Abstract
Organizers are regions of the embryo that can both induce new fates and impart pattern on other regions. So far, surprisingly few organizers have been discovered, considering the number of patterned tissue types generated during development. This may be because their discovery has relied on transplantation and ablation experiments. Here we describe a new approach, using chick embryos, to discover organizers based on a common gene expression signature, and use it to uncover the anterior intestinal portal (AIP) endoderm as a putative heart organizer. We show that the AIP can induce cardiac identity from non-cardiac mesoderm and that it can pattern this by specifying ventricular and suppressing atrial regional identity. We also uncover some of the signals responsible. The method holds promise as a tool to discover other novel organizers acting during development.
 Organizers are regions in the embryo that induce cell fate and impart pattern on neighbouring regions. Here, the authors search for new organizers based on a common gene signature, and show that the Anterior Intestinal Portal endoderm induces cardiac identity, specifies ventricle and inhibits atrial character.
Organizers are regions in the embryo that induce cell fate and impart pattern on neighbouring regions. Here, the authors search for new organizers based on a common gene signature, and show that the Anterior Intestinal Portal endoderm induces cardiac identity, specifies ventricle and inhibits atrial character.
Organizers are formally defined as signalling regions, unique in being able both to induce and to pattern adjacent tissue1. The dorsal lip of the blastopore, which can induce a complete secondary axis in amphibian embryos, was the first organizer to be discovered, in 1924 (ref. 2). Given the complexity of the vertebrate embryo, one might expect that many organizers should exist, but only a few others have been described3: Hensen’s node (the amniote equivalent of the dorsal lip of the blastopore, which can induce and pattern the central nervous system)4, the notochord/floor-plate (which can induce and organize different sets of neurons in the neural tube)5, the zone of polarizing activity (ZPA, which can induce a patterned set of limb elements)6 and the midbrain–hindbrain boundary (isthmus, which can specify and pattern the adjacent regions of the midbrain/tectum and hindbrain/cerebellum)7. One reason why so few organizers have been found could be that their discovery requires grafting appropriate tissues at the right time and place, and there are too many possible combinations.
It has been shown that in some cases organizers can substitute for each other. Most strikingly, a graft of Hensen’s node from an early (primitive streak stage) embryo into the anterior limb bud of a much later embryo can mimic the action of the limb organizer, the ZPA, by inducing and patterning a full set of skeletal elements including the digits8. This raises the intriguing possibility that organizers may share a genetic signature (‘synexpression’) that confers them with inducing and patterning properties. If this is the case, we should be able to use this genetic signature to point to potential new organizing regions. In this study, we tested this idea by comparing the transcriptomes of three known amniote organizers (Hensen’s node, the notochord/floor-plate and the ZPA). This defines a synexpression set of 48 transcripts that are either enriched or depleted in organizers, which we then used to explore the embryo for other regions of synexpression. This suggested that the endoderm of the anterior intestinal portal (AIP) might be a new organizer. The AIP is an endodermal invagination appearing with the head-fold, which moves caudally down the embryo to form the foregut9. As it does so, it is closely associated with the developing heart tube. We therefore performed a series of experiments to test whether the AIP can act as an organizer of the heart. Ablation and transplantation experiments in vivo, along with co-culture in vitro, revealed that the AIP endoderm can induce heart fate from non-cardiac mesoderm as well as pattern the heart field by specifying ventricular and suppressing atrial regional identity.
Results
Defining a gene signature for organizers
To investigate whether organizers have a common genetic signature of enriched and depleted transcripts, we designed a differential microarray screen in chick embryos, comparing three known organizers to their most similar non-organizer tissue. First, we chose stage (HH10) 3+/4 Hensen’s node, which induces and patterns the neural plate11, compared with the posterior primitive streak, which cannot (Fig. 1a). We also sampled the later node (HH6), which has lost its ability to induce a full neural plate11. This three-way comparison was designed to reveal transcripts associated with full organizer function. Second, we selected the notochord and floor-plate at HH10-11, responsible for organizing the dorso-ventral axis of the neural tube5, and compared this to the dorsal neural tube (Fig. 1b). Finally, we compared the posterior wing bud, containing the ZPA6, to the anterior wing bud at HH20-21 and HH24 (Fig. 1c). Each comparison generated a list of transcripts significantly enriched or depleted in that organizer (≥1.2-fold the log2 of the expression level, and a false discovery rate ≤0.05. The dataset was submitted to ArrayExpress with the title ‘Microarray analysis of chick embryonic tissues: gastrulation, neural tube/notochord and limb development’ and given accession number E-MTAB-4048). These lists were then combined using a Boolean algorithm to find enriched or depleted transcripts common to all three organizers. This approach uncovers relative changes in expression between organizer cells and neighbouring regions, even if the absolute level of expression is widely different at different stages: a putative organizer gene set of 31 enriched and 17 depleted transcripts is revealed (Supplementary Fig. 1a–d and Supplementary Data 1). Interestingly, over 60% of the enriched genes encode membrane-associated or secreted molecules and 7% represent transcription factors, whereas transcription factors are much more frequent (38%) among the depleted genes (Supplementary Fig. 1c). One possible interpretation of this is that the synexpression set is enriched for signalling molecules emitted by the organizer and depleted of transcriptional repressors that suppress the organizer state.
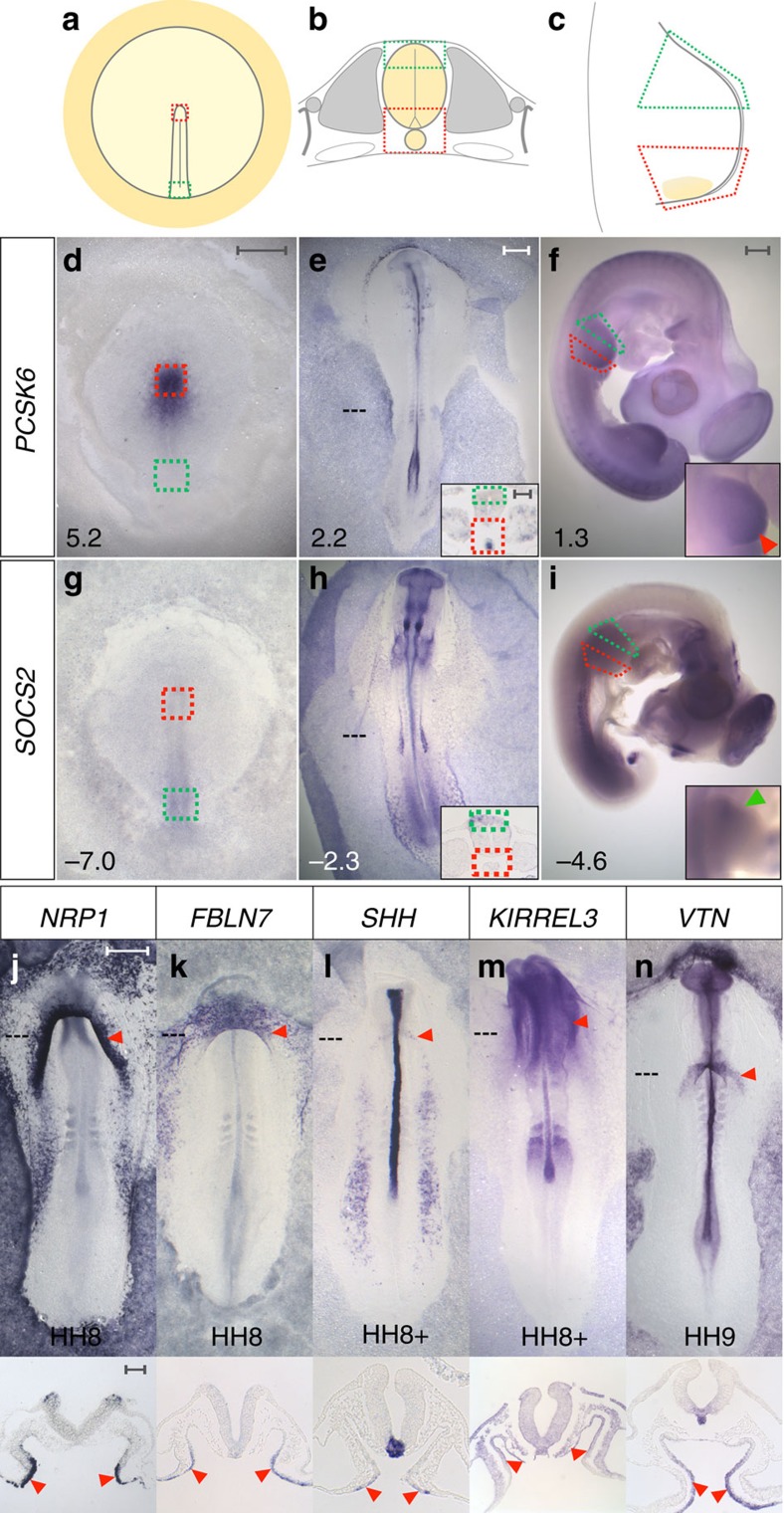
Figure 1. Transcriptome comparison of known organizers reveals genes co-expressed in organizers and in the Anterior Intestinal Portal endoderm.
(a–c) Experimental design. (a) Hensen’s node (red box) was compared with posterior primitive streak (green box); (b) notochord and ventral neural tube (red box) was compared with dorsal neural tube (green box) and (c) posterior wing bud (red box) was compared with anterior wing bud (green box). (d–i) One gene enriched in organizers is PCSK6 (d–f, red boxes, red arrowhead in f-inset) and SOCS2 exemplifies a depleted gene (g–i, green boxes, green arrowhead in i-inset). Fold-change values are indicated. (j–n) Examples of organizer-enriched transcripts expressed in the anterior intestinal portal endoderm (AIP) (red arrowheads): NRP1 (j), FBLN7 (k), SHH (l), KIRREL3 (m) and VTN (n). Scale bars, 0.5 mm in whole-mounts and 0.1 mm in sections.
The AIP endoderm as a candidate organizer
Next, we used this set to verify the expression of the selected genes (Fig. 1d–i, Supplementary Figs 2 and 3), as well as to explore whether the synexpression signature occurs in other places and stages during development. A large-scale in situ hybridization analysis of the 48 genes was undertaken from pre-primitive streak stage up to HH27; these expression patterns can be browsed on eChick atlas12 (www.echickatlas.org). One embryonic region appropriately expresses 35 genes from the organizer gene set: the endoderm of the AIP. At some stage between HH7-14, 20/31 enriched transcripts are detected (Supplementary Fig. 4a–t), 15/17 organizer-depleted genes are appropriately absent from the early AIP (Supplementary Fig. 4u–k), and SOCS2 and BTG2 are absent before HH10 and HH14 respectively (Supplementary Fig. 4l′,m′). Thus, the AIP shares a similar transcriptional profile to other known organizers.
There is substantial evidence that the early endoderm adjacent to the bilateral cardiac mesoderm is required for normal heart formation in chick, amphibian and fish embryos13,14, but less is known about the AIP endoderm of later stages. Genetic or manual ablation of the AIP endoderm in mouse and chick embryos results in cardia bifida15,16,17,18,19 (failure of the bilateral heart progenitors to fuse in the midline), rotation defects16 and downregulation of early cardiac genes15, indicating that a functional AIP endoderm is required for normal heart formation, but its inducing and patterning abilities have not been tested. Could the AIP be an organizer of the heart?
The AIP can induce cardiac identity from non-cardiac mesoderm
We first confirmed that ablation of the AIP at HH8 does indeed cause cardia bifida and abnormal heart rotation (Supplementary Fig. 5). Next, to test if the AIP can induce cardiac identity, a necessary prerequisite is to identify a suitable responding tissue. Fate and specification maps indicate that the paraxial mesoderm adjacent to Hensen’s node at HH5 (prospective head mesoderm) is neither fated nor specified as heart20,21,22,23,24, but is competent to respond to cardiac inducing signals22. We compared this mesoderm (named #3) with four other regions of HH5 mesoderm (Supplementary Figs 6a and 7a): #3 does not contribute to the heart when grafted homotopically (Supplementary Fig. 7b–h), and is the only region of mesoderm that does not express cardiac markers when explanted either in vitro (Fig. 2 i,l,o,r,u,x,a′ and Supplementary Table 1) or in a host embryo (Supplementary Fig. 7j–v and Supplementary Table 2). Mesoderm #3 is therefore suitable for testing whether the early AIP can induce heart fate. The AIP induces early cardiac markers, MYOCD, NKX2.5 (Fig. 2j,m and Supplementary Fig. 8b,c), GATA4, TBX5, ISL1 and MEF2C in explants of #3-mesoderm (Supplementary Fig. 8d–g), while co-culture with control (Cont.), non-AIP endoderm from the lateral embryo, largely does not (Fig. 2k,n, Supplementary Fig. 8b–h, Supplementary Tables 2 and 3). Strikingly, spontaneous beating is observed after 48 h of in vitro co-culture of #3-mesoderm with AIP (Supplementary Movie 1), but not with control, non-AIP endoderm. Together these results show that the AIP endoderm can induce cardiac identity in mesoderm that is not otherwise fated to become part of the heart.
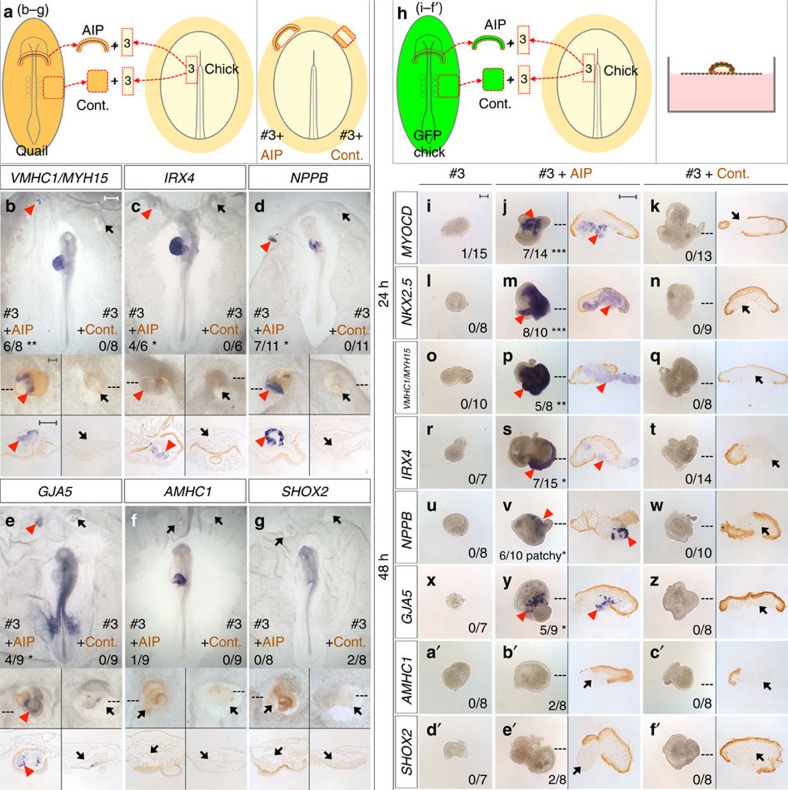
Figure 2. AIP induces cardiac and ventricular identity in non-cardiac mesoderm.
(a) AIP or control (Cont.), lateral endoderm from a HH8 quail co-cultured with anterior-medial mesoderm (#3) from a HH5 chick embryo overnight in the anterior area opaca of a host chick. (b–g) Regional myocardium markers VMHC1/MYH15 (b), IRX4 (c), NPPB (d) and GJA5 (e) are induced in #3-mesoderm by quail (brown) AIP (red arrowheads), but not by control endoderm (Cont., black arrows), whereas AMHC1 (f) and SHOX2 (g) are not induced (black arrows). (h) In vitro co-culture of #3-mesoderm with GFP-AIP (j,m,p,s,v,y,b’,e’) or -Cont. (k,n,q,t,w,z,c’,f’) for 24 (j,k,m,n) or 48 h (p,q,s,t,v,w,y,z,b’,c’,e’,f’). No expression is seen in #3-mesoderm cultured alone (i,l,o,r,u,x,a’,d’). AIP (brown) induces MYOCD (j), NKX2.5 (m), VMHC1/MYH15 (p), IRX4 (s), NPPB (v) and GJA5 (y) in #3-mesoderm (red arrowheads), but Cont. does not (brown, k,n,q,t,w,z, black arrows). Neither AMHC1 nor SHOX2 are induced (black arrows) by AIP (b’,e’) or by Cont. (c’,f’). *P≤0.05, **P≤0.005, ***P≤0.0005 using two-tailed Fisher's exact test. Scale bars, 0.5 mm in whole-mounts and 0.1 mm in insets, explants and sections.
Patterning by inducing ventricular identity and suppressing atrial character
Many of the known early heart markers are normally expressed in the bilateral cardiac mesoderm before the AIP forms (around stage HH5; Supplementary Fig. 6a–f), whereas regional markers of anterior–posterior heart tube patterning are observed later (after HH9; Supplementary Fig. 6v–n′). Ventricular markers (VMHC1/MYH15, IRX4 and NPPB) are initially expressed in the medial splanchnic mesoderm25,26,27 immediately adjacent to the AIP (Supplementary Fig. 6d′,f′,g′), and the atrial marker AMHC1 is in the lateral splanchnic mesoderm28 (Supplementary Fig. 6c′). In #3-mesoderm explants cultured either in a host embryo or in vitro, AIP induces VMHC1/MYH15, IRX4 and patchy NPPB expression (Fig. 2b–d,p,s,v, Supplementary Tables 2 and 3). AIP also induces GJA5 (Fig. 2e,y), which marks the anterior ventricular myocardium, endocardium and outflow tract (Supplementary Fig. 6h′). AIP does not induce AMHC1 (Fig. 2f,b′) or SHOX2 (Fig. 2g,e′), a marker of the sinoatrial node (Supplementary Fig. 6i′,p′). These results show that the AIP can induce ventricular cardiac identity in mesoderm not destined to this fate.
Previous tissue recombination experiments using HH5-6 chick explants have demonstrated that anterior-lateral endoderm adjacent to the early cardiogenic mesoderm can induce heart gene expression in posterior primitive streak29. Are the induction properties of the HH8 AIP endoderm distinct to the early anterior-lateral endoderm? To test this, we co-cultured HH5 anterior-lateral endoderm with non-cardiac #3-mesoderm (Supplementary Fig. 9a and Supplementary Table 4). Unlike AIP endoderm, anterior-lateral endoderm does not induce ventricular markers (Supplementary Fig. 9b–e) nor does it induce AMHC1 (Supplementary Fig. 9f). These data show that the anterior-lateral endoderm is different to the AIP endoderm and that the ability to induce ventricular character is a property specific to the AIP endoderm.
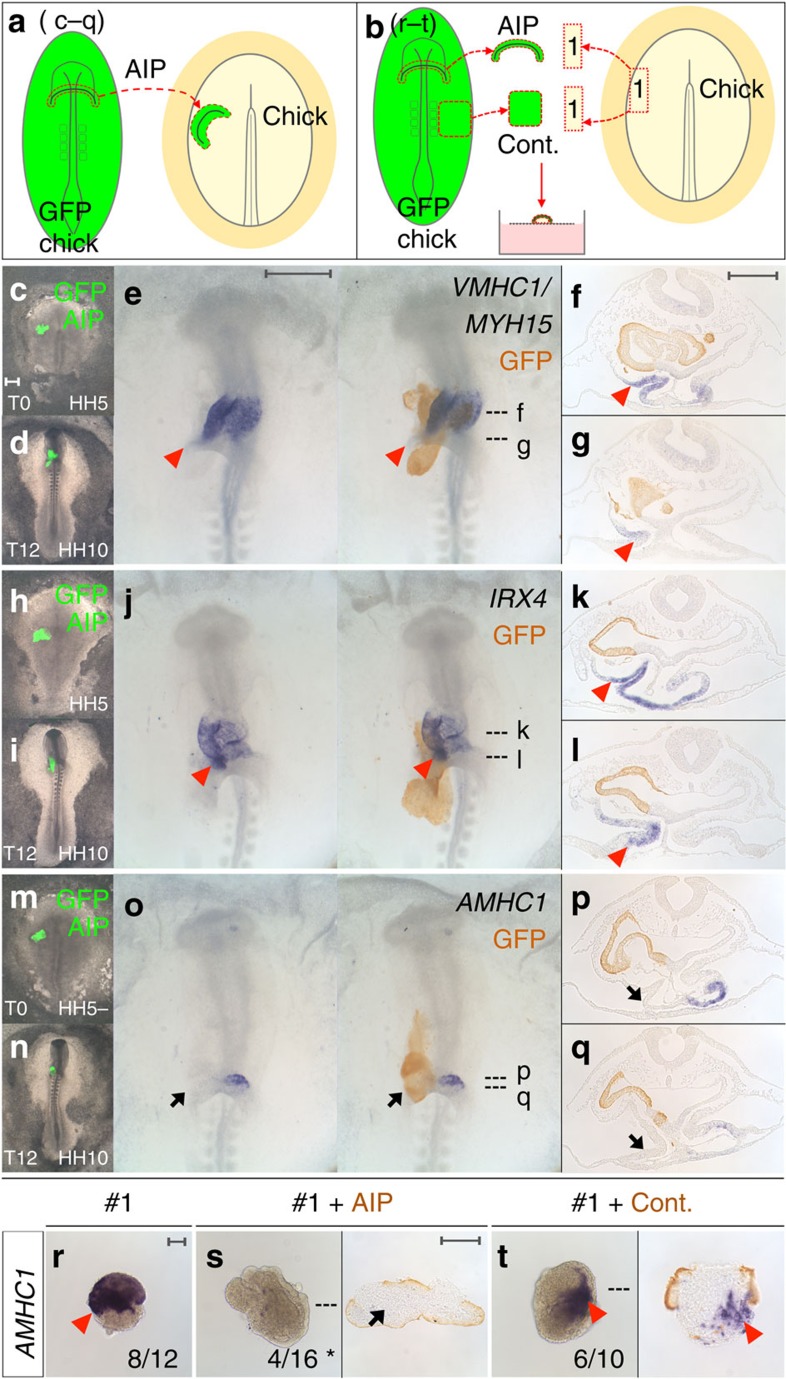
To test if the AIP patterns the heart tube by specifying ventricular identity, AIP from transgenic GFP donors was grafted over the cardiogenic mesoderm of a host embryo (Fig. 3a). Strikingly, this expands the expression of VMHC1/MYH15 and IRX4 posteriorly into the prospective atrial region (Fig. 3c–l), while expression of the atrial marker AMHC1 is abolished (Fig. 3m–q). Moreover, the AIP represses AMHC1 in #1-mesoderm which would normally express it (Fig. 3r–t and Supplementary Table 3); since IRX4 is known to repress AMHC1 (refs 26, 30), this repression by the AIP may be either direct or indirect, due to prior AIP-mediated induction of IRX4. Thus, the AIP not only induces ventricular identity, but also actively blocks atrial character. The ability to induce a new fate in responding tissues and to impart a spatial pattern are properties shared by other known organizers3. Taken together, these results show that the AIP endoderm is distinct from other endodermal regions, in that it can both induce cardiac identity in mesoderm not destined for this fate and also pattern heart mesoderm by promoting ventricular and suppressing atrial regional character.
Figure 3. AIP specifies ventricular regional identity and represses atrial character.
(a) AIP (green, c,d,h,i,m,n; brown, e–g,j–l,o–q) from a HH8 GFP chick was grafted heterotopically onto cardiogenic mesoderm of a HH5 wild-type chick host (c,h,m, T0) and grown for 12 h (d,i,n, T12). (c–q) AIP expands VMHC1/MYH15 (e–g) and IRX4 (j–l) expression (red arrowheads) and abolishes AMHC1 (o–q, black arrows). (b) In vitro co-culture of #1-mesoderm from a HH5 chick with HH8 GFP-AIP or control (Cont.) endoderm (brown) for 48 h. AMHC1 expression in #1-mesoderm (r, red arrowhead). AIP represses AMHC1 in #1-mesoderm (s, black arrow), Cont. does not (t, red arrowhead). *P≤0.05 using two-tailed Fisher's exact test. Scale bars, 0.5 mm in whole-mounts and 0.1 mm in explants and sections.
Common signals between organizers
Grafts of either Hensen’s node8, notochord or floor-plate31 into the anterior limb bud can induce extra digits, suggesting that at least some signals (an obvious one being SHH) may be common to different organizers. We therefore tested whether the heart-inducing or ventricle-specifying functions of the AIP can be mimicked by other organizers. We find that Hensen’s node induces patchy expression of VMHC1/MYH15 but not MYOCD in #3-mesoderm cultured in a host (Supplementary Fig. 10a,b and Supplementary Table 5), as well as in explants (Supplementary Fig. 10c,d and Supplementary Table 5). Hensen’s node has also been shown to induce ectopic VMHC1, but not AMHC1 in the germinal crescent and the lateral plate mesoderm32, comparable to the ability of the AIP to induce ventricular markers. Conversely, to test whether the AIP can mimic the neural inducing activity of Hensen’s node, we grafted AIP endoderm into the area opaca33 at HH3+: this induces the early neural marker SOX3, but not the later neural plate marker SOX2 (Supplementary Fig. 10e,f). Finally, we tested whether the AIP can mimic a ZPA graft into the anterior wing bud: strikingly, the AIP causes duplication of digit-2 (Supplementary Fig. 10h). Therefore, the AIP is partly interchangeable with other organizers at earlier and later stages of development, some of which could be related to its expression of SHH (Fig. 1l) or FGF8 (ref. 34). However it seems that the ability of organizers to substitute for each other is limited.
Signals from the AIP endoderm
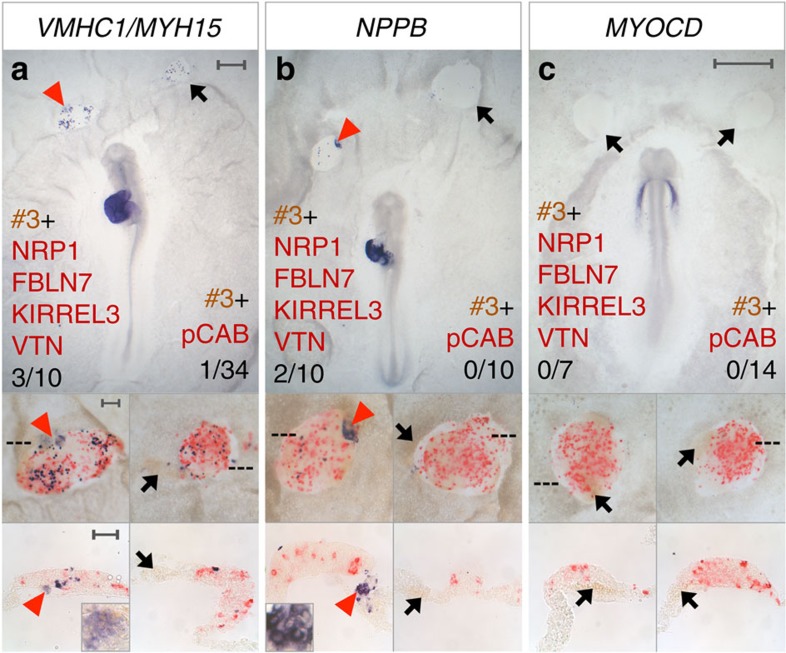
The inducing and patterning activities of other known organizers rely on multiple signals, acting together and/or sequentially35,36,37. FGF8 (refs 38, 39) and SHH (refs 40, 41) play important roles in other organizers; both have been implicated in cardiogenesis34,42 and both are expressed in the AIP. However, our synexpression set contains many other secreted or membrane-associated factors that have not hitherto been implicated either in the activity of other organizers or in cardiac gene induction or heart patterning. Four of these are expressed in the early AIP: NRP1, FBLN7, KIRREL3 and VTN (Fig. 1j,k,m,n). To investigate their activity, we co-cultured #3-mesoderm with pellets of cells transfected with expression plasmids encoding these four factors. This combination partly mimics the AIP, sometimes inducing patchy expression of the ventricular markers VMHC1/MYH15 (Fig. 4a) and NPPB (Fig. 4b), but not IRX4 (Supplementary Fig. 11f) or other early cardiac markers (Fig. 4c, Supplementary Fig. 11a–e and Supplementary Table 6).
Figure 4. Secreted molecules induce ventricular markers.
(a–c) HH5 quail (brown) #3-mesoderm co-cultured in a chick host with cell pellets transfected with NRP1+FBLN7+KIRREL3+VTN (red) either overnight (a,b) or for 6–9 h (c). VMHC1/MYH15 and NPPB are induced in #3-mesoderm (a,b, red arrowheads) but MYOCD is not (c, black arrows). Control pellets (red; pCAB) do not induce VMHC1/MYH15, NPPB or MYOCD (a–c, black arrows). Scale bars, 0.5 mm in whole-mounts and 0.1 mm in insets and sections.
These results suggest that other signals are required, possibly SHH and FGF8, but also considerable complexity, not unlike other organizers, which are believed to emit different signals at different times to account for their various inducing and patterning functions35,36,37. Accordingly, some gene expression profiles change over time as the organizer changes its properties. Although HH8 AIP endoderm was used in co-cultures, the AIP continues its developmental programme, expressing transcripts that are detected in the later AIP (Supplementary Fig. 12). HH8 AIP cultured either alone or with #3-mesoderm expresses genes, including those that encode secreted or membrane-associated molecules normally observed in the HH10 AIP (Supplementary Fig. 4j,k), after 24 h (Supplementary Fig. 12a–d) and those expressed in the HH12-13 AIP (Supplementary Fig. 4q,s) after 48 h (Supplementary Fig. 12e–h). These results suggest that the signalling properties of the AIP endoderm mature over the culture period as they do in the embryo.
Taken together, our results implicate the AIP as an organizer of the heart, and suggest that a complex combination of signals account for its heart inducing and patterning functions at early stages of heart tube formation.
Discussion
Our results suggest that organizers do share a common genetic signature, and that this property can be used to identify putative new organizers acting during development. The choice of the three organizers to compare was relatively arbitrary and perhaps we should have used a different well-characterized organizer, the mid-hindbrain boundary (MHB) or isthmic organizer3,7 instead of the comparison between notochord/floor-plate with dorsal neural tube (particularly as the latter is also a signalling region). The main reason why we did not use the MHB is that it is difficult to identify a non-organizer tissue that is similar enough to this boundary in every other respect. The organizer gene set could be refined further using this tissue in future. Nevertheless, this study is an important proof of principle and, based on the synexpression of 48 genes, we uncover the AIP endoderm as a putative organizer because it can both induce cardiac identity and pattern the heart field by specifying regional characteristics (inducing ventricle and suppressing atrial character).
Previous studies have concluded that the endoderm is required for heart development (reviewed in ref. 13). Many of these studies investigated the early anterior-lateral endoderm adjacent to the cardiac mesoderm at HH4-6 (refs 34, 43, 44, 45, 46, 47, 48). Tissue recombination experiments have shown that this endoderm can reprogram non-cardiac tissues to heart identity29 and BMPs, FGFs and inhibition of WNT signalling have been implicated in this13,22,32,34,49,50,51,52,53 (Fig. 5a). Some recombination experiments have used posterior primitive streak or posterior lateral plate (#4–5; Supplementary Figs 6a and 7a) explants as a responding tissue to test for cardiac induction29,49,50,53. However, it has since been shown that these tissues express MYOCD (ref. 43), GATA (ref. 54) and TBX20 (ref. 14) transcription factors, and can express MYOCD and VMHC1/MYH15 following culture in vitro (Supplementary Table 1) leaving open the possibility that these experiments do not demonstrate a true change of fate in this tissue. Widespread expression of some early cardiac markers makes it difficult to find a responding tissue suitable for an induction assay (neither being fated to give rise to heart nor express any of the markers being assessed as a result of the induction). Here we use anterior-medial mesoderm (#3), which is neither fated nor specified as heart20,21,22,23,24 and can be used to assess both induction and patterning rigorously.
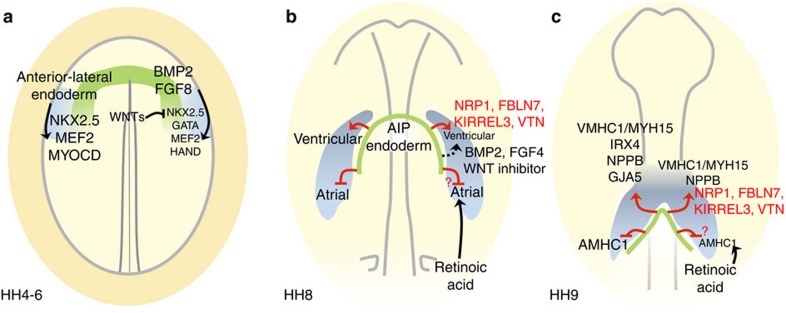
Figure 5. Successive roles of the endoderm in heart induction and patterning.
Each diagram represents a stage in development; in each, tissue interactions are depicted on the left, signals on the right. (a) Initially (HH 4–6), the anterior-lateral endoderm (including some prospective AIP56, green) is required for the adjacent cardiac mesoderm (light blue) to express NKX2.5, MEF2C29 and MYOCD43. BMP2 and FGF8 signalling from the anterior-lateral endoderm induce NKX2.5, GATA, MEF2 and HAND transcription factors22,34,51,52. The anterior endoderm expresses WNT inhibitors including Crescent, which can induce NKX2.5 (ref. 49). (b) At HH8, progenitor cells are becoming determined as either atrial or ventricular28,57,58. The AIP endoderm induces ventricular character and represses atrial identity. Signals from the AIP endoderm, including NRP1, FBLN7, KIRREL3 and VTN described here (red text and arrows) and previously reported BMP2, FGF4 and Wnt inhibitors22,32,49 (black text and arrows), induce some ventricular markers, which are integrated with atrial-inducing retinoic acid signalling from the posterior lateral plate mesoderm28,57,59. The signals from the AIP endoderm that repress atrial identity are unknown. (c) At HH9, when regional markers of anterior–posterior heart tube patterning begin to be expressed, AIP endoderm induces ventricular markers VMHC1/MYH15, IRX4, NPPB and GJA5 (the latter expressed from HH12) and represses the atrial marker AMHC1. Four secreted molecules from the AIP endoderm, NRP1, FBLN7, KIRREL3 and VTN can induce VMHC1/MYH15 and NPPB.
The expression patterns of early cardiac markers, like NKX2.5 (ref. 29), and the signalling molecules previously implicated in their induction (BMP2/4/7 (ref. 22), FGF8 (ref. 34) and CRESCENT (ref. 49)) indicate that initial signals act at an early stage of cardiogenesis (HH4-6, Fig. 5a) before the AIP forms. Our results show that the AIP from a later stage embryo can also induce heart identity (reprogramming non-heart #3-mesoderm from an earlier stage donor) as well as pattern it by specifying ventricular and suppressing atrial character. During normal development, the AIP is likely to be responsible for the later patterning functions (Fig. 5b), but our results show that it can also mimic the activity of earlier endoderm. This could be due to the presence of AIP precursors within this early endoderm21,55,56 and the continued expression of BMP2 (ref. 22) and FGF8 (ref. 34). This is reminiscent of other organizers, for example, Hensen’s node can initiate the entire process of neural induction, even though the first neural inducing signals begin before gastrulation, long before Hensen’s node can be defined38. This could be a general feature of organizers, perhaps because they produce many signals. However, the signals emitted by organizers also change over time as their patterning properties evolve, which is seen in organizers like Hensen’s node11,36, as well as in the AIP.
Heart progenitor cells become determined to either the ventricular or atrial compartment at around HH8 28,57,58(refs 28,57,58), consistent with the timing of signals from the AIP endoderm that induce ventricular and repress atrial character (Fig. 5b). Retinoic acid signalling from the posterior lateral plate mesoderm is known to control the allocation of cardiac progenitor cells to the atrial lineage28,57,59. We have shown that signals from the AIP endoderm, NRP1, FBLN7, KIRREL3 and VTN when combined can sometimes induce VMHC1/MYH15 and NPPB (Fig. 5c), but whether these signals also repress atrial identity remains to be tested. BMP2/4, FGF4 and Wnt inhibitors have also been shown to induce VMHC1 22,32,49(refs 22,32,49), although the results of these studies do not rule out induction of heart identity rather than regionalization. Additional regional markers need to be tested to resolve this. Interestingly, both VTN and NRP1 have other, much later roles in heart patterning.
There is conflicting evidence about the extent to which cells that make up the AIP roll around it (involution) and turn over rapidly, or whether they remain in place as it advances caudally down the embryo19,55,60. Neither possibility is incompatible with the AIP being an organizer. The cellular composition of Hensen’s node constantly changes as cells move in and out of it61 but it also contains resident cells62 and similar findings have been made for the ZPA of the limb63. In all these cases the organizer property, as well as molecular markers for it, remain in the region rather than moving with the cells: cells regulate their expression and functional properties according to their current position61. These findings raise the general principle that organizers define a position within the embryo where a set of properties come together. Using a ‘synexpression’ gene set for this state should not only allow us to seek new organizers, but also to investigate the clues that instruct cells to have such properties in particular places in the embryo.
Our novel approach of identifying genes common to different organizers offers great potential not only for uncovering new organizing centres during development, but also as a tool to identify new signalling molecules underlying organizer function, as well as transcription factors that confer cells with this property. Interestingly, we find that the synexpression set is enriched for signalling molecules (15 of the 31 enriched genes), whereas transcription factors are more abundant among the depleted genes (6/17), suggesting that some transcriptional repressors may function to suppress the organizer state in non-organizer tissues.
Methods
Differential microarray screen and analysis
Fertile Brown Bovan Gold hens' eggs (Henry Stewart, UK) were incubated at 38 °C and staged according to Hamburger and Hamilton (HH)10. The following tissues were collected (three separate samples of each) and RNA extracted by standard methods: Hensen’s Node at HH3+/4 (HH4 HN) and HH5/6 (HH6 HN), posterior primitive streak at HH3+/4 (HH4 PS), HH10/11 notochord and adjacent ventral neural tube/floor-plate (VNT) and the corresponding dorsal neural tube (DNT), the posterior third of the limb at HH20/21 (HH20 PL) and HH24 (HH24 PL) and the anterior third of the limb at HH20/21 (HH20 AL) and HH24 (HH24 AL)64.
Hybridization to Affymetrix microarrays was conducted by ARK-Genomics at the Roslin Institute, University of Edinburgh, as described in the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix, Inc.). Briefly, 15 μg of total RNA from each sample was reverse-transcribed using a T7-oligo (dT) primer in the first-strand complementary DNA (cDNA) synthesis. After RNAase-H treatment, second-strand cDNA synthesis was carried out. The cDNA was then purified and used as a template in the subsequent in vitro transcription reaction for linear amplification of each transcript, and incorporation of biotinylated CTP and UTP using T7 RNA polymerase. The biotinylated cRNA targets were fragmented and hybridized to the Affymetrix GeneChip Chicken Genome Array, which provides good coverage of the chicken genome (32,773 transcripts corresponding to over 28,000 genes). Twenty-seven arrays were used in total (samples from 9 tissues, each in triplicate). Hybridization was performed at 45 °C for 16 h with constant rotation (60 rpm). The microarrays were then automatically washed and stained with Streptavidin-phycoerythrin conjugate (SAPE; Invitrogen) in a GeneChip fluidics station (Affymetrix). Fluorescence intensities were measured with a GeneArray scanner 3,000 (Affymetrix). The scanned images were analysed as described in the Affymetrix GeneChip Command Console 3.0 User Manual.
Gene expression data generated from the GeneChip software (GCOS) were normalized using the probe logarithmic intensity error (PLIER) method65 within the Affymetrix Expression Console software package. The normalized data were then analysed using the Limma and FARMS66 packages within R in Bioconductor. Venn diagrams were generated using the R package Vennerable with dependencies, graph, RBGL, gplots, gtools and reshape. Weights used for scaling the Venn diagrams were based on a priori count data reflecting intersections of genes between different comparisons of datasets.
Dataset comparisons
Pair-wise comparison of samples was performed as follows:
HH4 HN versus HH4 PS;
HH6 HN versus HH4 PS;
HH4 HN versus HH6 HN;
VNT versus DNT;
HH20 PL versus HH20 AL;
HH24 PL versus HH24 AL.
Probes with a fold change (FC) ≥1.2 (log2) and a false discovery rate ≤0.05 were deemed significant. The results of these individual comparisons (using significant enrichment or depletion as the indicator, rather than absolute amounts) were then combined using the Boolean algorithm: ((HH4 HN versus HH4 PS AND (HH4 HN versus HH6 HN) OR HH6 HN versus HH4 PS)) AND VNT versus DNT AND (HH20 PL versus HH20 AL OR HH24 PL versus HH24 AL). Probes that are differentially enriched or depleted significantly in this algorithm comprise the organizer gene set. The first set of comparisons selects genes enriched or depleted in the early (HH3+/4) Hensen’s node compared with the posterior streak, some of which remain expressed the same way at the later (HH5/6) stage. The comparisons for the limb select genes enriched or depleted in the posterior limb bud (containing the ZPA) at either HH20/21 or HH24.
The dataset was submitted to ArrayExpress with the title ‘Microarray analysis of chick embryonic tissues: gastrulation, neural tube/notochord and limb development’ and given accession number E-MTAB-4048.
Embryo manipulation
Fertilized Brown Bovan Gold hens’ eggs (Henry Stewart, UK), Coturnix coturnix japonica quails’ eggs (B.C. Potter, Rosedean Farm, UK, and Blue Bridge Engineering Limited, Essex, UK) and transgenic GFP chicken eggs67 (Roslin Institute, Edinburgh, UK) were used. Embryo manipulations were performed in Pannett-Compton saline and modified New culture68,69. Tissues were excised from donor embryos using 0.12% w/v trypsin (porcine trypsin 1:250, Sigma) in Pannett-Compton saline and 30 G syringe needles, washed with saline and kept on ice until needed. Mesoderm (#1–5, from both the left and the right sides and used randomly) was harvested from HH5− to HH5+ donor embryos. Early AIP endoderm (AIP) and control (Cont.) lateral endoderm (the endoderm underlying the somites and proximal lateral plate mesoderm) were harvested from HH8− to HH8+ embryos. Early AIP endoderm was used because cardiac progenitors are closely associated with the AIP at this time, and because it is easier to isolate the AIP at this stage than later, when the heart is beating. Anterior-lateral endoderm (#1-2E) and anterior-medial endoderm (#3E) were harvested from HH5 donor embryos. Host embryos ranged from HH4+ to HH6. Grafts were placed on the left or the right side of host embryos; no differences were noted in the results. After grafting, the embryos were incubated for either 6–9 h or overnight (12–18 h). Limb grafts were performed in ovo at HH20-21 and incubated for 5 days70.
Delivery of factors using transfected cells
Chicken NRP1 (NM_204782.1; F primer CCGCTCTCGGAAGG, R primer CATCCCGATTCTCTG), FBLN7 (XP_003640934.1, formerly FIBULIN 7-LIKE; F primer GGACCATGGCTTCGGGGCTC, R primer CCTGGTCTGCCCTAGAACTCATAGGC), VTN (NM_205061.1; F primer CTCTGGATCCTGCTCAGTCACAGTAG with introduced BamHI site, R primer CTGGCGGTGAATTCGGGTCTAGC with introduced EcoRI site) and KIRREL3 (XP_004948018.1; F primer CCTGAGGAATGAGCGCTTTC, R primer GTTCAAACGTGCGTCTGCATC) were cloned by RT-PCR using cDNA synthesized from HH13/14 embryo mRNA. Full-length cDNAs were inserted into the pCAβ-IRES-GFP expression vector.
HEK293T cells were grown in DMEM (Gibco)+10% fetal bovine serum (FBS), 1% GlutaMAX (Gibco) and 1% penicillin/streptomycin (Gibco), and transfected with appropriate expression vectors using polyethylenimine71. Pellets of 500 cells were made by recombining cells from individual transfections in a hanging drop culture72.
In vitro culture of explants
Tissues were excised from chicken and quail embryos as described above, and cultured in vitro for 24 or 48 h at 37 °C with 5% CO2 on a 0.4 μm Nucleopore filter (Whatman, WHA110407) floating on DMEM (Gibco) with 10% FBS, 5% chicken embryo extract (US Biological), 1% GlutaMAX (Gibco) and 1% Penicillin/Streptomycin (Gibco)29. Tissues were arranged such that the basal surface of the AIP faced the mesoderm explants. Spontaneous beating in 48 h explant co-cultures was recorded with a QImaging Retiga 2000R camera and QCapture Pro software.
Probes and antibodies
Whole-mount in situ hybridization and immunohistochemistry were performed as described73,74. Probes used were as follows: MYOCD (ref. 43), TBX5 (ref. 75), IRX4 (ref. 26), ISL1 (ref. 76), PITX2 (ref. 77), NXPH1 (ref. 78), NRP1 (ref. 79), NOTO (cNOT1) (ref. 80), VTN (ref. 81), MOXD1 (DBHR) (ref. 82), CXCL12 (SDF1) (ref. 83), ID2 (ref. 84), DLX6 (ref. 85) and SHOX2 (ref 86), kindly provided by the originating laboratories. AMHC1 and NKX2.5 were kindly provided by T. Brand and GATA4 by B. Pain. The cDNA available for VMHC1 (MYH15)27 is a 2.5 kb cDNA, which also has significant (73%) homology to MYH7b (Slow Myosin 2, SM2); therefore, a short 0.6 kb probe (VMHC1/MYH15, generated after cutting with PstI, where the two sequences differ most) was used instead (see VMHC1/MYH15 short in Supplementary Fig. 6). Other probes, including MEF2C (ChEST776g19), NPPB (ANF; ChEST509m15) and GJA5 (CX40; ChEST304a4), were transcribed from EST clones (Supplementary Data 1)87. Following whole-mount in situ hybridization, embryos were incubated in QCPN (Developmental Studies Hybridoma Bank, diluted 1:5) and/or anti-GFP antibody (Life Technologies, A11122, diluted 1:2,000); secondary antibodies used were goat anti-mouse IgG peroxidase (Jackson, 115-035-003), goat anti-rabbit IgG peroxidase (Santa Cruz, sc-2004) or goat anti-rabbit IgG alkaline phosphatase (Upstate, 12–448) diluted 1:1,000. Cryosections of 15 μm or paraffin sections of 10 μm were prepared. For skeletal staining, embryos were stained with Alcian Blue88.
Scoring of co-cultures
Explant cultures (Supplementary Tables 1–6) were considered ‘positive’ if high-level expression was observed throughout the majority of the explant. The n-numbers in the figures represent those that were scored as positive. The n-numbers that include positive and patchy expression are indicated. Explants with no detectable expression or very weak expression (perhaps due to non-specific staining, constitutive expression and/or marginal induction) were scored as negative. Explants with high-level expression in a small patch (some of which could be due to contamination) were excluded from P-value calculations, unless indicated. To calculate P values, positive (plus patchy where specified) and negative counts were compared across two conditions (#3 alone versus #3+AIP or #3+Cont.) using two-tailed Fisher's exact test with a 2 × 2 contingency table. P values ≤0.05 were deemed significant.
Data availability
The microarray data have been deposited in the ArrayExpress database under accession code E-MTAB-4048. All other data supporting the findings of this study are available within the article and its Supplementary information files or from the corresponding author upon reasonable request.
Additional information
How to cite this article: Anderson, C. et al. A strategy to discover new organizers identifies a putative heart organizer. Nat. Commun. 7:12656 doi: 10.1038/ncomms12656 (2016).
Supplementary Material
Supplementary Figures 1-12, Supplementary Tables 1-6
Spontaneous beating in explants of #3-mesoderm after 48 hours of in vitro co-culture with AIP.
A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set.
Acknowledgments
This study was funded by BBSRC and ERC. We thank Angel Pang, Jiahui Liu, Andrew Bain, Monique Welten and Lynn McTeir for help with in situ hybridization, Megan Davey for comments on the manuscript, Duncan Davidson for an anatomical ontology, Michael Wicks for enhancing the eChickAtlas database, Federica Bertocchini for tissue collection, Melanie Krause for help with cell pellet grafts and Irene de Almeida for help with cloning.
Footnotes
The authors declare no competing financial interests.
Author contributions M.A.F.K. produced the Venn diagrams and heatmap. F.W., D.W.B. and M.A.F.K. analysed the microarray data. T.S. performed some of the limb stage in situ hybridizations and cloned the VTN expression plasmid. N.M.M.O. performed cell transfections and generated the cell pellets. C.D.S., D.W.B., C.T. and R.A.B. conceived the project and obtained funding. C.T. performed the limb stage tissue collections. C.A. performed all other experiments. C.D.S. designed the screen, performed the Boolean comparisons and participated in the analysis of other data. C.A. and C.D.S. wrote the manuscript.
References
- Gurdon J. B. Embryonic induction–molecular prospects. Development 99, 285–306 (1987). [DOI] [PubMed] [Google Scholar]
- Spemann H. & Mangold H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Roux' Arch. EntwMech. Org. 100, 599–638 (1924). [Google Scholar]
- Anderson C. & Stern C. D. Organizers in development. Curr. Top. Dev. Biol. 117, 435–454 (2016). [DOI] [PubMed] [Google Scholar]
- Waddington C. H. & Schmidt G. A. Induction by heteroplastic grafts of the primitive streak in birds. W. Roux Arch. Entwicklungsmech. Org. 128, 522–563 (1933). [DOI] [PubMed] [Google Scholar]
- van Straaten H. W., Hekking J. W., Thors F., Wiertz-Hoessels E. L. & Drukker J. Induction of an additional floor plate in the neural tube. Acta Morphol. Neerl. Scand. 23, 91–97 (1985). [PubMed] [Google Scholar]
- Saunders J. W. J. & Gasseling M. T. in Epithelial-Mesenchymal Interactions (eds Fleischmeyer, R. & Billingham, R. E.) 78–97 (Williams & Wilkins, 1968).
- Martinez S., Wassef M. & Alvarado-Mallart R. M. Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene en. Neuron 6, 971–981 (1991). [DOI] [PubMed] [Google Scholar]
- Hornbruch A. & Wolpert L. Positional signalling by Hensen's node when grafted to the chick limb bud. J. Embryol. Exp. Morphol. 94, 257–265 (1986). [PubMed] [Google Scholar]
- Bellairs R. Studies on the development of the foregut in the chick blastoderm. 1. The presumptive foregut area. J. Embryol. Exp. Morph. 1, 115–124 (1953). [Google Scholar]
- Hamburger V. & Hamilton H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951). [PubMed] [Google Scholar]
- Storey K. G., Crossley J. M., De Robertis E. M., Norris W. E. & Stern C. D. Neural induction and regionalisation in the chick embryo. Development 114, 729–741 (1992). [DOI] [PubMed] [Google Scholar]
- Wong F. et al. eChickAtlas: an introduction to the database. Genesis 51, 365–371 (2013). [DOI] [PubMed] [Google Scholar]
- Lough J. & Sugi Y. Endoderm and heart development. Dev. Dyn. 217, 327–342 (2000). [DOI] [PubMed] [Google Scholar]
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev. Biol. 258, 1–19 (2003). [DOI] [PubMed] [Google Scholar]
- Pfister S. et al. Sox17-dependent gene expression and early heart and gut development in Sox17-deficient mouse embryos. Int. J. Dev. Biol. 55, 45–58 (2011). [DOI] [PubMed] [Google Scholar]
- Kirby M. L. et al. Hensen's node gives rise to the ventral midline of the foregut: implications for organizing head and heart development. Dev. Biol. 253, 175–188 (2003). [DOI] [PubMed] [Google Scholar]
- Narita N., Bielinska M. & Wilson D. B. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev. Biol. 189, 270–274 (1997). [DOI] [PubMed] [Google Scholar]
- Li S., Zhou D., Lu M. M. & Morrisey E. E. Advanced cardiac morphogenesis does not require heart tube fusion. Science 305, 1619–1622 (2004). [DOI] [PubMed] [Google Scholar]
- Varner V. D. & Taber L. A. Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly. Development 139, 1680–1690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redkar A., Montgomery M. & Litvin J. Fate map of early avian cardiac progenitor cells. Development 128, 2269–2279 (2001). [DOI] [PubMed] [Google Scholar]
- Rosenquist G. C. A Radioautographic study of labelled grafts in the chick blastoderm. Development from primitive-streak stages to stage 12. Contrib. Embryol. Carnegie Inst. Wash. 38, 71–110 (1966). [Google Scholar]
- Schultheiss T. M., Burch J. B. & Lassar A. B. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 11, 451–462 (1997). [DOI] [PubMed] [Google Scholar]
- Rosenquist G. C. & DeHaan R. L. Migration of precardiac cells in the chick embryo: a radioautographic study. Contrib. Embryol. Carnegie Inst. Wash. 38, 111–121 (1966). [Google Scholar]
- Rawles M. E. The heart-forming areas of the early chick blastoderm. Physiol. Zool. 16, 22–43 (1943). [Google Scholar]
- Houweling A. C., Somi S., Van Den Hoff M. J., Moorman A. F. & Christoffels V. M. Developmental pattern of ANF gene expression reveals a strict localization of cardiac chamber formation in chicken. Anat. Rec. 266, 93–102 (2002). [DOI] [PubMed] [Google Scholar]
- Bao Z. Z., Bruneau B. G., Seidman J. G., Seidman C. E. & Cepko C. L. Regulation of chamber-specific gene expression in the developing heart by Irx4. Science 283, 1161–1164 (1999). [DOI] [PubMed] [Google Scholar]
- Bisaha J. G. & Bader D. Identification and characterization of a ventricular-specific avian myosin heavy chain, VMHC1: expression in differentiating cardiac and skeletal muscle. Dev. Biol. 148, 355–364 (1991). [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Rhee J. T. & Bader D. Expression of the atrial-specific myosin heavy chain AMHC1 and the establishment of anteroposterior polarity in the developing chicken heart. Development 120, 871–883 (1994). [DOI] [PubMed] [Google Scholar]
- Schultheiss T. M., Xydas S. & Lassar A. B. Induction of avian cardiac myogenesis by anterior endoderm. Development 121, 4203–4214 (1995). [DOI] [PubMed] [Google Scholar]
- Wang G. F., Nikovits W., Bao Z. Z. & Stockdale F. E. Irx4 forms an inhibitory complex with the vitamin D and retinoic X receptors to regulate cardiac chamber-specific slow MyHC3 expression. J. Biol. Chem. 276, 28835–28841 (2001). [DOI] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T. & Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature 345, 819–822 (1990). [DOI] [PubMed] [Google Scholar]
- Lopez-Sanchez C., Climent V., Schoenwolf G. C., Alvarez I. S. & Garcia-Martinez V. Induction of cardiogenesis by Hensen's node and fibroblast growth factors. Cell. Tissue. Res. 309, 237–249 (2002). [DOI] [PubMed] [Google Scholar]
- Streit A. et al. Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development 124, 1191–1202 (1997). [DOI] [PubMed] [Google Scholar]
- Alsan B. H. & Schultheiss T. M. Regulation of avian cardiogenesis by Fgf8 signaling. Development 129, 1935–1943 (2002). [DOI] [PubMed] [Google Scholar]
- Linker C. & Stern C. D. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development 131, 5671–5681 (2004). [DOI] [PubMed] [Google Scholar]
- Pinho S. et al. Distinct steps of neural induction revealed by Asterix, Obelix and TrkC, genes induced by different signals from the organizer. PLoS ONE 6, e19157 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle C. The number of polarizing region cells required to specify additional digits in the developing chick wing. Nature 289, 295–298 (1981). [DOI] [PubMed] [Google Scholar]
- Streit A., Berliner A. J., Papanayotou C., Sirulnik A. & Stern C. D. Initiation of neural induction by FGF signalling before gastrulation. Nature 406, 74–78 (2000). [DOI] [PubMed] [Google Scholar]
- Crossley P. H., Martinez S. & Martin G. R. Midbrain development induced by FGF8 in the chick embryo. Nature 380, 66–68 (1996). [DOI] [PubMed] [Google Scholar]
- Echelard Y. et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430 (1993). [DOI] [PubMed] [Google Scholar]
- Riddle R. D., Johnson R. L., Laufer E. & Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401–1416 (1993). [DOI] [PubMed] [Google Scholar]
- Zhang X. M., Ramalho-Santos M. & McMahon A. P. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell 105, 781–792 (2001). [PubMed] [Google Scholar]
- Warkman A. S., Yatskievych T. A., Hardy K. M., Krieg P. A. & Antin P. B. Myocardin expression during avian embryonic heart development requires the endoderm but is independent of BMP signaling. Dev. Dyn. 237, 216–221 (2008). [DOI] [PubMed] [Google Scholar]
- Orts-Llorca F. Influence of the endoderm on heart differentiation during the early stages of development of the chicken embryo. Roux' Arch. EntwMech. 154, 533–551 (1963). [DOI] [PubMed] [Google Scholar]
- Orts-Llorca F. Influence of the endoderm on heart differentiation. Roux' Arch. EntwMech. 156, 368–370 (1965). [DOI] [PubMed] [Google Scholar]
- Yamazaki Y. & Hirakow R. Factors required for differentiation of chick precardiac mesoderm cultured invitro. Proc. Jpn Acad. B–Phys. 67, 165–169 (1991). [Google Scholar]
- Sugi Y. & Lough J. Anterior endoderm is a specific effector of terminal cardiac myocyte differentiation of cells from the embryonic heart forming region. Dev. Dyn. 200, 155–162 (1994). [DOI] [PubMed] [Google Scholar]
- Gannon M. & Bader D. Initiation of cardiac differentiation occurs in the absence of anterior endoderm. Development 121, 2439–2450 (1995). [DOI] [PubMed] [Google Scholar]
- Marvin M. J., Di Rocco G., Gardiner A., Bush S. M. & Lassar A. B. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 15, 316–327 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough J. et al. Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev. Biol. 178, 198–202 (1996). [DOI] [PubMed] [Google Scholar]
- Schlange T., Andree B., Arnold H. H. & Brand T. BMP2 is required for early heart development during a distinct time period. Mech. Dev. 91, 259–270 (2000). [DOI] [PubMed] [Google Scholar]
- Andree B., Duprez D., Vorbusch B., Arnold H. H. & Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev. 70, 119–131 (1998). [DOI] [PubMed] [Google Scholar]
- Barron M., Gao M. & Lough J. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Dev. Dyn. 218, 383–393 (2000). [DOI] [PubMed] [Google Scholar]
- Jiang Y., Tarzami S., Burch J. B. & Evans T. Common role for each of the cGATA-4/5/6 genes in the regulation of cardiac morphogenesis. Dev. Genet. 22, 263–277 (1998). [DOI] [PubMed] [Google Scholar]
- Bellairs R. Studies on the development of the foregut in the chick blastoderm. 2. The morphogenetic movements. J. Embryol. Exp. Morphol. 1, 369–385 (1953). [Google Scholar]
- Kimura W., Yasugi S., Stern C. D. & Fukuda K. Fate and plasticity of the endoderm in the early chick embryo. Dev. Biol. 289, 283–295 (2006). [DOI] [PubMed] [Google Scholar]
- Hochgreb T. et al. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development 130, 5363–5374 (2003). [DOI] [PubMed] [Google Scholar]
- Orts-Llorca F., Collado J. J. & Dehaan R. L. Determination of heart polarity (arterio venous axis) in the chicken embryo. W. Roux Arch. Entwicklungsmech. Org. 158, 147–163 (1967). [DOI] [PubMed] [Google Scholar]
- Heine U. I., Roberts A. B., Munoz E. F., Roche N. S. & Sporn M. B. Effects of retinoid deficiency on the development of the heart and vascular system of the quail embryo. Virchows. Arch. B. Cell. Pathol. Incl. Mol. Pathol. 50, 135–152 (1985). [DOI] [PubMed] [Google Scholar]
- Stalsberg H. & DeHaan R. L. Endodermal movements during foregut formation in the chick embryo. Dev. Biol. 18, 198–215 (1968). [DOI] [PubMed] [Google Scholar]
- Joubin K. & Stern C. D. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell 98, 559–571 (1999). [DOI] [PubMed] [Google Scholar]
- Selleck M. A. & Stern C. D. Fate mapping and cell lineage analysis of Hensen's node in the chick embryo. Development 112, 615–626 (1991). [DOI] [PubMed] [Google Scholar]
- Harfe B. D. et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528 (2004). [DOI] [PubMed] [Google Scholar]
- Bangs F. et al. Identification of genes downstream of the Shh signalling in the developing chick wing and syn-expressed with Hoxd13 using microarray and 3D computational analysis. Mech. Dev. 127, 428–441 (2010). [DOI] [PubMed] [Google Scholar]
- Affymetrix. Guide to probe logarithmic error (PLIER) estimation. http://media.affymetrix.com/support/technical/technotes/plier_technote.pdf (2005).
- Talloen W. et al. I/NI-calls for the exclusion of non-informative genes: a highly effective filtering tool for microarray data. Bioinformatics 23, 2897–2902 (2007). [DOI] [PubMed] [Google Scholar]
- Sang H. Prospects for transgenesis in the chick. Mech. Dev. 121, 1179–1186 (2004). [DOI] [PubMed] [Google Scholar]
- New D. A. T. A new technique for the cultivation of the chick embryo invitro. J. Embryol. Exp. Morphol. 3, 320–332 (1955). [Google Scholar]
- Stern C. D. & Ireland G. W. An integrated experimental study of endoderm formation in avian embryos. Anat. Embryol. 163, 245–263 (1981). [DOI] [PubMed] [Google Scholar]
- Tickle C. Grafting of apical ridge and polarizing region. Methods Mol. Biol. 97, 281–291 (1999). [DOI] [PubMed] [Google Scholar]
- Boussif O. et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA 92, 7297–7301 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. & Stern C. D. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech. Dev. 85, 85–96 (1999). [DOI] [PubMed] [Google Scholar]
- Streit A. & Stern C. D. Combined whole-mount in situ hybridization and immunohistochemistry in avian embryos. Methods 23, 339–344 (2001). [DOI] [PubMed] [Google Scholar]
- Stern C. D. Detection of multiple gene products simultaneously by in situ hybridization and immunohistochemistry in whole mounts of avian embryos. Curr. Top. Dev. Biol. 36, 223–243 (1998). [DOI] [PubMed] [Google Scholar]
- Isaac A. et al. Tbx genes and limb identity in chick embryo development. Development 125, 1867–1875 (1998). [DOI] [PubMed] [Google Scholar]
- Pfaff S. L., Mendelsohn M., Stewart C. L., Edlund T. & Jessell T. M. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84, 309–320 (1996). [DOI] [PubMed] [Google Scholar]
- Logan M., Pagan-Westphal S. M., Smith D. M., Paganessi L. & Tabin C. J. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell 94, 307–317 (1998). [DOI] [PubMed] [Google Scholar]
- Apostolova G. et al. Neurotransmitter phenotype-specific expression changes in developing sympathetic neurons. Mol. Cell. Neurosci. 35, 397–408 (2007). [DOI] [PubMed] [Google Scholar]
- Herzog Y., Kalcheim C., Kahane N., Reshef R. & Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech. Dev. 109, 115–119 (2001). [DOI] [PubMed] [Google Scholar]
- Stein S., Niss K. & Kessel M. Differential activation of the clustered homeobox genes CNOT2 and CNOT1 during notogenesis in the chick. Dev. Biol. 180, 519–533 (1996). [DOI] [PubMed] [Google Scholar]
- Pons S. & Marti E. Sonic hedgehog synergizes with the extracellular matrix protein vitronectin to induce spinal motor neuron differentiation. Development 127, 333–342 (2000). [DOI] [PubMed] [Google Scholar]
- Knecht A. K. & Bronner-Fraser M. DBHR, a gene with homology to dopamine beta-hydroxylase, is expressed in the neural crest throughout early development. Dev. Biol. 234, 365–375 (2001). [DOI] [PubMed] [Google Scholar]
- Rehimi R. et al. Stromal-derived factor-1 (SDF-1) expression during early chick development. Int. J. Dev. Biol. 52, 87–92 (2008). [DOI] [PubMed] [Google Scholar]
- Martinsen B. J. & Bronner-Fraser M. Neural crest specification regulated by the helix-loop-helix repressor Id2. Science 281, 988–991 (1998). [DOI] [PubMed] [Google Scholar]
- Hsu S. H. et al. Dlx5- and Dlx6-mediated chondrogenesis: differential domain requirements for a conserved function. Mech. Dev. 123, 819–830 (2006). [DOI] [PubMed] [Google Scholar]
- Tiecke E. et al. Expression of the short stature homeobox gene Shox is restricted by proximal and distal signals in chick limb buds and affects the length of skeletal elements. Dev. Biol. 298, 585–596 (2006). [DOI] [PubMed] [Google Scholar]
- Boardman P. E. et al. A comprehensive collection of chicken cDNAs. Curr. Biol. 12, 1965–1969 (2002). [DOI] [PubMed] [Google Scholar]
- McLeod M. J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299–301 (1980). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-12, Supplementary Tables 1-6
Spontaneous beating in explants of #3-mesoderm after 48 hours of in vitro co-culture with AIP.
A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set.
Data Availability Statement
The microarray data have been deposited in the ArrayExpress database under accession code E-MTAB-4048. All other data supporting the findings of this study are available within the article and its Supplementary information files or from the corresponding author upon reasonable request.