
A specific measure of muscle blood flow during exercise was made possible with the 133Xenon clearance method (99) confirming a value of ∼70 ml·min−1·100 g−1, although larger values can be measured if the collimator is placed “looking away” from the direction of blood flow (185), thus minimizing the influence of absorption 133Xenon by intramuscular fat that slows its clearance. Considering a muscle mass of ∼30 kg in males, the 133Xenon-measured muscle blood flow demand, even when “all” muscles are engaged in exercise, would be estimated to ∼21 l/min and that is within the cardiac output capacity of both sedentary subjects (∼22 l/min; Ref. 12) and especially athletes (∼42 l/min; Ref. 39).
Accordingly, as demonstrated already by Nicolai and Zuntz (122) using X-ray evaluation of the dimensions of the heart during treadmill walking, exercise enhances the central blood volume and, as illustrated during electrically evoked (36) or arm exercise (201) in spinal cord injured subjects, a reduction in abdominal blood volume compensates for the increase in muscle blood volume and skin blood flow required for thermoregulation (32). These observations are in support of the position that muscles have “unlimited” access to blood even during maximal whole body exercise (111).
A different view, however, came about when thermodilution (9) and ultrasound (147) blood flow methods were applied to evaluate leg flow during one-legged knee-extensor exercise. Assuming that the measured blood flow of, e.g., 5.7 l/min was reflecting the drainage of, or flow to, the quadriceps muscle during one-legged knee extensor exercise, with occlusion of flow to the lower leg, and by using an anthropometric estimate of the active muscle mass, a remarkable muscle perfusion of ∼250 ml·min−1·100 g−1 was estimated. Furthermore, even larger muscle perfusion has been determined during hypoxic exercise (∼310 ml·min−1·100 g−1; Ref. 168) and in highly trained cyclists (∼385 ml·min−1·100 g−1; Ref. 158).
Yet, methodological considerations regarding the magnitude of the muscle perfusion values obtained with thermodilution need to be addressed. First, when evaluating femoral venous flow, the proximity of the artery and the vein, combined with the diffusion of heat in the tissue, may “contaminate” the thermodilution estimate of flow by prohibiting temperature equilibration within the vein and thus overestimate the flow rate (185). Furthermore, it should be considered that these studies have not addressed muscle blood flow per se but leg blood flow during exercise. The determination of flow includes also more or less inactive muscle blood flow, eventual changes in bone blood flow, and, probably more importantly, skin blood flow during exercise as body temperature increases, e.g., to 38° within a few minutes of exercise, depending on initial temperatures and the rate of heat production or exercise intensity (54, 172). However, even though the estimated ∼300-400 ml/min thigh skin blood flow during heat stress (176) would overestimate femoral venous flow as an index of muscle blood flow, most likely it would not be significant since the accuracy of the thermodilution estimate is ∼10%, and hence, skin blood flow would be less than the detection threshold when the leg blood flow is >4 l/min (176).
Another concern for the estimate of muscle perfusion is the determination of the engaged muscle mass. With the use of imaging techniques, such as magnetic resonance and computed tomography, combined with electrical muscle stimulation, it was established that during maximal knee extension exercise the quadriceps muscle is the sole contributor to the work produced (90) as it gradually gets fully recruited (152). In fact, it seems that the anthropometric muscle mass evaluation overestimates the engaged muscle volume, compared with evaluations by computed tomography, suggesting that peak muscle perfusion estimated with the use of anthropometric muscle mass evaluation may be up to ∼30% larger (148).
Accepting these limitations, the magnitude of the thermodilution measurements of femoral venous blood flow has been verified by ultrasound Doppler of the femoral artery flow (147). Furthermore, these femoral venous flows are in agreement with values obtained with both continuous (80) and bolus (140) tracer injection of indocyanine green dye or radiolabeled albumin (179, 92). Also, these high perfusion values are in line with reports from animal exercise models as reviewed by Laughlin et al. (100).
Thus, if leg muscle blood flow values during exercise are representative of all muscles in the body, there is an obvious conflict between the cardiac output that can be established (∼42 l/min; Ref. 39, or at the most ∼44 l/min as estimated from the highest V̇o2 max reported; Ref. 170) and the capacity for blood flow of the muscles during maximal whole body exercise, i.e., a demand of more than 100 l/min, expressed by Rowell's metaphor (165) when he compared the muscles to a “sleeping giant.” Consequently, even if maximal blood flow capacity varies within different muscle groups, a “competition” for a share of cardiac output develops during whole body exercise, and thus the cardiac output “pie” needs to be carefully “sliced” for all the active muscles to be adequately perfused (178).
Competition for Blood Flow Among Different Vascular Beds During Exercise
The second line of evidence for flow limitation to skeletal muscles during exercise comes by considering whether exercise with several muscle groups affects blood flow to each of these muscle groups compared with when they are working in isolation. This question has been addressed by addition of handgrip exercise on plantar flexion exercise demonstrating that “competition” for flow between these two muscle groups attenuates the postexercise hyperemic response (84). Also, calf blood flow is reduced when occlusion of the active forearm provokes ischemia and increases sympathetic activity to the calf (169). Calf blood flow is reduced only when the intensity of the added handgrip exercise exceeds ∼50% of maximum voluntary contraction (86) or when exercise is performed to exhaustion (85). Similar blood flow reduction is observed when elbow-flexion exercise is added to low intensity plantar flexion exercise (84). The flow reduction seems to depend on the relative intensity of the working muscles rather than on the specific muscles involved in exercise, i.e., whether fatiguing muscle contractions are provoking sufficient sympathoexcitation to elicit muscle vasoconstriction. Also, the duration of the attenuated vascular conductance after exercise suggests that the responsible mechanism is related to a metabo-receptor-mediated sympathetic activation and, therefore, vasoconstriction rather than to central neural drive to the muscle, or an effect of mechano-receptors (84), and pointing to the metabolic contribution to the exercise pressor reflex as the key cardiovascular regulator during exercise with several muscle groups. It seems that the addition of high-intensity fatiguing exercise of even a small muscle mass induces vasoconstriction in active small muscles.
Respiratory Muscles
The consideration that the exercise pressor reflex dictates regulation of muscle blood flow during exercise has also been evaluated during whole body exercise including flow regulation to the respiratory muscles. During exercise ventilation increases exponentially with workload and the respiratory muscles need to work intensively to establish minute ventilation values that may exceed 260 l/min (76, 112). Thus, considering the remarkable activation of the respiratory muscles during exercise, their role for such “competition” for flow among different muscle groups has been of interest (2, 3). Even though the metabolic requirements of the respiratory muscles are assumed to increase with ventilation, the mechanical work performed by these muscles during exercise is probably underestimated since displacement of the heavy abdominal content and ineffective forces due to thoracic distortion are not included in the estimate (137).
The intensity of sympathetic activation elicited from fatiguing respiratory muscles (195) can reduce limb blood flow at rest (188) and implies that sympathetic activity reduces blood flow even in large muscles during maximal exercise and, thereby, has detrimental effects on performance. A ∼10% reduction of leg blood flow during cycling with elevated respiratory muscle work supports that breathing is prioritized over locomotion (62, 63). Such observations may contribute to the performance improvement associated with respiratory muscle training that reduces fatigue of these muscles as summarized in two meta-analyses (74, 60) and therefore, at least presumably, also sympathetic activation.
The reverse question has also been addressed, i.e., whether exercise affects respiratory muscle blood flow. During progressive isocapnic ventilation, intercostal muscle blood flow increased from ∼20 ml·min−1·100 g−1 [as determined by indocyanine (“cardiac”) green and near infrared spectroscopy] during quiet breathing to ∼70 ml·min−1·100 g−1 at a ventilation equal to that observed during maximal exercise (211). These muscle flow values seem low, probably reflecting that evaluation by near infrared spectroscopy is influenced by skin blood flow (e.g., Ref. 194). Nevertheless, when that ventilation was established during exercise, the intercostal muscle blood flow increased to only 80% of the maximum value observed during isocapnic ventilation, and it declined further to ∼25 ml·min−1·100 g−1 during maximal exercise (211), which maybe reflecting that even during maximal exercise-induced ventilation the diaphragm does not use its blood flow capacity (145). Furthermore, as suggested by animal studies, diaphragmatic blood flow is more resistant to sympathetic vasoconstriction than other skeletal muscles (1). If this finding applies also to humans, it could be that during whole body exercise the enormous ventilation required for CO2 elimination is prioritized over the blood flow demands of locomotor muscles.
Cardiac Output
The potentially limiting role of cardiac output for peripheral circulation has been evaluated in heart failure patients (CHF) during one- and two-legged dynamic knee extensor exercise (105). When only one leg is engaged in dynamic exercise, patients with moderate CHF achieve an equally high peak muscle perfusion as healthy age-matched controls, while when both legs are engaged a lower peak muscle perfusion is established. Similarly, when in healthy subjects, the ability to increase cardiac output is constrained by administration of a β1-adrenergic blocker (metoprolol), leg blood flow is attenuated during exercise and attributed to sympathetic activation as indicated by increased norepinephrine “spillover” from the leg (140).
Thus the third line of evidence for the limitation of cardiac output to satisfy active muscle blood flow demand is represented by evaluation of regional blood flow during exercise, where a discrepancy between flow to a muscle group working exclusively and when it works together with other muscles exists. When either arm or leg blood flow is determined during combined arm and leg exercise, blood flow to the arms or the legs is lower than when these limbs are working exclusively, provided that during the combined exercise the additional workload represents a substantial part of the total work performed (Fig. 1; Ref. 179).
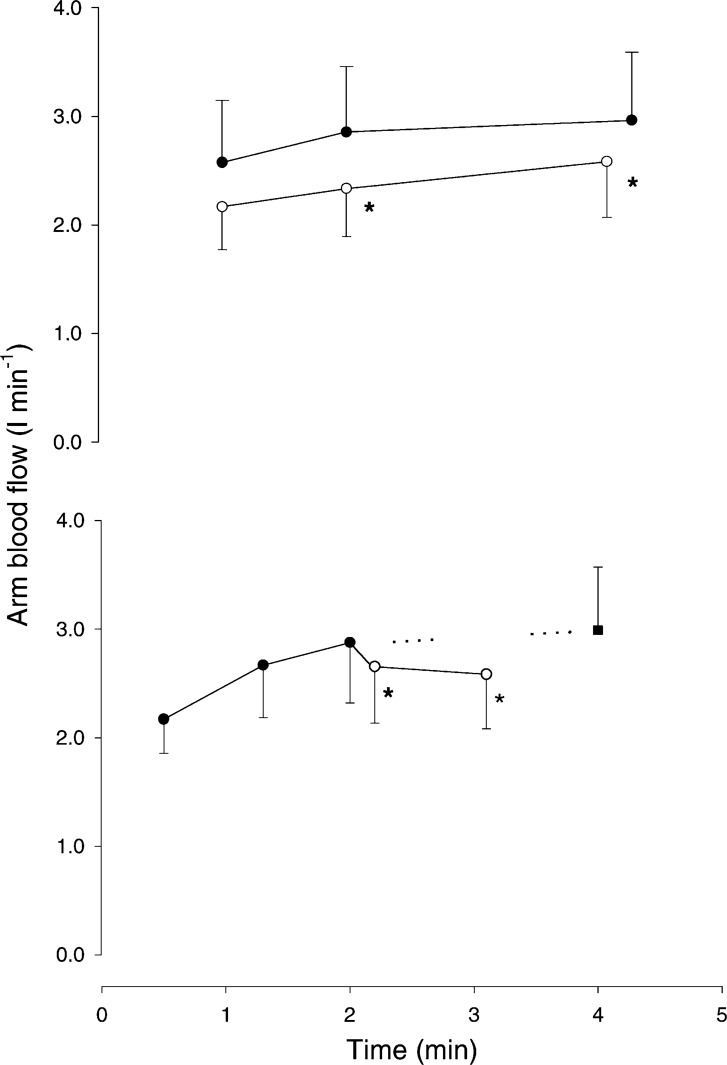
Fig. 1.
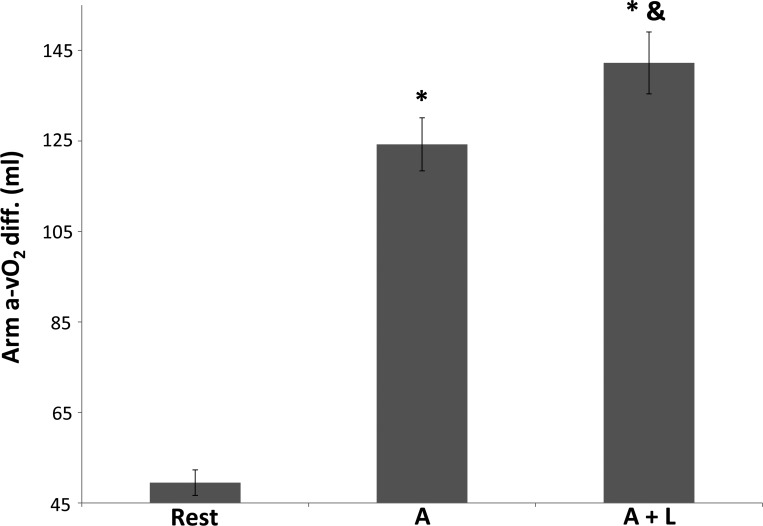
When arm blood flow is determined during combined arm and leg exercise, blood flow to the arm is lower than when arms are working exclusively, provided that during the combined exercise the additional workload represents a substantial part of the total work performed. Arm blood flow during arm (A; ●), arm and leg (A + L, ○; top) and the transition (bottom) from A to A + L trials. Measurements were taken at 60, 120 and 270 s during the independent trials and at 30, 60, 110, and 180 s during the transition trial. The projected value (■) is from the data during the A and A + L independent trials. Values are means ± SE; n = 7. *P < 0.05 different from A. (Reproduced from Ref. 215 with permission.)
Although several subsequent studies failed to reproduce these findings (13, 157, 159, 177, 199), when the results from the studies are summarized, a ∼10% reduction is revealed (212, 215). Furthermore, the blood flow reduction induced by arm and leg exercise may be large enough to reach statistical significance with a small sample size, i.e., a 20–30% blood flow reduction is reported (24) and that is of importance for oxygen delivery to the muscles as here exemplified for the arm muscles with an estimate of their diffusion capacity for oxygen (do2).
Arm do2 has been calculated as the slope of the line of best fit that passes through the origin of the regression between arm V̇o2 and axillary venous oxygen pressure (PvO2), as proposed by Wagner (220). The relative roles of blood flow vs. diffusion for the arm V̇o2 reduction can be estimated using a model of diffusion (142). According to that model, the equilibrium index Y, reflecting the relative components of perfusion and diffusion limitation for muscle V̇o2, is then calculated as Y = do2/(QA·γ), where QA is the arm blood flow and γ is the mean slope of the oxygen dissociation curve. Such estimation reveals that about half of the ∼13% reduction in arm V̇o2 (mean value of studies: 212, 215, 218) following the addition of leg to arm cycling is contributed from blood flow reduction, while the other half is contributed from a reduction in the diffusing capacity of the muscles.
Considering that diffusion depends on perfusion, it is recognized that a complete separation between the two components is difficult. If arm oxygen diffusion is calculated using the diffusion equation do2 = armV̇o2/(PaO2−PvO2) where armV̇o2 decreases (21%, Ref. 218) during combined arm and leg exercise, while the a-v difference increases by 4% resulting in an ∼24% calculated arm diffusion reduction. Considering that during combined arm and leg exercise arm flow is reduced by ∼25% this estimate supports that both diffusion and perfusion contribute to the reduction in armV̇o2.
Similarly, during ergometer cycling leg blood flow is attenuated compared with the value achieved during one-legged knee extension (118), illustrating the restraint placed on the peripheral circulation when increased active muscle mass is competing for the available cardiac output. Furthermore, the competition for flow between different vascular beds, both between the two legs (92) and upper and lower body (Fig. 2A; Ref. 218), is manifested even following endurance training. This observation suggests that both the enhanced cardiac output and central and local structural changes that accompany endurance training, including cardiac hypertrophy (e.g., 34, 113), arteriolar density (101), and number of capillaries in the muscles (e.g., 67, 91), facilitate peak muscle perfusion, nevertheless, cannot satisfy the peripheral blood flow demands during maximal whole body exercise. Even though increased number of capillaries in the muscle following endurance training is improving muscle do2 (221), the mass-normalized muscle do2 is higher in the leg than in the arm muscles (23, 218). In support, the twofold higher muscle do2 in the legs compared with the arms is confirmed across exercise modalities (Fig. 3).
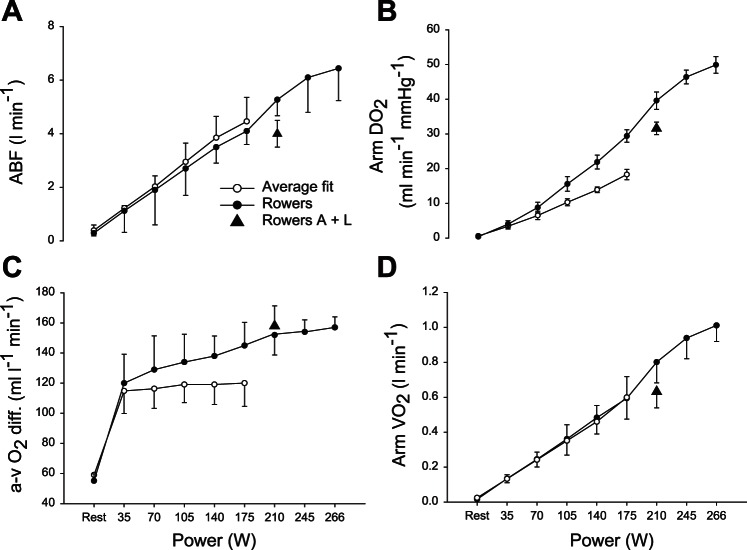
Fig. 2.
Peripheral circulatory variables during arm cranking to exhaustion in well-trained rowers and average fit subjects. The competition for flow between different vascular beds when the active muscle mass increase is manifested even following endurance training. ABF, arm blood flow (A); do2, diffusional O2 conductance (B); a-v O2 diff, arteriovenous O2 difference (C);V̇o2, oxygen uptake (D); A + L, addition of leg exercise to arm cranking in the rowers. Values are means ± SE for 8 average fit subjects and 7 rowers. (Reproduced from Ref. 218 with permission.)
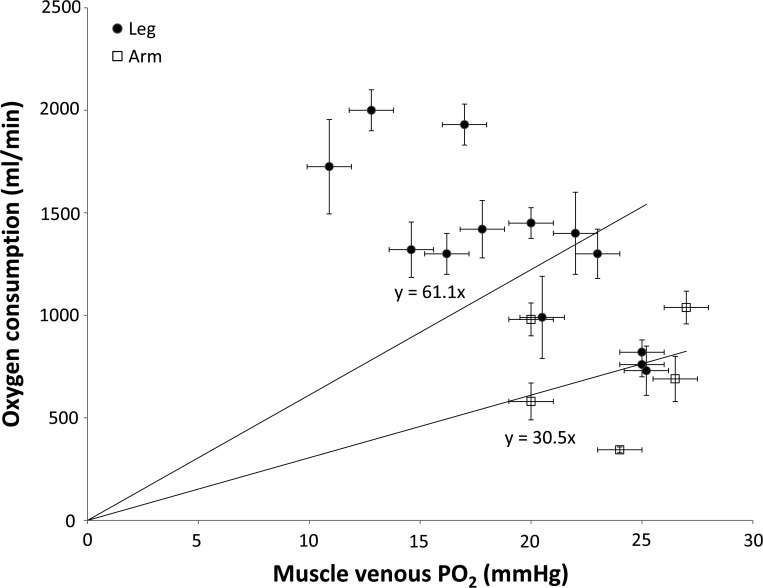
Fig. 3.
Diffusive O2 conductance (do2) in the leg and the arm. do2 is defined as the slope of the regression lines extended to the origin (220). Each data point is the calculated mean DO2 from knee extension (9, 118, 20, 157, 168, 157), skiing (23), cycling (22, 25, 93, 160), arm cranking (19, 77, 207, 218), and arm skiing (22). The data confirm across exercise modalities a twofold higher muscle do2 in the legs compared with the arms.
Yet, muscle blood flow depends not only on metabolism and thereby vasodilation in the vessels feeding the muscle but also on mechanical factors such as the duration of contraction relative to relaxation (duty cycle) and the effective pressure head, i.e., the perfusion pressure. Thus perfusion pressure to a given muscle is not determined only by the exercise-induced mean arterial pressure but also affected by the hydrostatic difference between the heart and that muscle (47). During upright arm and leg exercise, arm blood flow is expected to be smaller than leg blood flow when related to the estimated muscle mass, also by virtue of the height difference between arms and legs.
Furthermore, arm blood flow is influenced both by the lower perfusion pressure that the arms are subjected to when they work together with the legs (179, 218) and possibly sympathetically mediated vasoconstriction, as indicated by increased norepinephrine spill-over over the arms when leg exercise is added (Fig. 4, Ref. 212). The resultant lower arm blood flow is provoking a larger arterial to venous oxygen difference to meet the oxygen demands of the working arms (Fig. 5; Refs. 179, 212, 215, 218). Also, when during running the legs are swung back and forth, leg blood flow is promoted (189) while a similar “gravitational swing” is unlikely to be established for the arms.
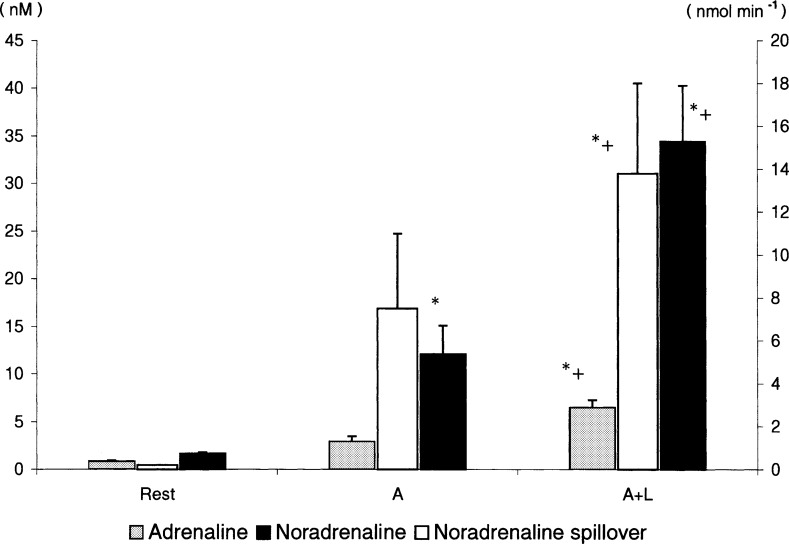
Fig. 4.
Arm blood flow is possibly influenced also by sympathetically mediated vasoconstriction, as indicated by increased norepinephrine spillover over the arms when leg exercise is added. Catecholamines and noradrenaline spillover at rest and during arm (A) and combined arm and leg (A + L) exercise. Values are means ± SE; n = 10. *P < 0.05, different from rest; +P < 0.05, different from A. (Reproduced from Ref. 212 with permission.)
Fig. 5.
The addition of leg exercise to on-going arm exercise decreases arm blood flow and provokes a larger arterial to venous oxygen difference to meet the oxygen demands of the working arms. Arterial-venous O2 difference (a-vO2 diff.) average mean data ± SE (179, 212, 215, 218); n = 31. *P < 0.05, different from rest. &Different from A.
Accordingly during arm cranking, arm blood flow does not increase to a similar level as leg blood flow at an equal power (5, 6) indicating that the arms are not as perfused as the legs (23). When arm blood flow during maximal exercise is related to the active muscle mass, evaluated by dual X-ray absorptiometry (DXA), a perfusion of ∼140 ml·min−1·100 g−1 in nonarm trained subjects and ∼185 ml·min−1·100 g−1 in rowers is revealed (Fig. 2; Ref. 218). The effect of endurance training on increasing peak arm blood flow has been confirmed, albeit to a lesser extent due to the low intensity and relatively short training period in a longitudinal study (19). Even though computerized tomography and magnetic resonance imaging are standards for measuring skeletal muscle mass, the availability and the minimal exposure to radiation makes DXA an attractive alternative (222). The DXA approach provides skeletal muscle estimates that agree closely with measurements by computerized tomography, although DXA tends to overestimate total body skeletal muscle by ∼5% (222), and thus peak arm perfusion may be proportionally larger. However, even if these arm perfusion values (∼160 ml·min−1·100 g−1) are corrected for the DXA overestimation of muscle mass, they are still well below the maximal leg blood flow reported (158).
Since blood flow for a given oxygen uptake is higher during contraction in a muscle comprised predominantly with slow twitch (ST) fibers (soleus) compared with a muscle with primarily fast twitch (FT) fibers (gastrocnemius) in rats (110), it could be considered that the differences in blood flow capacity between arms and legs are reflecting the different fiber-type composition between arms and legs. Even though fiber types in animal skeletal muscle are highly compartmentalized (11), and there is significant variation in human muscle fiber type distribution, as shown by Johnson et al. (79), nevertheless, the relevant muscles for the consideration of arm and leg blood flow, i.e., the deltoid and biceps of the arms and the vastus lateralis, rectus femoris, and gastrocnemius of the legs, contain ∼50% slow and 50% FT fibers with little regional differences (102). On the other hand, the soleus muscle has 25–40% more ST fibers than the other leg muscles while the arm triceps muscle has 10–30% more FT fibers than the other arm muscles (40, 171). Overall, since arm or leg exercise is performed with the majority of the muscles in these extremities, we suggest that the difference in blood flow capacity between arms and legs cannot be attributed to a different fiber type distribution.
Yet, if the arm-derived values for muscle blood flow are applied to a hypothetical total body muscle mass of ∼30 kg (75), the cardiac output capacity (regardless of training status) would still be surpassed during whole body dynamic exercise. Taken together, muscle blood flow represents a balance between metabolically mediated vasodilation and sympathetically induced vasoconstriction. While engaging only a small muscle mass sympatholysis attenuates sympathetic control and, thus, secures a large muscle blood flow. On the other hand, with coactivation of multiple muscle groups, or with a restraint on cardiac output [for example, by lower body negative pressure (197)], there is an increase in sympathetic activity that cannot be overruled by sympatholysis. Even though such partial sympatholysis has not been evaluated during combined arm and leg exercise by, e.g., intra-arterial tyramine infusion or α-adrenergic blockade, it has been evaluated during knee extension exercise (119). Further support for restricted muscle blood flow during whole body exercise comes from the association of the reduced leg blood flow during exercise with β-adrenergic blockade-restrained cardiac output with increased norepinephrine spill-over from the legs (140).
Skin Blood Flow
Limited cardiac output may also affect skin blood flow during exercise. With skin blood flow that can reach 7–8 l/min, or ∼300 to 400 ml·min−1·100 g−1 during passive heating (167), the flow capacity of the cutaneous circulation is similar to that of skeletal muscles. Thus it has been considered that during exercise in a hot environment the elevated skin blood flow required for thermoregulatory homeostasis, besides the implication for right atrial pressure and ventricular end-diastolic volume, according to Krogh's model when a large fraction of cardiac output is distributed to a compliant region like skin, can reduce blood flow to active muscles (164). During prolonged exercise in a hot environment, skin blood flow increases gradually and may amount to ∼3 l/min, as estimated from forearm skin values (78). This additional skin blood flow demand cannot be satisfied by the further ∼20% reduction in splanchnic (166) or renal (149) blood flows that are already reduced by ∼75% during exercise, as indicated by the reduction in venous oxygenation (213). Considering that splanchnic and renal circulations, combined with the modest vasoconstriction in inactive skeletal muscles, can contribute to the systemic circulation at most a total of ∼1 l/min (164), it is deemed that active muscles are needed as a circulatory “donor” (78).
However, blood flow to limb muscles and tissues is either maintained or increased when heat stress is superimposed upon light to moderate intensity prolonged exercise (123, 124, 125, 176). Using positron emission tomography Heinonen et al. (65) found that heat increases muscle blood flow as evaluated by the partitioning of blood flow between muscle and skin under passive heat stress. The implication is that when the metabolic heat production is substantial during prolonged or high intensity exercise, an increase in muscle blood flow may compromise skin blood flow. In support, the cutaneous circulatory demand has a ceiling as skin blood flow plateaus at ∼ 55% of maximal level when core temperature reaches ∼38°C (20). This plateau manifests by a restraint in active vasodilation, as shown by selective local blockade of noradrenergic vasoconstrictor nerves (89), implying that oxygen delivery to muscles is prioritized over skin blood flow with adverse consequence for thermoregulation (55). Support for that postulate comes from the increased risk for death in the elderly associated with a heat wave (186), probably reflecting the limited capacity of the elderly to increase cardiac output and thus skin blood flow and thereby provoking their vulnerability to hyperthermia.
Cerebral Blood Flow
Whole body exercise poses not only a circulatory challenge to splanchnic, muscle, and cutaneous vascular beds but also to the brain with potential metabolic consequences and possibly also implication for performance. Cerebral activation and thus cerebral metabolism are heterogeneous (i.e., brain area specific; Ref. 98) and therefore provokes a differential blood flow response, depending on the type of mental activity. Moreover, regional specificity of CBF combined with the different methods used have led to contrasting observations that fuelled a controversy as to whether CBF increases during exercise (73, 180). However, considering that regional CBF is more sensitive than the global CBF to brain activation during small muscle exercise with no increase in global CBF, e.g., handgrip exercise (48, 49, 134, 182), it is now demonstrated by several methods that whole body exercise provokes a marked increase in CBF (82, 83, 174, 204), e.g., by ∼35% during cycling (from 58 to 79 ml·min−1·100 g−1; Ref. 181).
Similarly to the peripheral vasculature, however, CBF and O2 delivery to the brain are compromised when cardiac output is restricted with, for example, administration of a β1-adrenergic blocking agent (70, 183), heat stress (130, 131, 224), or because of cardiac disease (71). This circulatory challenge during exercise is further exaggerated with the addition of large muscle mass as shown in CHF patients who achieve a normal increase in middle cerebral artery mean flow velocity during one-legged exercise, but during two-legged exercise that response is attenuated (66).
Additionally, with the marked hyperventilation associated with maximal whole body exercise, PaCO2 tension decreases and reduces CBF (82, 83, 104, 183, 204, 223) although that does not seem to manifest in the posterior cerebral vasculature (174). Together with the possible lowering of the arterial oxygen saturation developed during maximal whole body exercise (37) and especially rowing (150, 217), the reduction in CBF may provoke a decrease in cerebral oxygenation by 10% (126).
The increase of the cerebral metabolic rate for oxygen during intense whole body exercise (181) indicates that, besides the decrease in CBF, all the factors that influence cerebral oxygen delivery, and thus oxygenation, are important. In that respect, the reduction in arterial oxygen saturation should be mentioned, although it is a rare event in healthy subjects due to the s-shape of the oxyhemoglobin dissociation curve that maintains oxygen saturation with mild reductions in PaO2. Due to the Bohr-effect on that curve, the resulting arterial oxygen saturation depends critically on how low arterial pH becomes during maximal exercise, as manifested during (ergometer) rowing (217) rather than during, e.g., skiing (69). Also, even though the oxygen carrying capacity of blood is enhanced by the exercise-induced increase in hematocrit, it should be considered that the inverse relationship between CBF and hematocrit (202) indicates that an increase in hematocrit does not necessarily promote oxygen delivery to the tissue.
Near infrared spectroscopy (NIRS) is often used to monitor cerebral oxygenation during intense exercise and deserves a comment even though its reliability and validity are questioned, since the signal can be disturbed by motion artifacts and is influenced by skin blood flow (e.g., 194). A reduction in the NIRS-determined cerebral oxygenation is a consistent finding during maximal exercise (56, 126) and manifests despite the increase in skin blood flow established in parallel with the increase in body temperature. NIRS integrates arterial, capillary, and venous oxygen saturation and likely the NIRS-detected decrease in cerebral oxygenation is dominated by the reduction in venous oxygen saturation when CBF decreases (73, 214, 216, 181), rather than the eventual decrease in arterial oxygen saturation. Nevertheless, the recovery of the cerebral NIRS signal with hyperoxic breathing suggests that it is a sensitive index of changes in arterial oxygen saturation (126).
In support, hypoxia and the associated reduction in cerebral oxygenation appear to affect performance, i.e., elicit so-called central fatigue as illustrated by the ability to perform repeated handgrip exercise, which requires mostly the recruitment of ST muscle fibers rather than rapid contraction as exemplified by computer “mouse click” task that requires FT muscle fiber activation (151). Conversely, when oxygen is added to the inspired air during whole body exercise and hinders the reduction in cerebral oxygenation, performance is enhanced according to the increase in oxygen carrying capacity of blood (by ∼5%). The unchanged muscle deoxygenation (8, 126) suggests that the enhanced oxygen delivery to the muscles facilitated higher work rates while it preserved muscle oxygenation to the level of normoxic exercise. Although the performance enhancement with oxygen supplementation is not always statistically significant in single studies (129, 214; Fig. 6), when these results are summarized an average enhancement by ∼3.5% is revealed (effect size Glass' Δ = 2.2; Ref. 193) albeit an effect on “peripheral fatigue” may be also contributing (8).
Fig. 6.
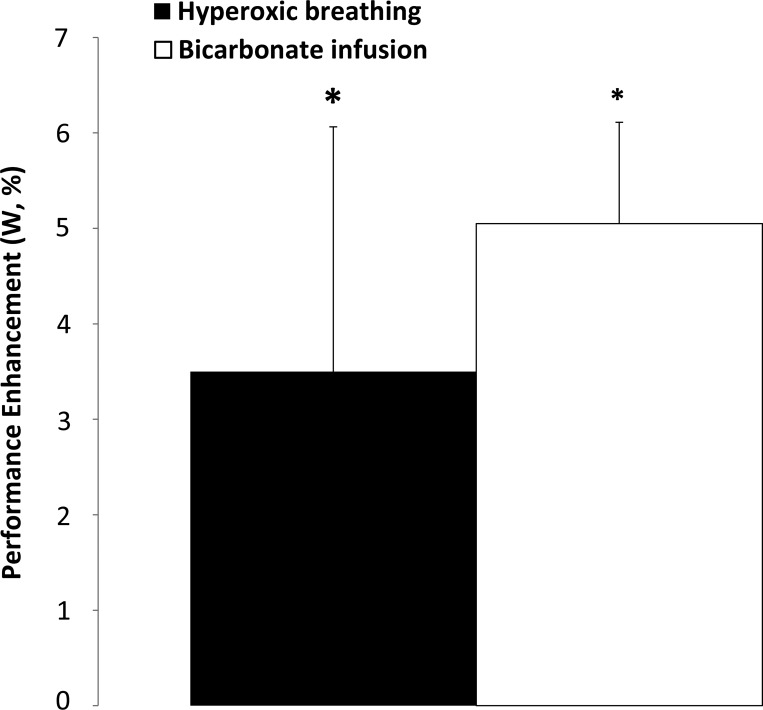
Meta-analysis of studies on performance enhancement with 0.28–0.30 O2 oxygen supplementation in the inspired air (8, 126, 129, 214) or bicarbonate infusion (127, 216) demonstrating an average enhancement of ∼3.5 or ∼5% of power, respectively (effect size Glass' Δ = 2.2; Ref. 193). Mean data ± SE from studies. *P < 0.05, different from normoxic or saline trial, respectively.
The opposite view that a reduction in CBF during maximal exercise does not affect performance has, however, also been advanced. Since PaCO2 influences CBF, it has been evaluated whether addition of CO2 to inspired air improves the reduced work capacity in hypoxia. At altitude, cerebral oxygen delivery is reduced, both by the hypoxia-induced arterial hemoglobin desaturation and attenuation of CBF, as a result of hypoxia-stimulated hyperventilation and the consequent hypocapnia (46, 135, 192, 200). However, when CBF and cerebral oxygenation are restored, by adding CO2 to the inspired air, performance is not improved. An explanation for the absence of performance improvement by addition of CO2 in hypoxia could be that elevated ventilation in response to increased PaCO2 limits muscle blood flow (62) and hence, performance is limited by “peripheral” rather than “central” fatigue. However, addition of CO2 increases ventilation only during submaximal exercise in hypoxia and, therefore, a possible “competition” for flow between the brain and the respiratory muscles during maximal exercise in hyper- and normocapnia would be considered to be similar. On the other hand, during exercise with bicarbonate infusion that reduces ventilation by ∼12 l/min despite an increase in PaCO2 and therefore presumably in CBF, performance is enhanced by ∼5% (216; Fig. 6), while the NIRS determined muscle oxygenation is not different compared with the control trial (127).
Considering that bicarbonate administration restores muscle pH during exercise (128), it seems that the performance enhancement during exercise with bicarbonate administration is effected by both a “central” ergogenic effect of enhanced CBF and attenuation of “peripheral” fatigue as a consequence of reduced ventilation (62) and may be a direct effect of pH on muscle metabolism. Thus an explanation for the absence of performance enhancement with CO2 supplementation during exercise in hypoxia may be related to “peripheral” fatigue outweighing an eventual ergogenic effect of enhanced CBF.
While attenuated increase in cardiac output during exercise may affect CBF, it remains unsettled how that restraint is established. Cholinergic vasodilatation has been considered with regard to the cerebral perfusion response to both handgrip and cycling exercise (182) but has not been substantiated with magnetic resonance scanning during moderate handgrip exercise (149). Alternatively, similar to findings for exercising muscles (140, 212), increased sympathetic activity may restrain flow to the brain. Even though it remains debated whether sympathetic activity influences CBF in humans (198, 208), sympathetic influence on CBF in humans is illustrated during exercise as stellate ganglion blockade hinders the restriction in CBF on the blocked side when the increase of cardiac output is limited with administration of a β-adrenergic blocking agent (70). Furthermore, sympathetic nerve activity of the cerebral vasculature is assessed by transcranial plasma noradrenaline spillover. Specifically, by modifying sympathetic nerve firing (with trimethaphan or clonidine infusion) and neuronal noradrenaline uptake (with desipramine infusion) in healthy and autonomic failure subjects, the occurrence of sympathetically mediated cerebral vasoconstriction is substantiated (115). It follows that sympathetic vasoconstriction may explain the lack of exercise-induced increase in CBF when the increase in cardiac output is small or absent during exercise (181) but that possibility has not been evaluated during exercise (or orthostasis) with restrained cardiac output.
Arterial and Cardiopulmonary Baroreflex
The fourth line of evidence for restricted muscle blood flow during whole body exercise is derived from considering blood pressure regulation. The maintenance, or increase, of blood pressure during exercise requires that the increase in cardiac output matches the elevated skeletal muscle and skin vascular conductance. Since with administration of plasma expander (87) cardiac output during exercise increases, it can be argued that it is not the pumping capacity of the heart but rather venous return that is limiting cardiac output capacity (117).
However, how is blood pressure controlled when there is limited cardiac output to distribute to the tissues? In 1972, Guyton et al. (59) presented a model for long-term blood pressure control. The model links blood pressure and sodium balance, where imbalance between salt intake and renal excretion leads to alteration in filling of the vascular system and thus influences blood pressure. The critical role of the kidney in Guyton's model of blood pressure control is relevant to exercise. The increased sympathetic activity elicited when exercise intensity approaches ∼75% V̇o2 max (51) provokes not only release of arginine vasopressin, to promote water reabsorption and increase blood pressure, but reduces also renal blood flow in proportion to exercise intensity (57).
Renal vasoconstriction during exercise relates to the conservation of sodium and water but the amounts conserved are small compared with the loss by sweating. Similarly, redistribution of blood flow from the kidneys, which is ∼300 ml/min, is also small compared with a ∼20–25 l/min cardiac output increase during exercise. Rather, renal vasoconstriction is of significance for the maintenance of arterial pressure (164). In support, patients with autonomic failure who have compromised capacity for sympathetic vasoconstriction show a pronounced fall in blood pressure with exercise (107).
Even though the role of the kidney is important for long-term blood pressure regulation, short-term regulation (e.g., during exercise) is manifested by baroreceptor control. During exercise, the main contributor to peripheral resistance comes from constraining the extent of peripheral hyperemia through the arterial baroreflex, which includes both the carotid bifurcation and the aortic arch and is stimulated by feedback from stretch sensing unencapsulated free nerve endings located at the medial-adventitial border (187).
At first, it was thought that the arterial baroreflex is “deactivated” and does not regulate blood pressure during exercise as deduced from the fact that both heart rate and blood pressure rise during exercise, an observation that is in direct opposition to the inverse relationship between heart rate and blood pressure, the essential characteristic of the arterial baroreflex (106).
An explanation for the parallel increase of heart rate and blood pressure during exercise was found to be a “resetting” of the arterial baroreflex that continues to regulate both heart rate and blood pressure at the higher levels established during exercise (45, 146; Fig. 7) by influence from both central command (52) and the exercise pressor reflex (53, 138, 53). Support for that explanation comes with the use of variable pressure manipulations of the carotid baroreceptors with a neck chamber during exercise where the reflex heart rate and blood pressure responses have the same magnitude as at rest (16). Among the different quantitative approaches available for evaluation of the arterial baroreflex function the application of variable negative/positive pressures to the anterior neck region (42) is of special interest because it allows for noninvasive, nonpharmacological and selective evaluation of the carotid baroreflex (i.e., void from the contributions of the aortic and cardiopulmonary baroreceptors to the blood pressure responses) by altering carotid sinus intramural pressure. Thus, with the application of variable negative/positive pressures in humans during exercise, it was shown that the baroreflex tonically opposes vasodilation by imposing sympathetic vasoconstriction to active muscles and, thereby, increases peripheral resistance to support blood pressure (132, 165). In support, similar findings have been shown in dogs during exercise, albeit with invasive carotid occlusion (30, 133).
Fig. 7.
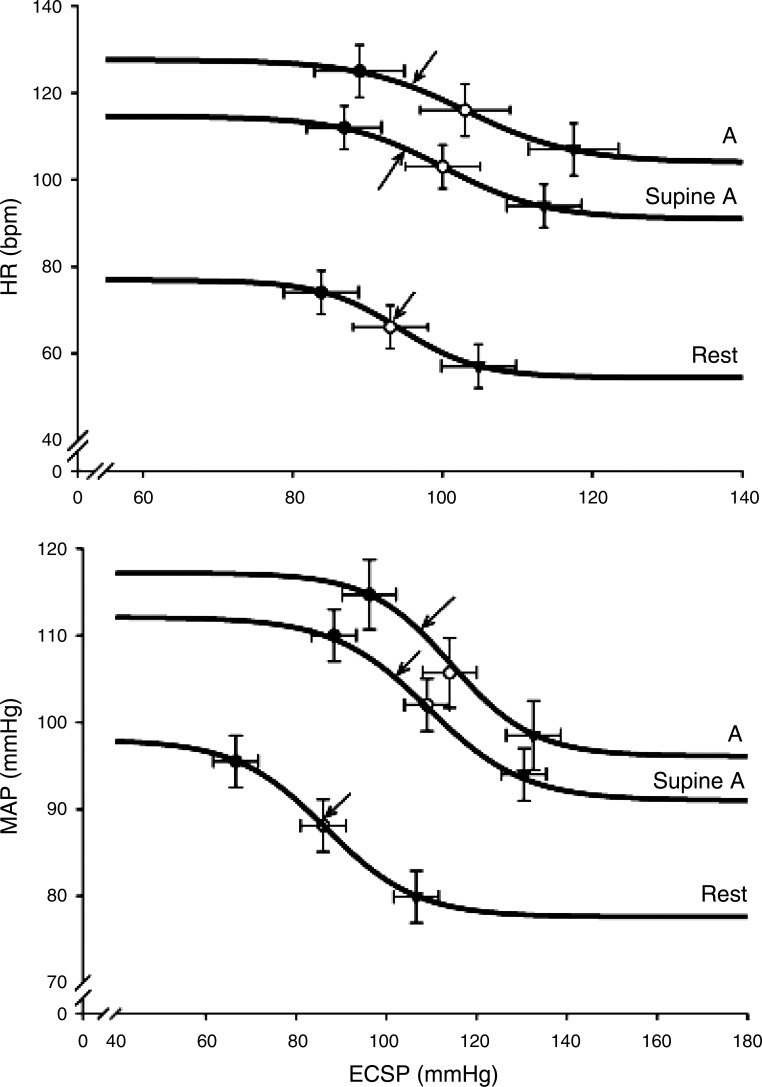
The carotid-cardiac (top) and carotid-vasomotor (bottom) reflex response during upright and supine arm exercise (A). Reflex responses in heart rate (HR) and mean arterial pressure (MAP) after stimulation of the carotid sinus baroreceptors, with a variable neck-pressure chamber that produced different estimated carotid sinus pressure (ECSP) at rest and during upright and supine arm exercise. Data represent mean ± SE. Lines represent mean fit of data from individual subjects. Arrows indicate the prevailing blood pressure (operating point of the baroreflex). (Reproduced from Ref. 219 with permission.)
The hemodynamic response to whole body exercise is influenced also by cardiopulmonary baroreceptors. Higher heart rate and blood pressure during sitting compared with supine exercise at similar level of V̇o2 (196) have hinted to the contribution of cardiopulmonary baroreceptors to the blood pressure response during exercise. Importantly, the cardiopulmonary baroreceptors not only contribute in establishing the prevailing blood pressure but are also involved in the resetting of the arterial baroreflex (219). Specifically, increasing the load of the cardiopulmonary baroreceptors by assuming the supine position resets the operating point of the arterial baroreflex to a lower blood pressure during exercise (Fig. 7). Furthermore, the increased load to the cardiopulmonary baroreceptors in the supine posture is established by enhanced venous return as implied by plasma atrial natriuretic peptide that is released in response to atrial stretch (Fig. 8; Ref. 210). Thus, variation in the central blood volume may explain the lower blood pressure during combined arm and leg exercise than when the arms are working alone (179, 212, 215, 218) by concomitant adjustment of the arterial baroreflex function (45, 219).
Fig. 8.
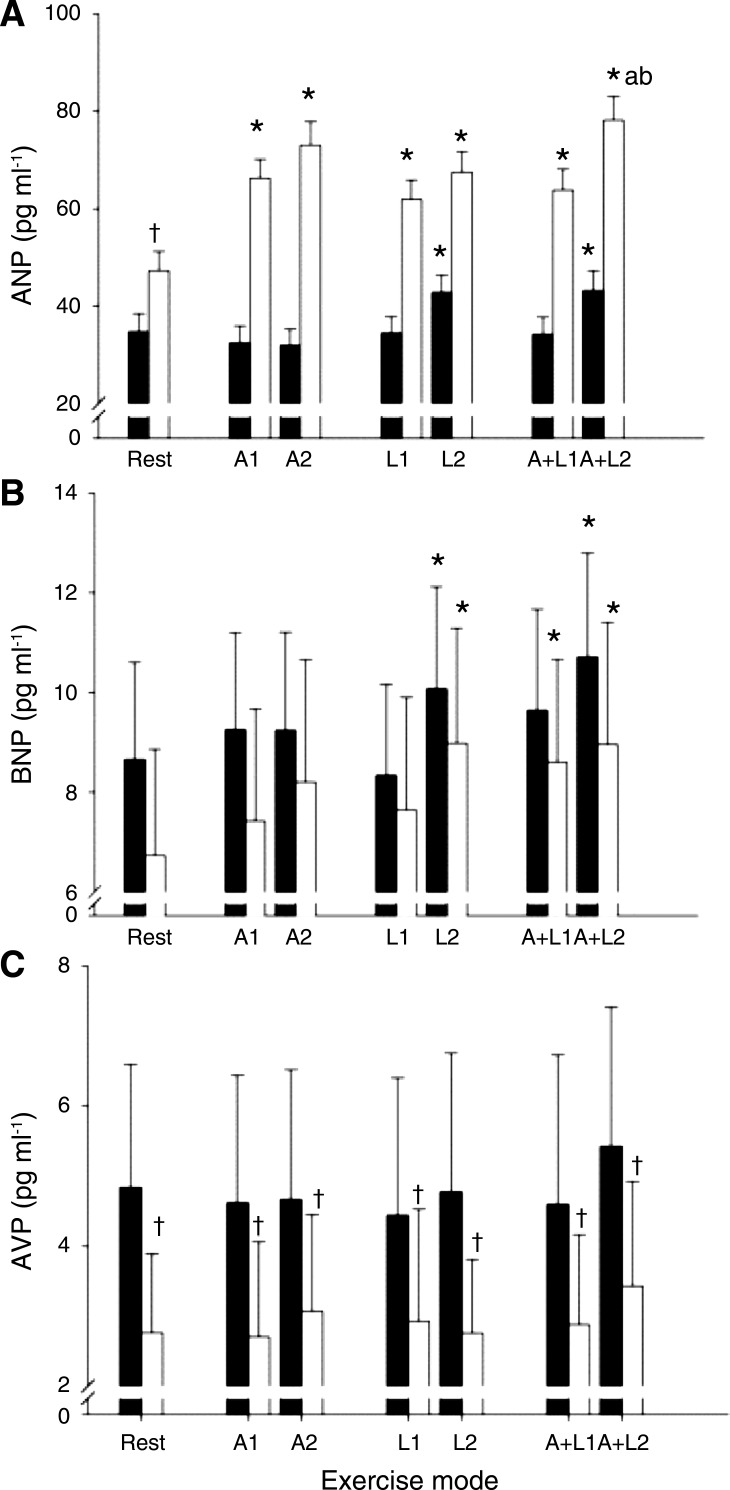
The increased load to the cardiopulmonary baroreceptors in the supine posture is established by enhanced venous return as implied by plasma atrial natriuretic peptide that is released in response to atrial stretch. Plasma atrial natriuretic peptide (ANP) at rest and during arm (A), leg (L), and combined A and L (A + L) exercise in upright seated (filled bars) and supine exercise (open bars). Blood samples were taken at the first (1) and the last minute of exercise (2). Values are means ± SD; n = 11. *P < 0.05, different from rest; †P < 0.05, different from upright posture; aP < 0.05, different from A; bP < 0.05, different from L. (Reproduced with permission from Ref. 210.)
Conclusions
During whole body exercise competition between the cardiac output capacity and blood flow demands of different vascular beds presents a potential challenge to blood pressure regulation. Despite the lower blood flow capacity of the arms compared with that of the legs, the blood flow demand of active muscles presents a challenge to the capacity of the heart to provide sufficient cardiac output during intense whole body exercise. Mass-specific muscle blood flow in humans seems to approach the values established in animals only when small muscle exercise is performed. Even though cardiac output is supported by an increase in venous return, mediated by the muscle pump of primarily the legs, the blood flow demand surpasses venous return, and hence, cardiac output redistribution is necessary to preserve systemic blood pressure. The arterial baroreflex is critical in the cardiac output redistribution during exercise as it provokes sympathetic vasoconstriction to regulate systemic resistance, not only to the abdominal organs (and inactive vascular beds) but also to active muscles and the skin and, perhaps equally importantly, maybe also to the brain.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.V. and N.H.S. conception and design of research; S.V. analyzed data; S.V. and N.H.S. interpreted results of experiments; S.V. prepared figures; S.V. and N.H.S. drafted manuscript; S.V. and N.H.S. edited and revised manuscript; S.V. and N.H.S. approved final version of manuscript; S.V. and N.H.S. performed experiments.
REFERENCES
- 1.Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to α1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol 92: 1808–1816, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Aaron EA, Johnson BD, Seow CK, Dempsey JA. Oxygen cost of exercise hyperpnea: measurement. J Appl Physiol 72: 1810–1817, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol 72: 1818–1825, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Ahlborg G, Jensen-Urstad M. Arm blood flow at rest and during arm exercise. J Appl Physiol 70: 928–933, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Ahlborg G, Jensen-Urstad M. Metabolism in exercising arm vs. leg muscle. Clin Physiol 11: 459–468, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575: 937–952, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 189–208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol 59: 1322–1328, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Asmussen E, Nielsen M. The cardiac output in rest and work determined simultaneously by the Acetylene and the dye injection methods. Acta Physiol Scand 27: 217–230, 1952. [DOI] [PubMed] [Google Scholar]
- 13.Bangsbo J, Juel C, Hellsten Y, Saltin B. Dissociation between lactate and proton exchange in muscle during intense exercise in man. J Physiol 504: 489–499, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barcroft H, Dornhorst AC. The blood flow through the human calf during rhythmic exercise. J Physiol 109: 402–411, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcroft H, Samaan S. Explanation of the increase in systemic flow caused by occluding the descending thoracic aorta. J Physiol 85: 47–61, 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevegård BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest 45: 132–142, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black JE. Blood flow requirements of the human calf after walking and running. Clin Sci (Lond) 18: 89–93, 1959. [PubMed] [Google Scholar]
- 18.Bonde-Petersen F, Mork AL, Nielsen E. Local muscle blood flow and sustained contractions of human arm and back muscles. Eur J Appl Physiol Occup Physiol 34: 43–50, 1975. [DOI] [PubMed] [Google Scholar]
- 19.Boushel R, Ara I, Gnaiger E, Helge JW, González-Alonso J, Munck-Andersen T, Sondergaard H, Damsgaard R, van Hall G, Saltin B, Calbet JA. Low-intensity training increases peak arm VO2 by enhancing both convective and diffusive O2 delivery. Acta Physiol 211: 122–134, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Brengelmann GL, Johnson JM, Hermansen L, Rowell LB. Altered control of skin blood flow during exercise at high internal temperatures. J Appl Physiol 43: 790–794, 1977. [DOI] [PubMed] [Google Scholar]
- 21.Brøndum E, Hasenkam JM, Secher NH, Bertelsen MF, Grøndahl C, Petersen KK, Buhl R, Aalkjaer C, Baandrup U, Nygaard H, Smerup M, Stegmann F, Sloth E, Ostergaard KH, Nissen P, Runge M, Pitsillides K, Wang T. Jugular venous pooling during lowering of the head affects blood pressure of the anesthetized giraffe. Am J Physiol Regul Integr Comp Physiol 297: R1058–R1065, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Calbet JA, Gonzalez-Alonso J, Helge JW, Søndergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol 103: 969–978, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Calbet JA, Holmberg HC, Rosdahl H, van Hall G, Jensen-Urstad M, Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol 289: R1448–R1458, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol 558: 319–331, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardús J, Marrades RM, Roca J, Barberà JA, Diaz O, Masclans JR, Rodriguez-Roisin R, Wagner PD. Effects of F(I)O2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc 30: 697–703, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Chance B, Dait MT, Zhang C, Hamaoka T, Hagerman F. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am J Physiol Cell Physiol 262: C766–C775, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4: 825–850, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Clausen JP. Effect of physical training on cardiovascular adjustments to exercise in man. Physiol Rev 57: 779–815, 1977. [DOI] [PubMed] [Google Scholar]
- 29.Clausen JP, Trap-Jensen J. Arteriohepatic venous oxygen difference and heart rate during initial phases of exercise. J Appl Physiol 37: 716–719, 1974. [DOI] [PubMed] [Google Scholar]
- 30.Collins HL, Augustyniak RA, Ansorge EJ, O'Leary DS. Carotid baroreflex pressor responses at rest and during exercise: cardiac output vs. regional vasoconstriction. Am J Physiol Heart Circ Physiol 280: H642–H648, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Crandall CG. Heat stress and baroreflex regulation of blood pressure. Med Sci Sports Exerc 40: 2063–2070, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crandall CG, González-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol 199: 407–423, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 586: 293–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darling E. The effects of training: a study of the Harvard University Crew. Boston Med Surg J 141: 229–233, 1899. [Google Scholar]
- 35.De Jager S. Experiments and considerations on haemodynamics. J Physiol 7: 130–215, 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dela F, Mohr T, Jensen CM, Haahr HL, Secher NH, Biering-Sørensen F, Kjaer M. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation 107: 2127–2133, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 355: 161–175, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res 27: 669–678, 1970. [DOI] [PubMed] [Google Scholar]
- 39.Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol 25: 619–625, 1968. [DOI] [PubMed] [Google Scholar]
- 40.Elder GC, Bradbury K, Roberts R. Variability of fiber type distributions within human muscles. J Appl Physiol Respir Environ Exercise Physiol 53: 1473–1480, 1982. [DOI] [PubMed] [Google Scholar]
- 41.Endo MY, Suzuki R, Nagahata N, Hayashi N, Miura A, Koga S, Fukuba Y. Differential arterial blood flow response of splanchnic and renal organs during low intensity cycling exercise in women. Am J Physiol Heart Circ Physiol 294: H2322–H2326, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Ernsting J, Parry DJ. Some observations of the effects of stimulating the stretch receptors in the carotid artery in man. J Physiol 137: 454P–456P, 1957. [Google Scholar]
- 43.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11: 3–20, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Euler von US. A specific sympathomimetic ergone in adrenergic nerve fibres (sympathin) and its relations to adrenaline and noradrenaline. Acta Physiol Scand 12: 73–97, 1946. [Google Scholar]
- 45.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan JL, Bourdillon N, Kayser B. Effect of end-tidal CO2 clamping on cerebrovascular function, oxygenation, and performance during 15-km time trial cycling in severe normobaric hypoxia: the role of cerebral O2 delivery. Physiol Rep 1: e00066, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folkow B, Haglund U, Jodal M, Lundgren O. Blood flow in the calf muscle of man during heavy rhythmic exercise. Acta Physiol Scand 81: 157–163, 1971. [DOI] [PubMed] [Google Scholar]
- 48.Friedman DB, Friberg L, Mitchell JH, Secher NH. Effect of axillary blockade on regional cerebral blood flow during static handgrip. J Appl Physiol 71: 651–656, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Friedman DB, Friberg L, Payne G, Mitchell JH, Secher NH. Effects of axillary blockade on regional cerebral blood flow during dynamic hand contractions. J Appl Physiol 73: 2120–2125, 1992. [DOI] [PubMed] [Google Scholar]
- 50.Gaffney FA, Sjøgaard G, Saltin B. Cardiovascular and metabolic responses to static contraction in man. Acta Physiol Scand 138: 249–258, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma responses to graded and prolonged exercise in man. J Appl Physiol 38: 70–76, 1975. [DOI] [PubMed] [Google Scholar]
- 52.Gallagher KM, Fadel PJ, Strømstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol 533: 861–870, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallagher KM, Fadel PJ, Strømstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol 533: 871–880, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.González-Alonso J, Calbet JA, Nielsen B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J Physiol 520: 577–589, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol 586: 45–53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 557: 331–42, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grimby G. Renal clearances during prolonged supine exercise at different loads. J Appl Physiol 20: 1294–1298, 1965. [DOI] [PubMed] [Google Scholar]
- 58.Guyton AC, Douglas BH, Langston JB, Richardson TQ. Instantaneous increase in mean circulatory pressure and cardiac output at onset of muscular activity. Circ Res 11: 431–441, 1962. [DOI] [PubMed] [Google Scholar]
- 59.Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Ann Rev Physiol 34, 13–46, 1972. [DOI] [PubMed] [Google Scholar]
- 60.HajGhanbari B, Yamabayashi C, Buna TR, Coelho JD, Freedman KD, Morton TA, Palmer SA, Toy MA, Walsh C, Sheel AW, Reid WD. Effects of respiratory muscle training on performance in athletes: a systematic review with meta-analyses. J Strength Cond Res 27: 1643–1663, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Hansen J, Sander M, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiol Scand 168: 489–503, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82: 1573–1583, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol 85: 609–618, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Harms MP, van Lieshout JJ, Jenstrup M, Pott F, Secher NH. Postural effects on cardiac output and mixed venous oxygen saturation in humans. Exp Physiol 88: 611–616, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Heinonen I, Brothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall CG. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol 111: 818–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellström G, Magnusson B, Wahlgren NG, Gordon A, Sylven C, Saltin B. Physical exercise may impair cerebral perfusion in patients with chronic heart failure. Cardiol Elderly 4: 191–194, 1996. [Google Scholar]
- 67.Hermansen L, Wachtlova M. Capillary density of skeletal muscle in well-trained and untrained men. J Appl Physiol 30: 860–863, 1971. [DOI] [PubMed] [Google Scholar]
- 68.Higgins CB, Vatner SF, Franklin D, Braunwald E. Effects of experimentally produced heart failure on the peripheral vascular response to severe exercise in conscious dogs. Circ Res 31: 186–194, 1972. [DOI] [PubMed] [Google Scholar]
- 69.Holmberg HC, Rosdahl H, Svedenhag J. Lung function, arterial saturation and oxygen uptake in elite cross country skiers: influence of exercise mode. Scand J Med Sci Sports 17: 437–44, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Ide K, Boushel R, Sørensen HM, Fernandes A, Cai Y, Pott F, Secher NH. Middle cerebral artery blood velocity during exercise with beta-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol Scand 170: 33–38, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Ide K, Gulløv AL, Pott F, Van Lieshout JJ, Koefoed BG, Petersen P, Secher NH. Middle cerebral artery blood velocity during exercise in patients with atrial fibrillation. Clin Physiol 19: 284–289, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Ide K, Pott F, Van Lieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand 162: 13–20, 1998. [DOI] [PubMed] [Google Scholar]
- 73.Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol 61: 397–414, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Illi SK, Held U, Frank I, Spengler CM. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med 42: 707–724, 2012. [DOI] [PubMed] [Google Scholar]
- 75.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89: 81–88, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Jensen K, Johansen L, Secher NH. Influence of body mass on maximal oxygen uptake: effect of sample size. Eur J Appl Physiol 84: 201–205, 2001. [DOI] [PubMed] [Google Scholar]
- 77.Jensen-Urstad M, Ahlborg G, Sahlin K. High lactate and NH3 release during arm vs. leg exercise is not due to beta-adrenoceptor stimulation. J Appl Physiol 74: 2860–2867, 1993. [DOI] [PubMed] [Google Scholar]
- 78.Johnson JM, Rowell LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. J Appl Physiol 39: 920–924, 1975. [DOI] [PubMed] [Google Scholar]
- 79.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18: 111–129, 1973. [DOI] [PubMed] [Google Scholar]
- 80.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 41: 459–473, 1971. [DOI] [PubMed] [Google Scholar]
- 81.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jørgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol 73: 1825–1830, 1992. [DOI] [PubMed] [Google Scholar]
- 83.Jørgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol 72: 1123–1132, 1992. [DOI] [PubMed] [Google Scholar]
- 84.Kagaya A, Ogita F, Koyama A. Vasoconstriction in active calf persists after discontinuation of combined exercise with high-intensity elbow flexion. Acta Physiol Scand 157: 85–92, 1996. [DOI] [PubMed] [Google Scholar]
- 85.Kagaya A, Saito M, Ogita F, Shinohara M. Exhausting handgrip exercise reduces the blood flow in the active calf muscle exercising at low intensity. Eur J Appl Physiol Occup Physiol 68: 252–257, 1994. [DOI] [PubMed] [Google Scholar]
- 86.Kagaya A. Relative contraction force producing a reduction in calf blood flow by superimposing forearm exercise on lower leg exercise. Eur J Appl Physiol Occup Physiol 66: 309–314, 1993. [DOI] [PubMed] [Google Scholar]
- 87.Kanstrup IL, Ekblom B. Acute hypervolemia, cardiac performance, and aerobic power during exercise. J Appl Physiol Respir Environ Exercise Physiol 52: 1186–1191, 1982. [DOI] [PubMed] [Google Scholar]
- 88.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002. [DOI] [PubMed] [Google Scholar]
- 89.Kellogg DL Jr, Johnson JM, Kenney WL, Pérgola PE, Kosiba WA. Mechanisms of control of skin blood flow during prolonged exercise in humans. Am J Physiol Heart Circ Physiol 265: H562–H568, 1993. [DOI] [PubMed] [Google Scholar]
- 90.Kim CK, Strange S, Bangsbo J, Saltin B. Skeletal muscle perfusion in electrically induced dynamic exercise in humans. Acta Physiol Scand 153: 279–287, 1995. [DOI] [PubMed] [Google Scholar]
- 91.Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand 113: 9–16, 1981. [DOI] [PubMed] [Google Scholar]
- 92.Klausen K, Secher NH, Clausen JP, Hartling O, Trap-Jensen J. Central and regional circulatory adaptations to one-leg training. J Appl Physiol 52: 976–983, 1982. [DOI] [PubMed] [Google Scholar]
- 93.Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol 75: 2586–2594, 1993. [DOI] [PubMed] [Google Scholar]
- 94.Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol 52: 409–415, 1919a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 52: 457–474, 1919b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol 52: 391–408, 1919c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krogh A. Die Regulierung des blutzuflusses zum rechten Herzen (mit Beshreibung eines neuen Kreislaufmodells). Regulation of the supply of blood to the right heart (with the description of a new circulation model). Skand Arch Physiol 27: 227–248, 1912. [Google Scholar]
- 98.Lassen NA, Ingvar DH, Skinhøj E. Brain function and blood flow. Sci Am 239: 62–71, 1978. [DOI] [PubMed] [Google Scholar]
- 99.Lassen NA, Linbjerg I, Munck O. Measurement of blood flow through skeletal muscle by intramuscular injection of xenon 133. Lancet 1: 686–689, 1964. [DOI] [PubMed] [Google Scholar]
- 100.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. [DOI] [PubMed] [Google Scholar]
- 101.Laughlin MH, Cook JD, Tremble R, Ingram D, Colleran PN, Turk JR. Exercise training produces nonuniform increases in arteriolar density of rat soleus and gastrocnemius muscle. Microcirculation 13: 175–186, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. [DOI] [PubMed] [Google Scholar]
- 103.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Linkis P, Jørgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. J Appl Physiol 78: 12–16, 1995. [DOI] [PubMed] [Google Scholar]
- 105.Magnusson G, Kaijser L, Sylvén C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res 33: 297–306, 1997. [DOI] [PubMed] [Google Scholar]
- 106.Marey EJ. Physiologie Medicale de la Circulation du Sang. Paris, France: Delahaye 202–226, 1863. [Google Scholar]
- 107.Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation 24: 76–81, 1961. [DOI] [PubMed] [Google Scholar]
- 108.Matzen S, Knigge U, Schütten HJ, Warberg J, Secher NH. Atrial natriuretic peptide during head-up tilt induced hypovolaemic shock in man. Acta Physiol Scand 140: 161–166, 1990. [DOI] [PubMed] [Google Scholar]
- 109.Matzen S, Perko G, Groth S, Friedman DB, Secher NH. Blood volume distribution during head-up tilt induced central hypovolaemia in man. Clin Physiol 11: 411–422, 1991. [DOI] [PubMed] [Google Scholar]
- 110.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563: 903–913, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mellander S, Johansson B. Control of resistance, exchange, and capacitance functions in the peripheral circulation. Pharmacol Rev 20: 117–196, 1968. [PubMed] [Google Scholar]
- 112.Mikulic P. Maturation to elite status: a six-year physiological case study of a world champion rowing crew. Eur J Appl Physiol 111: 2363–2368, 2011. [DOI] [PubMed] [Google Scholar]
- 113.Milliken MC, Stray-Gundersen J, Peshock RM, Katz J, Mitchell JH. Left ventricular mass as determined by magnetic resonance imaging in male endurance athletes. Am J Cardiol 62: 301–305, 1988. [DOI] [PubMed] [Google Scholar]
- 114.Mitchell JH, Victor RG. Neural control of the cardiovascular system: insights from muscle sympathetic nerve recordings in humans. Med Sci Sports Exerc 28: 60–69, 1996. [DOI] [PubMed] [Google Scholar]
- 115.Mitchell DA, Lambert G, Secher NH, Raven PB, van Lieshout J, Esler MD. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J Physiol 587: 2589–2597, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Momen A, Cui J, McQuillan P, Sinoway LI. Local prostaglandin blockade attenuates muscle mechanoreflex-mediated renal vasoconstriction during muscle stretch in humans. Am J Physiol Heart Circ Physiol 294: H2184–H2190, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mortensen SP, Damsgaard R, Dawson EA, Secher NH, González-Alonso J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high-intensity whole-body exercise in humans. J Physiol 586: 2621–2635, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, González-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol 566: 273–285, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mortensen SP, Saltin B. Regulation of the skeletal muscle blood flow in humans. Exp Physiol 99: 1552–1558, 2014. [DOI] [PubMed] [Google Scholar]
- 120.Mourtzakis M, González-Alonso J, Graham TE, Saltin B. Hemodynamics and O2 uptake during maximal knee extensor exercise in untrained and trained human quadriceps muscle: effects of hyperoxia. J Appl Physiol 97: 1796–1802, 2004. [DOI] [PubMed] [Google Scholar]
- 121.Musch TI, Haidet GC, Ordway GA, Longhurst JC, Mitchell JH. Training effects on regional blood flow response to maximal exercise in foxhounds. J Appl Physiol 62: 1724–1732, 1987. [DOI] [PubMed] [Google Scholar]
- 122.Nicolai GE, Zuntz N. Füllung und entleerung des herzens bei ruhe und arbeit. Berliner Klinische Wochenschrift 51: 821–824, 1914. [Google Scholar]
- 123.Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 460: 467–485, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol 69: 1040–1046, 1990. [DOI] [PubMed] [Google Scholar]
- 125.Nielsen B, Strange S, Christensen NJ, Warberg J, Saltin B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflügers Arch 434: 49–56, 1997. [DOI] [PubMed] [Google Scholar]
- 126.Nielsen HB, Boushel R, Madsen P, Secher NH. Cerebral desaturation during exercise reversed by O2 supplementation. Am J Physiol Heart Circ Physiol 277: H1045–H1052, 1999. [DOI] [PubMed] [Google Scholar]
- 127.Nielsen HB, Bredmose PP, Strømstad M, Volianitis S, Quistorff B, Secher NH. Bicarbonate attenuates arterial desaturation during maximal exercise in humans. J Appl Physiol 93: 724–731, 2002. [DOI] [PubMed] [Google Scholar]
- 128.Nielsen HB, Hein L, Svendsen LB, Secher NH, Quistorff B. Bicarbonate attenuates intracellular acidosis. Acta Anaesthesiol Scand 46: 579–584, 2002. [DOI] [PubMed] [Google Scholar]
- 129.Nielsen HB, Madsen P, Svendsen LB, Roach RC, Secher NH. The influence of PaO2, pH and SaO2 on maximal oxygen uptake. Acta Physiol Scand 164: 89–97, 1998. [DOI] [PubMed] [Google Scholar]
- 130.Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol 534: 279–286, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol 545: 697–704, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ogoh S, Fadel PJ, Nissen P, Jans Ø, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O'Leary DS, Rowell LB, Scher AM. Baroreflex-induced vasoconstriction in active skeletal muscle of conscious dogs. Am J Physiol Heart Circ Physiol 260: H37–H41, 1991. [DOI] [PubMed] [Google Scholar]
- 134.Olesen J. Contralateral focal increase of cerebral blood flow during arm work. Brain 94: 635–646, 1971. [DOI] [PubMed] [Google Scholar]
- 135.Olin JT, Dimmen AC, Subudhi AW, Roach RC. Cerebral blood flow and oxygenation at maximal exercise: the effect of clamping carbon dioxide. Respir Physiol Neurobiol 175: 176–180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Osada T, Katsumura T, Hamaoka T, Inoue S, Esaki K, Sakamoto A, Murase N, Kajiyama J, Shimomitsu T, Iwane H. Reduced blood flow in abdominal viscera measured by Doppler ultrasound during one-legged knee extension. J Appl Physiol 86: 709–719, 1999. [DOI] [PubMed] [Google Scholar]
- 137.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol 2: 592–607, 1950. [DOI] [PubMed] [Google Scholar]
- 138.Papelier Y, Escourrou P, Helloco F, Rowell LB. Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol 82: 577–583, 1997. [DOI] [PubMed] [Google Scholar]
- 139.Parks CM, Manohar M. Distribution of blood flow during moderate and strenuous exercise in ponies (Equus caballus). Am J Vet Res 44: 1861–1866, 1983. [PubMed] [Google Scholar]
- 140.Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol 73: 1838–1846, 1992. [DOI] [PubMed] [Google Scholar]
- 141.Perko MJ, Nielsen HB, Skak C, Clemmesen JO, Schroeder TV, Secher NH. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J Physiol 513: 907–913, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Piiper J. Perfusion, diffusion and their heterogeneities limiting blood-tissue O2 transfer in muscle. Acta Physiol Scand 168: 603–607, 2000. [DOI] [PubMed] [Google Scholar]
- 143.Pittman RN. Oxygen supply to contracting skeletal muscle at the microcirculatory level: diffusion vs. convection. Acta Physiol Scand 168: 593–602, 2000. [DOI] [PubMed] [Google Scholar]
- 144.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98: 1645–1658, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol 88: 186–194, 2000. [DOI] [PubMed] [Google Scholar]
- 146.Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 265: H1928–H1938, 1993. [DOI] [PubMed] [Google Scholar]
- 147.Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83: 1383–1388, 1997. [DOI] [PubMed] [Google Scholar]
- 148.Rådegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol 87: 2375–2380, 1999. [DOI] [PubMed] [Google Scholar]
- 149.Radigan LR, Robinson S. Effects of environmental heat stress and exercise on renal blood flow and filtration rate. J Appl Physiol 2: 185–191, 1949. [DOI] [PubMed] [Google Scholar]
- 150.Rasmussen J, Hanel B, Diamant B, Secher NH. Muscle mass effect on arterial desaturation after maximal exercise. Med Sci Sports Exerc 23: 1349–1352, 1991. [PubMed] [Google Scholar]
- 151.Rasmussen P, Dawson EA, Nybo L, van Lieshout JJ, Secher NH, Gjedde A. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J Cereb Blood Flow Metab 27: 1082–1093, 2007. [DOI] [PubMed] [Google Scholar]
- 152.Ray CA, Dudley GA. Muscle use during dynamic knee extension: implication for perfusion and metabolism. J Appl Physiol 85: 1194–1197, 1998. [DOI] [PubMed] [Google Scholar]
- 153.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol Heart Circ Physiol 264: H1–H7, 1993. [DOI] [PubMed] [Google Scholar]
- 154.Rein H. Die interferenz der vasomotorischen regulationen. Klin Wochenschr 9: 1485, 1930. [Google Scholar]
- 155.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Circ Res 11: 370–380, 1962. [DOI] [PubMed] [Google Scholar]
- 156.Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc 30: 28–33, 1998. [DOI] [PubMed] [Google Scholar]
- 157.Richardson RS, Kennedy B, Knight DR, Wagner PD. High muscle blood flows are not attenuated by recruitment of additional muscle mass. Am J Physiol Heart Circ Physiol 269: H1545–H1552, 1995. [DOI] [PubMed] [Google Scholar]
- 158.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75: 1911–1916, 1993. [DOI] [PubMed] [Google Scholar]
- 159.Richter EA, Kiens B, Hargreaves M, Kjaer M. Effect of arm-cranking on leg blood flow and noradrenaline spillover during leg exercise in man. Acta Physiol Scand 144: 9–14, 1992. [DOI] [PubMed] [Google Scholar]
- 160.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at V̇o2 max. J Appl Physiol 73: 1067–1076, 1992. [DOI] [PubMed] [Google Scholar]
- 161.Rokamp KZ, Olesen ND, Larsson HB, Hansen AE, Seifert T, Nielsen HB, Secher NH, Rostrup E. Glycopyrrolate does not influence the visual or motor-induced increase in regional cerebral perfusion. Front Physiol 5: 45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rowell LB, Blackmon JR, Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest 43: 1677–1690, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rowell LB. Regulation of splanchnic blood flow in man. Physiologist 16: 127–142, 1973. [PubMed] [Google Scholar]
- 164.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. [DOI] [PubMed] [Google Scholar]
- 165.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol 97: 384–392, 2004. [DOI] [PubMed] [Google Scholar]
- 166.Rowell LB, Blackmon JR, Martin RH, Mazzarella JA, Bruce RA. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. J Appl Physiol 20: 384–394, 1965. [DOI] [PubMed] [Google Scholar]
- 167.Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969. [DOI] [PubMed] [Google Scholar]
- 168.Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986. [DOI] [PubMed] [Google Scholar]
- 169.Saito M, Kagaya A, Ogita F, Shinohara M. Changes in muscle sympathetic nerve activity and calf blood flow during combined leg and forearm exercise. Acta Physiol Scand 146: 449–456, 1992. [DOI] [PubMed] [Google Scholar]
- 170.Saltin B. The physiology of competitive cross country skiing across a four decade perspective; with a note on training induced adaptations and role of training at medium altitude. In: Science and Skiing, edited by Muller E, Schwameder H, Kornxl E, Raschner C. London: E & FN Spon, 1996, p. 435–469. [Google Scholar]
- 171.Saltin B, Henriksson J, Nygaard E, Andersen P, Jansson E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann NY Acad Sci 301: 3–29, 1977. [DOI] [PubMed] [Google Scholar]
- 172.Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol 21: 1757–1762, 1966. [DOI] [PubMed] [Google Scholar]
- 173.Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. [DOI] [PubMed] [Google Scholar]
- 174.Sato K, Sadamoto T. Different blood flow responses to dynamic exercise between internal carotid and vertebral arteries in women. J Appl Physiol 109: 864–869, 2010. [DOI] [PubMed] [Google Scholar]
- 175.Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, Ogoh S. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol 590: 3277–3290, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol 64: 649–657, 1988. [DOI] [PubMed] [Google Scholar]
- 177.Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol Heart Circ Physiol 257: H1812–H1818, 1989. [DOI] [PubMed] [Google Scholar]
- 178.Secher NH, Richardson RS. A large slice of cardiac output or humble pie for the respiratory muscles? J Physiol 587: 3411, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand 100: 288–297, 1977. [DOI] [PubMed] [Google Scholar]
- 180.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 104: 306–314, 2008. [DOI] [PubMed] [Google Scholar]
- 181.Seifert T, Secher NH. Sympathetic influence on cerebral blood flow and metabolism during exercise in humans. Prog Neurobiol 95: 406–426, 2011. [DOI] [PubMed] [Google Scholar]
- 182.Seifert T, Fisher JP, Young CN, Hartwich D, Ogoh S, Raven PB, Fadel PJ, Secher NH. Glycopyrrolate abolishes the exercise-induced increase in cerebral perfusion in humans. Exp Physiol 95: 1016–1025, 2010. [DOI] [PubMed] [Google Scholar]
- 183.Seifert T, Rasmussen P, Secher NH, Nielsen HB. Cerebral oxygenation decreases during exercise in humans with beta-adrenergic blockade. Acta Physiol 196: 295–302, 2009. [DOI] [PubMed] [Google Scholar]
- 184.Sejrsen P. Convection and diffusion of inert gases in cutaneous, subcutaneous, and skeletal muscle tissue. In: Capillary Permeability (Alfred Benzon Symp. II), edited by Crone C, Lassen NA. Copenhagen, Denmark: Munksgaard, 1970, p. 586–596. [Google Scholar]
- 185.Sejrsen P, Bülow J. Blood flow rate measurements with indicator techniques revisited. Clin Physiol Funct Imaging 29: 385–391, 2009. [DOI] [PubMed] [Google Scholar]
- 186.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90, 1996. [DOI] [PubMed] [Google Scholar]
- 187.Sheehan D, Mulholland JH, Safiroff B. Surgical anatomy of the carotid sinus nerve. Anat Rec 80: 431–442, 1941. [Google Scholar]
- 188.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol 537: 277–289, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sheriff DD, Mullin TM, Wong BJ, Ladouceur M. Does limb angular motion raise limb arterial pressure? Acta Physiol 195: 367–374, 2009. [DOI] [PubMed] [Google Scholar]
- 190.Shibata M, Ichioka S, Ando J, Togawa T, Kamiya A. Nonlinear regulation of capillary perfusion in relation to ambient pO(2) changes in skeletal muscle. Eur J Appl Physiol 94: 352–355, 2005. [DOI] [PubMed] [Google Scholar]
- 191.Shibata M, Ichioka S, Togawa T, Kamiya A. Arterioles' contribution to oxygen supply to the skeletal muscles at rest. Eur J Appl Physiol 97: 327–331, 2006. [DOI] [PubMed] [Google Scholar]
- 192.Siebenmann C, Sørensen H, Jacobs RA, Haider T, Rasmussen P, Lundby C. Hypocapnia during hypoxic exercise and its impact on cerebral oxygenation, ventilation and maximal whole body O2 uptake. Respir Physiol Neurobiol 185: 461–467, 2013. [DOI] [PubMed] [Google Scholar]
- 193.Smith ML, Glass GV. Meta-analysis of psychotherapy outcome studies. Am Psychol 32: 752–760, 1977. [DOI] [PubMed] [Google Scholar]
- 194.Sørensen H, Secher NH, Siebenmann C, Nielsen HB, Kohl-Bareis M, Lundby C. Cutaneous vasoconstriction affects near-infrared spectroscopy determined cerebral oxygen saturation during administration of norepinephrine. Anesthesiology 117: 263–270, 2012. [DOI] [PubMed] [Google Scholar]
- 195.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529: 493–504, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stenberg J, Astrand PO, Ekblom B, Royce J, Saltin B. Hemodynamic response to work with different muscle groups, sitting and supine. J Appl Physiol 22: 61–70, 1967. [DOI] [PubMed] [Google Scholar]
- 197.Strandell T, Shepherd JT. The effect in humans of increased sympathetic activity on the blood flow to active muscles. Acta Med Scand Suppl 472: 146–167, 1967. [DOI] [PubMed] [Google Scholar]
- 198.Strandgaard S, Sigurdsson ST. Point: Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol 105: 1366–1367, 2008. [DOI] [PubMed] [Google Scholar]
- 199.Strange S. Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. J Physiol 514: 283–291, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Subudhi AW, Olin JT, Dimmen AC, Polaner DM, Kayser B, Roach RC. Does cerebral oxygen delivery limit incremental exercise performance? J Appl Physiol 111: 1727–1734, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thijssen DH, Steendijk S, Hopman MT. Blood redistribution during exercise in subjects with spinal cord injury and controls. Med Sci Sports Exerc 41: 1249–1254, 2009. [DOI] [PubMed] [Google Scholar]
- 202.Thomas DJ, Marshall J, Russell RW, Wetherley-Mein G, du Boulay GH, Pearson TC, Symon L, Zilkha E. Effect of haematocrit on cerebral blood-flow in man. Lancet 2: 941–943, 1977. [DOI] [PubMed] [Google Scholar]
- 203.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 97: 731–738, 2004. [DOI] [PubMed] [Google Scholar]
- 204.Thomas SN, Schroeder T, Secher NH, Mitchell JH. Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J Appl Physiol 67: 744–748, 1989. [DOI] [PubMed] [Google Scholar]
- 205.Vallbo AB, Hagbarth KE. Impulses recorded with micro-electrodes in human muscle nerves during stimulation of mechanoreceptors and voluntary contractions. Electroencephalogr Clin Neurophysiol 23: 392, 1967. [PubMed] [Google Scholar]
- 206.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 207.Van Hall G, Jensen-Urstad M, Rosdahl H, Holmberg HC, Saltin B, Calbet JA. Leg and arm lactate and substrate kinetics during exercise. Am J Physiol Endocrinol Metab 284: E193–E205, 2003. [DOI] [PubMed] [Google Scholar]
- 208.Van Lieshout JJ, Secher NH. Point: Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Point: Sympathetic activity does influence cerebral blood flow. J Appl Physiol 105: 1364–1366, 2008. [DOI] [PubMed] [Google Scholar]
- 209.VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol 550: 563–574, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vogelsang TW, Yoshiga CC, Højgaard M, Kjaer A, Warberg J, Secher NH, Volianitis S. The plasma atrial natriuretic peptide response to arm and leg exercise in humans: effect of posture. Exp Physiol 91: 765–771, 2006. [DOI] [PubMed] [Google Scholar]
- 211.Vogiatzis I, Athanasopoulos D, Habazettl H, Kuebler WM, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol 587: 3665–3677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol 544: 977–984, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Volianitis S, Dawson EA, Dalsgaard M, Yoshiga C, Clemmesen J, Secher NH, Olsen N, Nielsen HB. Renal lactate elimination is maintained during moderate exercise in humans. J Sports Sci 30: 149–153, 2012. [DOI] [PubMed] [Google Scholar]
- 214.Volianitis S, Fabricius-Bjerre A, Overgaard A, Strømstad M, Bjarrum M, Carlson C, Petersen NT, Rasmussen P, Secher NH, Nielsen HB. The cerebral metabolic ratio is not affected by oxygen availability during maximal exercise in humans. J Physiol 586: 107–112, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Volianitis S, Krustrup P, Dawson E, Secher NH. Arm blood flow and oxygenation on the transition from arm to combined arm and leg exercise in humans. J Physiol 547: 641–648, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Volianitis S, Rasmussen P, Seifert T, Nielsen HB, Secher NH. Plasma pH does not influence the cerebral metabolic ratio during maximal whole body exercise. J Physiol 589: 423–429, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Volianitis S, Secher NH. Rowing, the ultimate challenge to the human body–implications for physiological variables. Clin Physiol Funct Imaging 29: 241–244, 2009. [DOI] [PubMed] [Google Scholar]
- 218.Volianitis S, Yoshiga CC, Nissen P, Secher NH. Effect of fitness on arm vascular and metabolic responses to upper body exercise. Am J Physiol Heart Circ Physiol 286: H1736–H1741, 2004. [DOI] [PubMed] [Google Scholar]
- 219.Volianitis S, Yoshiga CC, Vogelsang T, Secher NH. Arterial blood pressure and carotid baroreflex function during arm and combined arm and leg exercise in humans. Acta Physiol Scand 181: 289–295, 2004. [DOI] [PubMed] [Google Scholar]
- 220.Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58: 21–50, 1996. [DOI] [PubMed] [Google Scholar]
- 221.Wagner PD. Diffusive resistance to O2 transport in muscle. Acta Physiol Scand 168: 609–614, 2000. [DOI] [PubMed] [Google Scholar]
- 222.Wang ZM, Visser M, Ma R, Baumgartner RN, Kotler D, Gallagher D, Heymsfield SB. Skeletal muscle mass: evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol 80: 824–831, 1996. [DOI] [PubMed] [Google Scholar]
- 223.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 592: 841–859, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest 5: 2967–2972, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]