Abstract
MR findings of early infectious spondylodiscitis are non-specific and may be confused with those of other conditions. Therefore, it is important to recognize early MR signs of conditions, such as inappreciable cortical changes in endplates, confusing marrow signal intensities of vertebral bodies, and inflammatory changes in paraspinal soft tissues, and subligamentous and epidural spaces. In addition, appreciation of direct inoculation, such as in iatrogenic spondylodiscitis may be important, because the proportion of patients who have undergone recent spine surgery or a spinal procedure is increasing. In this review, the authors focus on the MR findings of early spondylodiscitis, atypical findings of iatrogenic infection, and the differentiation between spondylodiscitis and other disease entities mimicking infection.
Keywords: Spine infection, Magnetic resonance imaging, Spondylodiscitis, Iatrogenic infection
INTRODUCTION
The early presentations of spondylodiscitis may be ignored or mistaken for a neoplasm, traumatic condition, or other acute inflammatory conditions, such as Modic change or an acute Schmorl's node, because magnetic resonance (MR) findings can be non-specific or atypical. Furthermore, iatrogenic infectious conditions may cause abnormal MR signal intensities at unusual sites, and laboratory evaluations are not always reliable, although erythrocyte sedimentation rate (ESR) and C-reactive protein are often elevated (1).
MRI should be performed as soon as infection is suspected because it has high sensitivity and specificity for the detection of early infection (2). Furthermore, when the diagnosis is uncertain, early follow-up MRI may enable visualization of early changes, which is critical because delayed diagnosis can increase the mortality and morbidity rates (3,4,5). Typically, spinal infection presents as involvement of two contiguous vertebrae and inflammatory change in the intervening disc, but it can take weeks or months for these findings to develop (6). The other imaging features, such as paraspinal or epidural enhancement, a hyperintense T2 disc signal, and erosion of at least one vertebral endplate have good sensitivity (> 80%) for diagnosing spinal infections (7), but they are not always seen on MR images in the early stage.
The purpose of this review was to evaluate the MR findings of early spondylodiscitis, atypical findings of iatrogenic infection, and the differentiation between spondylodiscitis and other disease entities mimicking infection.
Pathogenesis
Vascular development in the spine exhibits age-related patterns. In children, intraosseous arteries display extensive anastomoses and vascular channels penetrate the discs, and therefore, a septic embolus is unlikely to produce a substantial osseous infarct and infection is often limited to the disc (Fig. 1). In contrast, in adults, the disc is avascular and intraosseous anastomosis is complexified by the third decade of life to create end arteries, in which a septic embolus might lead to a large infarct in the vertebral body (8). In addition, subsequent spread of infection to the neighboring disc or vertebra can create a lesion typical of spondylodiscitis (Fig. 2) (9,10,11). In the elderly, disc degeneration may be followed by the ingrowth of vascularized granulation tissue, causing secondary vascularization of the disc material, which is the reason why primary discitis is possible in older patients (Fig. 3) (2). However, single discitis is an uncommon finding on images regardless of patient age.
Fig. 1. Pyogenic discitis.
9-year-old girl received chemotherapy for leukemia. Sagittal fat-suppressed, contrast-enhanced T1-weighted image showed peripheral rim enhancement (arrows) of L4–5 and L5–S1 discs.
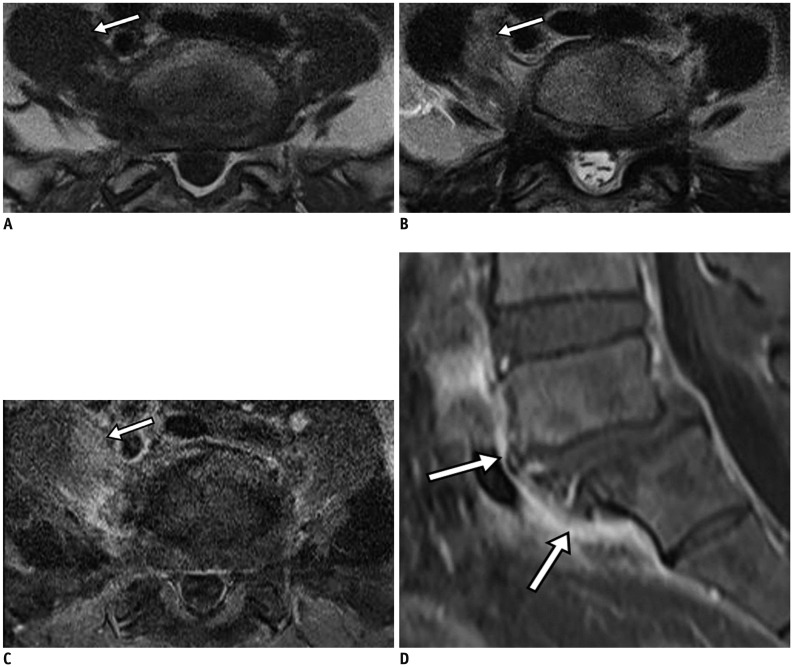
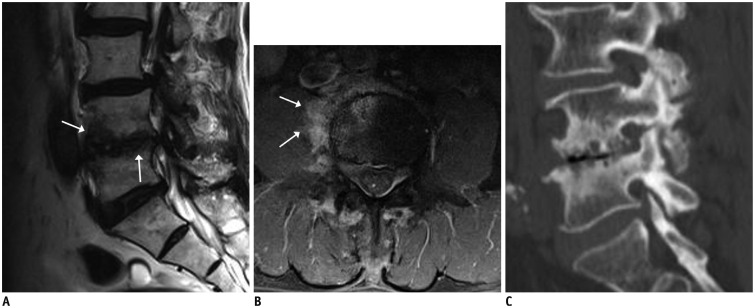
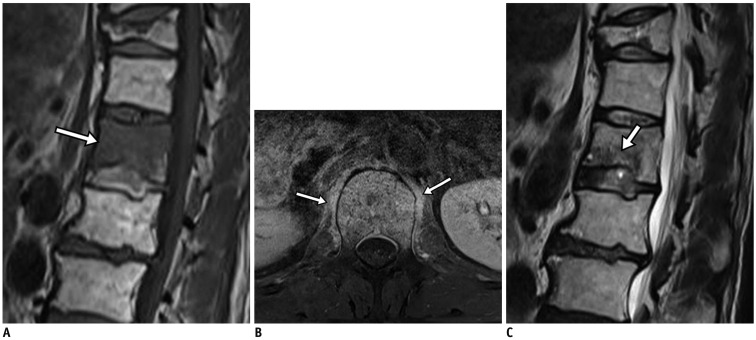
Fig. 2. Pyogenic (enterococcal infection) spondylodiscitis.
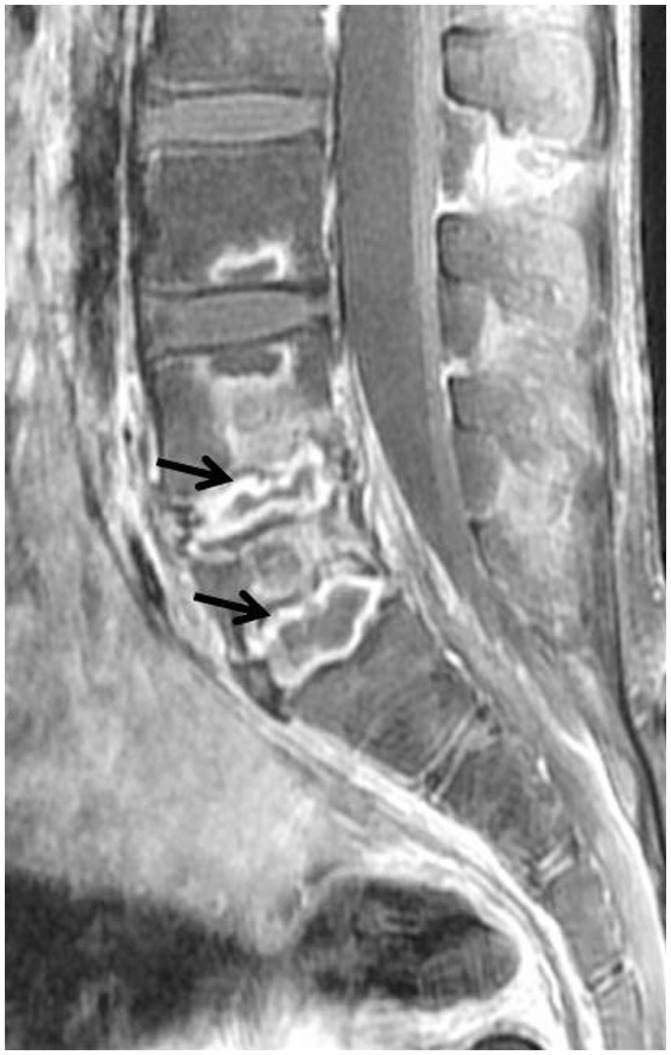
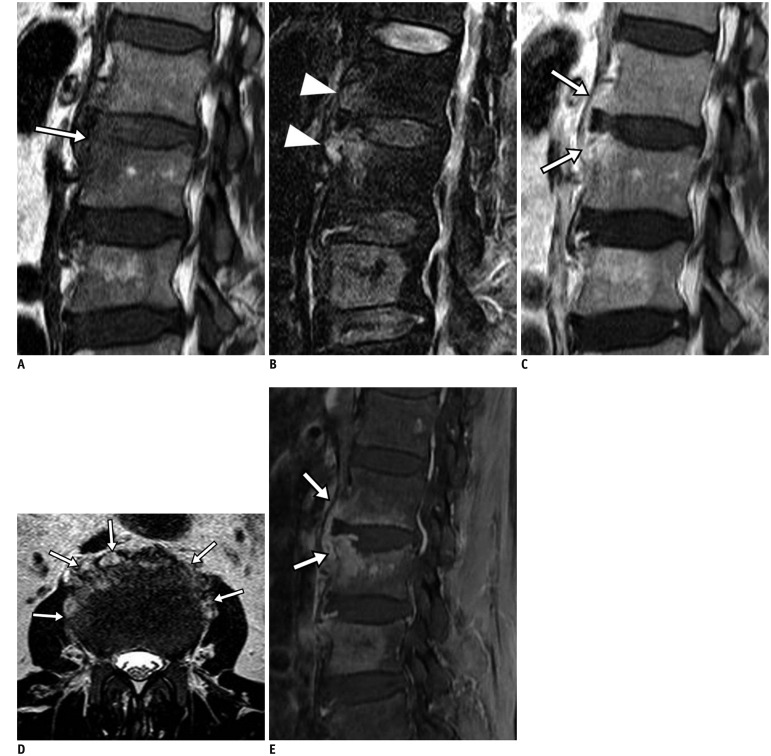
75-year-old man with back pain and absence of fever. Focal cortical discontinuity (arrow) was visualized on sagittal T1-weighted imaging (WI) (A). Subtle marrow signal changes (arrowheads) were noted in anterior subchondral areas of L2 and L3 bodies on fat-suppressed, sagittal T2WI (B). Also, faint subligamentous enhancement (arrows) was observed on fat-suppressed, contrast-enhanced sagittal T1WI (C), representing high degree of suspicion of early infectious condition. However, formation of multiple spurs (arrows), representing more degenerative changes, were prominent on axial T2WI (D). On sagittal fat-suppressed, contrast-enhanced T1WI (E) obtained 2 weeks later, infectious process was found to be aggravated.
Fig. 3. Pyogenic spondylodiscitis.
68-year-old man with lower back pain. Fat-suppressed sagittal T2-weighted image (A) showed bright high signal intensity (arrow) of L1–2 disc, which was well enhanced (arrow) on fat-suppressed, contrast-enhanced sagittal T1-weighted imaging (B). Fat-suppressed, contrast-enhanced axial T1-weighted image (C) demonstrated enhancement of paraspinal soft tissue and peripheral portion of disc (arrows).
Spondylodiscitis may result from hematogenous spread, direct inoculation, or inoculation from nearby infective tissues (4,9,12,13), and the most common cause is hematogenous spread via an arterial route (1,2).
Arteries supplying the vertebrae are derived from vertebral, intercostal, lumbar, or sacral arteries, and they are located on the anterolateral surfaces of vertebral bodies. Fine arterioles from this network ramify within the vertebral bodies and through central nutrient foramen and are most abundant at the endplates, especially in the anterior subchondral region where the primary focus of infective change usually begins (Fig. 4) (2,14,15). Vertebral osteomyelitis resulting from an infected microembolus in the arterial system, which becomes lodged in a metaphyseal artery and causes infarction and subsequent infection is the most widely accepted hypothesis for the pathogenesis of spondylodiscitis (11). Hematogenous infection rarely causes pyogenic infection in the posterior vertebral elements due to their relatively poor blood supply as compared with vertebral bodies (16), and posterior involvement is more common in tuberculous and fungal spondylitis than in pyogenic spondylitis, which can make it difficult to differentiate infection from tumor (9,17). However, in cases with single involvement of a posterior element, we usually consider tumorous conditions rather than infectious conditions (18).
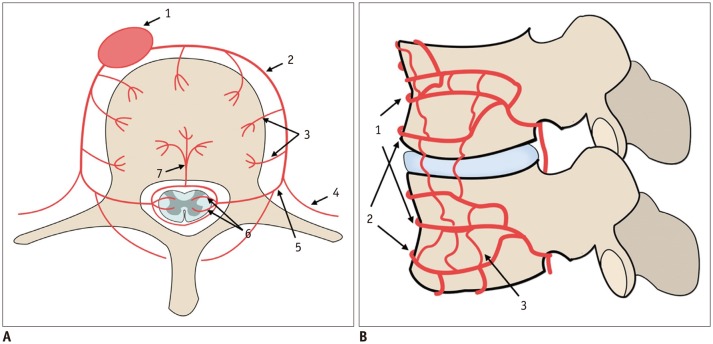
Fig. 4. Schematic illustrations representing vertebral arterial system.
A. Axial schematic figure of lumbar vertebra: 1, aorta; 2, segmental artery; 3, direct vertebral body feeding artery; 4, intercostal/muscular artery; 5, dorsal spinal artery; 6, radicular artery; 7, nutrient artery. B. Sagittal schematic figure of lumbar vertebra: 1, segmental artery; 2, metaphyseal anastomosis; 3, intermetaphyseal anastomosis.
Direct inoculation develops in response to a local contaminating event affecting the discs or vertebrae (19), and it may occur in cases of penetrating trauma or direct exposure related to skin breakdown or an open wound (20). Iatrogenic direct inoculation is usually caused during surgery, interventional or diagnostic procedures (e.g., lumbar puncture or discography), pain management procedures (e.g., epidural, nerve, or facet block), vertebroplasty, kyphoplasty, or indwelling catheter use (4,21,22,23). The vertebral structures that are primarily involved are determined by the procedure used (2), and therefore, posterior regions are usually affected. An appreciation of iatrogenic spondylodiscitis is important because the number of patients who have undergone spine surgery or some other procedure continues to increase (24).
Inoculation from nearby infectious tissues is rare and it is often difficult to distinguish it from direct inoculation (2), which may occur from an adjacent infective focus, but more commonly from an aortic graft infection, a ruptured esophagus, or a retropharyngeal abscess (9).
MR Imaging Protocols
MRI is the most useful modality in imaging of spine infections. The MRI protocols used and their clinical efficacies for evaluating spondylodiscitis are summarized in Table 1. Standard MRI protocols should routinely include fluid-sensitive sequences (i.e., fat-suppressed T2-weighted imaging [T2WI] or short-tau inversion recovery). These sequences are highly sensitive for detecting early inflammatory edema and are better than T2W spin-echo sequences in terms of demonstrating initial infectious foci (2). Moreover, the addition of pre- and post-contrast fat-suppressed T1-weighted imaging (T1WI), allows visualization of anatomical details and differentiation of vascularized and nonvascularized necrotic inflammatory components, such as abscesses (2,25). On the other hand, differentiation of true inflammatory and fatty signal changes in the psoas muscle and in the paraspinal area is important, and therefore, a comparison of T1WI and T2WI is necessary, especially in the absence of contrast-enhanced images (Fig. 5) (3). T1WI is also useful for detecting inflammation when a fat-suppression technique is not included or incomplete fat-suppression is suspected. Therefore, it is essential to obtain T1WI and T2WI in the sagittal and axial planes. However, although spinal epiduritis can be easily identified on contrast-enhanced images, it is often missed on unenhanced T1WI and T2WI (26).
Table 1. MRI Protocols and Their Efficacies for Evaluating Spondylodiscitis.
| Protocol | Efficacies |
|---|---|
| Fat suppression sequence | Applied to T2WI & post-gadolinium T1WI |
| Improves contrast between hyperemic osseous or soft tissue components and surrounding normal structures -improves conspicuity of infected areas | |
| T1WI | Edema-type - hypointensity of bone marrow |
| Cortical change of endplates | |
| T2WI/STIR | Edema-type - hyperintensity of bone marrow |
| Disc involvement - hyperintensity (fluid-like signal) of disc, loss of normal intranuclear cleft | |
| Involved paraspinal soft tissues - hyperintensity | |
| Frank abscess - hyperintense fluid collection | |
| Contrast enhancement | Edema-type - bone marrow enhancement |
| Disc involvement - variable enhancement pattern of disc | |
| Involved paraspinal or epidural inflammatory tissues - diffuse enhancement (phlegmon) | |
| Frank abscess - peripheral rim enhancement | |
| PDWI | May be helpful for visualizing epidural extension |
PDWI = proton-density weighted imaging, STIR = short-tau inversion recovery, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging
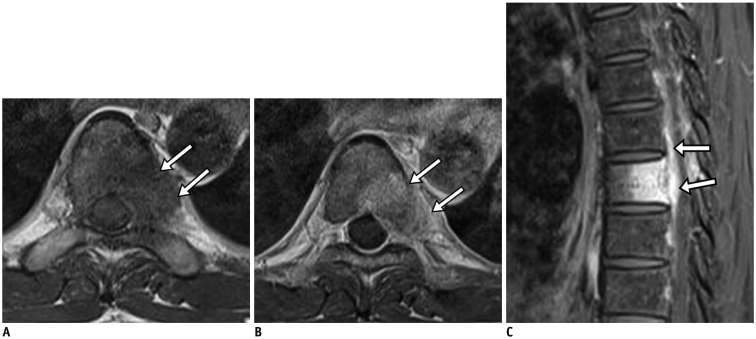
Fig. 5. Pyogenic spondylodiscitis.
67-year-old woman with lower back pain. Axial T1-weighted imaging (T1WI) showed abnormal low signal intensity (arrow) (A), and axial T2-weighted imaging showed corresponding high signal intensity (arrow) (B) in right paraspinal area. Fat-suppressed, contrast-enhanced axial T1WI showed diffusely enhanced lesion (arrow) (C). Fat-suppressed, contrast-enhanced sagittal T1WI showed linear subligamentous enhancement (arrows) at L5–S1 level (D).
Diffusion-weighted imaging (DWI) is also considered to be useful for early and accurate diagnoses of spinal and paraspinal infections (27). Eastwood et al. (28) reported that osseous or epidural abscesses showed marked hyperintensity on DWI, and that they appeared dark on apparent diffusion coefficient maps (Fig. 6). DWI demonstrates the extension and multiplicity of abscesses, even when they are diminutive, in any spinal or paraspinal area (29). Important differential diagnoses include hemorrhage, disc herniation, granulation tissue, tumor, degeneration and fluid collection (29).
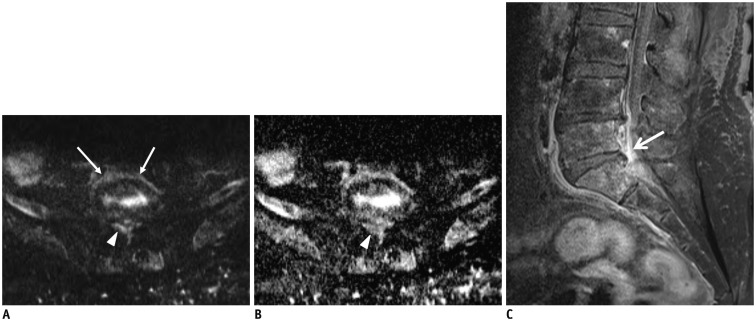
Fig. 6. Pyogenic spondylodiscitis with epidural abscess.
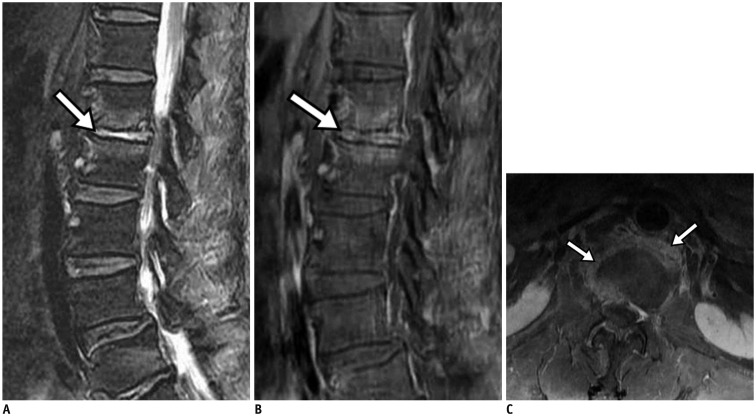
72-year-old woman with lower back pain and underlying hepatocellular carcinoma. Axial diffusion-weighted imaging (A) showed high signal intensity in paraspinal area (arrows) and epidural area (arrowhead) at L5–S1 level. Epidural lesion demonstrated signal loss (arrowhead) on axial apparent diffusion coefficient (ADC) map (B) and had lower ADC value (0.93 x 10-3 mm2/s). Fat-suppressed, contrast-enhanced sagittal T1-weighted imaging (C) showed marked epidural enhancement (arrow) at L5–S1 level.
Imaging Features of Early Spondylodiscitis Caused by Hematogenous Spread
Hematogenous infection in adults may start within the disc, soft tissue anterior to the disc, or epidural space (30); however, the anterior subchondral region of the vertebral body is the usual primary focus of infection. In the absence of signal changes in vertebral endplates, enhancement in paravertebral soft tissue or the epidural space may be the only MR finding of early spondylodiscitis (3). In fact, the earliest sign of an infectious process may be an altered marrow signal within only one vertebra.
Usually, high signal intensity and loss of the intranuclear cleft on T2WI are reliable findings of infectious spondylitis (31), but it is difficult to detect these findings in the early stage. In this stage, MR findings may show atypical appearances or inappreciable changes, such as indistinct or erosive cortical changes in endplates, bone marrow signal change in an isolated vertebral body, and inflammatory changes in paraspinal soft tissue and subligamentous and epidural spaces without bone marrow signal changes.
Cortical Changes in Endplates
The destruction of endplates, which is seen as marked thinning or absence of the hypointense band of cortical bone on T1WI, is a relatively typical, but it is a delayed finding of spinal infection (Fig. 2) (14). During the early stage, inappreciable cortical discontinuity or erosion may be seen at the endplates without any apparent T2 signal change in bodies or disc (3). Therefore, spondylodiscitis may be missed or misinterpreted as another disease, such as erosive intervertebral osteochondrosis, Schmorl's node, or ankylosing spondylitis. When the cortical hypointense endplate band is preserved, sterile spondyloarthropathy should be considered (14).
Erosive intervertebral osteochondrosis, which has been defined as aggressive disc degeneration with widespread erosions of the vertebral body endplates, may mimic infectious spondylodiscitis (Fig. 7) (32). However, MR findings of the vacuum phenomenon, T2-hypointensity within the disc, band-like subchondral edema, fat accumulation or bone sclerosis, and lack of epidural or paraspinal inflammatory changes, are more common in intervertebral osteochondrosis than in infection (2).
Fig. 7. 77-year-old woman with erosive intervertebral osteochondrosis at L1–2 and L2–3 levels.
Sagittal T1-weighted imaging showed numerous erosions (arrows) surrounded by low and high signal intensity bone marrow consistent with osteosclerosis and fat transformation.
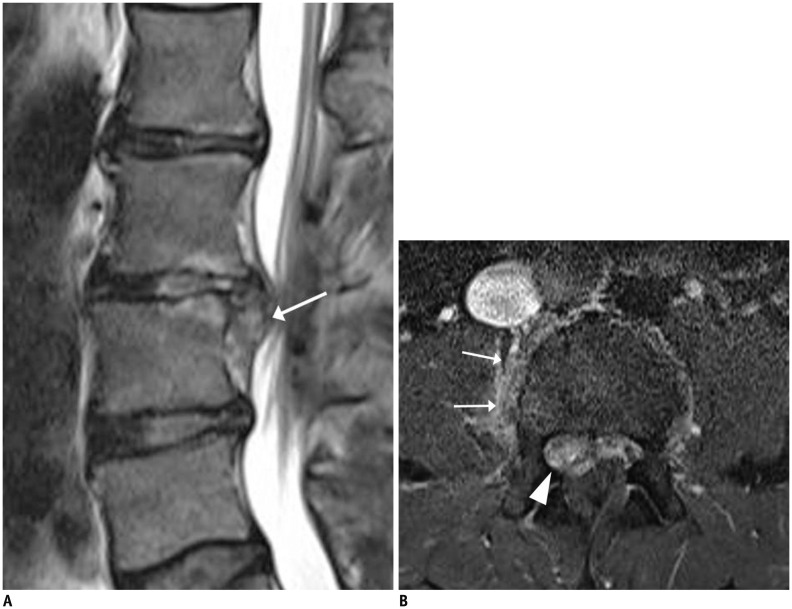
Acute Schmorl's nodes are characterized by surrounding concentric rings, resembling an edema-like signal, involvement of only the endplate with a herniated node, lack of diffuse abnormal signal changes within a disc (30), and predominant peripheral nodal enhancement (Fig. 8). Furthermore, with the exception of the osseous defect, the vertebral endplate remains defined (33).
Fig. 8. Acute Schmorl's node.
36-year-old woman with lower back pain. Sagittal T1-weighted imaging (T1WI) (A) showed relatively well-defined, large concentric ring (arrow) located in subchondral area of L4 body and smaller ring (arrowhead) in L3 body. Fat-suppressed, contrast-enhanced sagittal T1WI showed irregular, well enhanced concentric rings (B).
On the other hand, central and peripheral discovertebral erosions can occur in ankylosing spondylitis and can mimic infectious spondylitis. These erosions are usually bordered by a sclerotic margin and the remaining endplate may be preserved with no evidence of epidural or paraspinal inflammation (25,34). Advanced discovertebral junction destruction (Andersson lesion) causes pseudoarthrosis and it may also be confused with infectious spondylitis. At this stage, identification of a fracture line in posterior elements, syndesmophytosis, squaring of vertebral bodies, and apophyseal joint fusion favor the presence of Andersson lesion over infection (34,35).
Bone Marrow Signal Change in an Isolated Vertebral Body
During the early stage, abnormal signal intensity may be observed in an isolated vertebral body, especially in its endplate, and adjacent discs may be normal (3). These findings may cause diagnostic difficulty and adversely affect adequate treatment planning. Pathophysiologically, the accumulation of extracellular fluid within the marrow is the earliest response to infection in a vertebral body (2). Earliest MR findings are blurred margins of inflammatory bone marrow edema adjacent to an endplate exhibiting low signal intensity on T1WI and high signal intensity on T2WI with contrast enhancement (Fig. 2). Furthermore, these findings are in contrast with degenerative changes, which are represented by relatively sharp margins (36,37). However, these findings may be non-specific. Moreover, although abnormal signal intensity in the vertebral bodies usually involves the whole vertebra in infectious spondylitis, it is not a consistent finding, and therefore, type 1 Modic change, traumatic edema, or a tumorous condition should be considered in the differential diagnosis when an indistinct marrow signal change in a single vertebral body is the only MR finding.
Type 1 Modic changes may cause a diagnostic dilemma in patients with low back pain, because sometimes they resemble the MRI features of spondylodiscitis. In particular, they include bone marrow edema visualized as high signal intensity on T2WI (38,39). These bone marrow changes may also be enhanced on contrast-enhanced images. However, bone marrow edema is limited to the subchondral region, and it presents as a sharp margin, a well-defined vertebral endplate, and a degenerated disc showing low signal intensity rather than a disc showing increased signal intensity, which is not infrequently seen on T2WI of spinal infections (2). Also, gas may be present within severely degenerated discs (31). In such cases, CT might aid in differentiation by allowing visualization of the discal vacuum phenomenon or well-defined sclerosis and erosions of vertebral endplates without bone destruction (Fig. 9) (38).
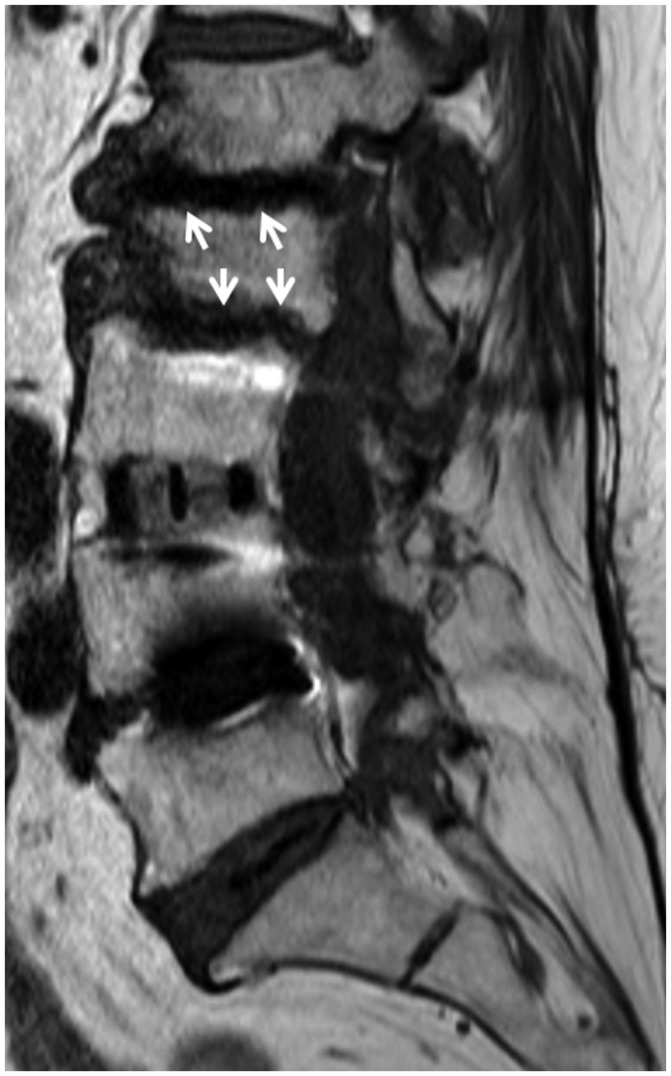
Fig. 9. Type 1 Modic change.
66-year-old man with history of lung cancer and lower back pain. Findings of multifocal cortical discontinuities or erosions of endplates (arrows) on sagittal T2-weighted imaging (A) and paraspinal soft tissue enhancement (arrows) on fat-suppressed, contrast-enhanced axial T1-weighted imaging (B) were confused with infection. However, reformatted sagittal CT (C) findings of vacuum disc, well-defined sclerosis, and erosions of vertebral endplates reduced possibility of infection.
Acute osteoporotic fractures usually show a band-like area of low signal intensity adjacent to the fractured endplates on T1WI with spared normal marrow signal intensity (40). Also, an area of low signal intensity corresponding to the fracture line or trabecular impaction can be seen on T2WI with a preserved disc (19,41). However, an acute traumatic fracture may cause diffuse low signal intensity of the body on T1WI, mimicking a malignant fracture or an atypical infection (42).
On the other hand, the involvement of a single vertebral body without disc involvement may lead to diagnostic confusion; in most cases of this type, the differential diagnosis includes metastatic disease and mycobacterial infection (7). Usually a destructive bone lesion associated with a well-preserved disc space with sharp endplates or the involvement of only one vertebral body or posterior elements suggests neoplastic infiltration, whereas a destructive bone lesion associated with a poorly defined vertebral body endplate, with or without loss of disc height, suggests infection (33,34,43). However, in the earliest stage, these changes are mild and variable, and therefore, they are not easily detected (Fig. 10). Shih et al. (44) found that the presence of anterior cortical disruption of the vertebra and upward subligamentous spread of infection most often aids in the early diagnosis of single-segment spondylodiscitis. Hence, mild changes in paravertebral soft tissue and vertebral endplates may aid in the differentiation between infection and malignancy.
Fig. 10. 46-year-old man with tuberculous spondylitis mimicking malignancy.
Axial T1-weighted imaging (T1WI) (A) depicted ill-defined low signal intensity lesion (arrows) with subtle cortical disruption on left posterolateral aspect of T11 body and pedicle. Corresponding lesion showed diffuse enhancement (arrows) on contrast-enhanced axial T1WI (B). Prominent enhancement of adjacent epidural space (arrows) was observed on fat-suppressed, contrast-enhanced sagittal T1WI (C).
Acute Schmorl's nodes sometimes mimic infectious spondylodiscitis because they are associated with MR signal changes corresponding to an inflammatory type in the adjacent bone marrow and enhancement of the disc material. Bone marrow edema surrounding acute Schmorl's nodes on initial MR images is believed to be associated with the recent occurrence of disc extrusion into the endplate (45). On the other hand, the presence of an infectious process is usually visualized as diffuse involvement of the entire disc and both endplates, whereas Schmorl's nodes are typically focal (46,47). Furthermore, for Schmorl's nodes, the signal within the affected disc is usually normal or reduced and the remaining vertebral endplate is well defined. In addition, T1WI show definite intraosseous disc herniation (Fig. 8) (2).
Inflammatory Changes in Paraspinal Soft Tissue and Subligamentous and Epidural Spaces
During the early stage of vertebral osteomyelitis, abnormal signal intensity involving the endplates may be observed without an appreciable increase in signal from the body or the affected disc on T2WI, and these findings may cause diagnostic confusion. Therefore, radiologists should look for abnormalities in paraspinal soft tissue and subligamentous (Fig. 5) and epidural spaces (Fig. 11). Paravertebral enhancement supports a diagnosis of infection, while the absence of enhancement in the subendplate or disc space indicates that infection is unlikely.
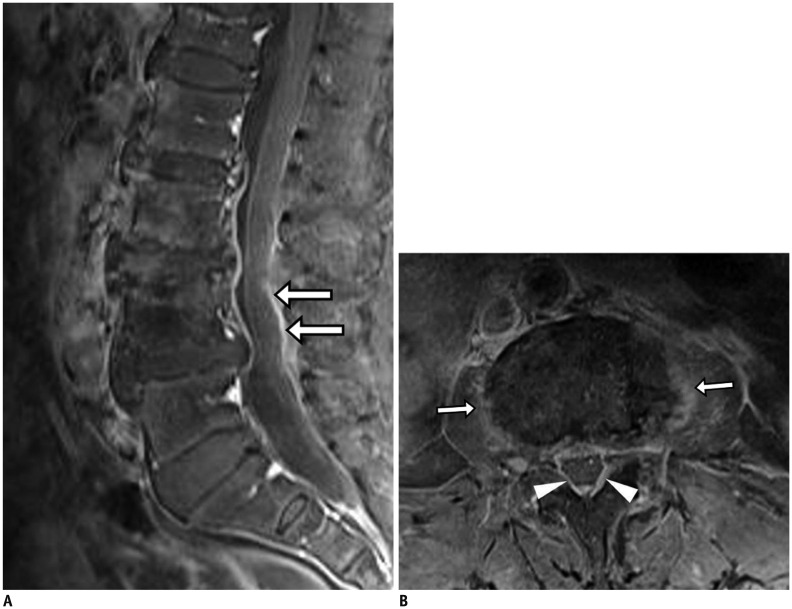
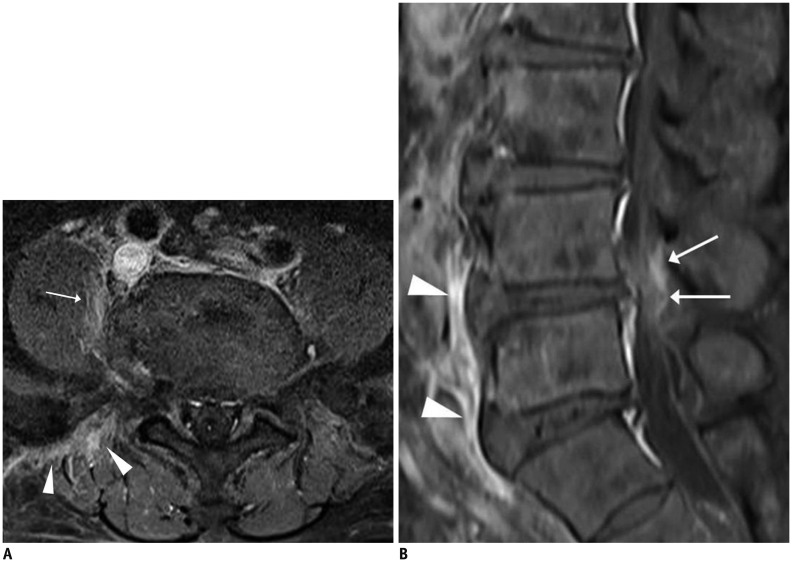
Fig. 11. Pyogenic (staphylococcal infection) spondylodiscitis.
73-year-old woman with mild fever and back pain. Fat-suppressed, contrast-enhanced sagittal T1-weighted imaging (T1WI) showed only thin linear enhancement of epidural space (arrows) without signal changes in bone marrow or cortical changes (A). However, fat-suppressed, contrast-enhanced axial T1WI (B) showed faint paraspinal (arrows) and epidural (arrowheads) enhancement. Two months later, follow-up MRI (not shown) demonstrated aggravation of infectious process.
During the early stage, mild paravertebral soft tissue changes are usually detected on contrast-enhanced images. Furthermore, a true inflammatory signal change should be differentiated from a fatty change in the psoas muscle or the paraspinal area, and therefore, a comparison of signal intensities on T1WI, T2WI, and contrast-enhanced images is necessary (Fig. 5). On the other hand, early MRI may show prevertebral and epidural inflammation with normal vertebral bodies (Figs. 5, 11) (30). Although signs of concomitant infective spondylitis, such as enhancement of the involved discs, vertebral bodies, and paravertebral soft tissues, are observed frequently, these signs may be absent (48,49) and epidural infection can occur in isolation. In most cases, epidural enhancement is located anteriorly and is usually close to the primary infectious site, but distant epidural involvement or an isolated epidural abscess may also occur rarely (2,48). In addition, posterior disc protrusion may impair venous return within the epidural venous plexus and promote the deposition of bacteria in the epidural space (30). Hence, a small epidural abscess related to the posterior annulus may sometimes be misdiagnosed as an extruded disc (Fig. 12). Also, some enhancement of the annulus is not specific for infection as it is also observed in annular tears; however, epidural enhancement in association with an annular tear has been shown to indicate the presence of infection (6). These unusual findings further confuse the diagnosis of infection.
Fig. 12. Epidural abscess (streptococcal infection) mimicking disc herniation.
54-year-old man with severe lower back pain and mild fever. Sagittal T2-weighted imaging showed mild collapse of L3–4 disc with high signal intensity continuous with epidural lesion showing similar signal intensity (arrow) (A). Fat-suppressed, contrast-enhanced axial T1-weighted imaging (B) showed diffusely enhanced epidural lesion (arrowhead) and faint paraspinal soft tissue enhancement (arrows). One month later, follow-up MRI (not shown) revealed that infectious changes had progressed.
Vertebral endplate injury caused by osteoporotic vertebral compression fractures may be associated with an epidural or paraspinal soft tissue lesion, and when these findings are present in the adjacent vertebra, acute endplate injury may mimic infectious spondylodiscitis (50). The MRI features suggestive of osteoporotic fractures are retropulsion, a low signal intensity band, spared normal marrow signal intensity, and the fluid sign, whereas the MRI features suggestive of fracture caused by infectious spondylitis are contiguous vertebral involvement, endplate disruption, disc involvement, and the presence of a paraspinal or epidural abscess (51). The most accurate diagnostic feature of fracture caused by infectious spondylitis is disc involvement, which has a sensitivity, specificity, and accuracy of 97%, 98%, and 98%, respectively (51). However, when MR findings show only vertebral endplate injury and paraspinal soft tissue inflammation, the differentiation between acute osteoporotic compression fracture and early infectious spondylodiscitis may be challenging (Fig. 13).
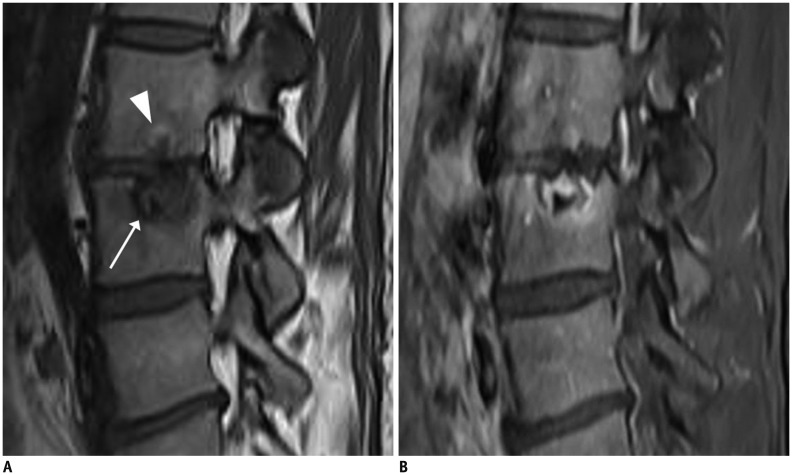
Fig. 13. Acute compression fracture with bone marrow edema mimicking infection.
62-year-old man with history of medication for osteoporosis. Sagittal T1-weighted imaging (T1WI) (A) revealed L1 vertebral body with subchondral bone defect at its lower endplate with marked bone marrow edema (arrow). Fat-suppressed, contrast-enhanced axial T1WI (B) depicted paraspinal soft tissue edema (arrows). Sagittal T2-weighted imaging (C) exhibited faint fracture line (arrow) with low signal intensity, highly suspicious for compression fracture.
Modic type 1 degeneration may also exhibit paraspinal soft tissue changes (Fig. 9), and degenerative or reactive bone changes related to disc degeneration cannot always be confidently distinguished from infection by imaging. Clinical and laboratory findings, such as white blood cell count, ESR, and elevated body temperature, provide supportive but not confirmatory evidence of infectious spondylodiscitis (38,52). Accordingly, when the clinical impression, laboratory findings, and imaging do not allow the exclusion of infection, a biopsy should be performed (1).
Atypical Imaging Features of Early Spondylodiscitis Caused by Direct Inoculation
Iatrogenic infection may occur by direct inoculation in the postoperative setting or during discography, epidural catheterization, laser or percutaneous discectomy, or therapeutic spinal injections (4).
After percutaneous selective nerve root block, diffuse inflammatory changes may be observed along the needle track, mainly in back muscles. Furthermore, inflammation may occur at injection sites or around the targeted nerve root ganglion and spread into paraspinal soft tissues and the adjacent epidural space. More rarely, inflammation can begin at the posterolateral aspect of a vertebral body adjacent to the injection site (Fig. 14). Also, facet joint infection (facetitis) is an uncommon, but not rare condition (53), which may result from direct inoculation during an interventional procedure, such as adjacent surgery or facet block, or from direct contiguous or hematogenous spread (1,54). MRI typically demonstrates erosive changes on the subchondral surface of a facet joint or edema around a fluid-filled facet joint, with facet capsular enhancement or associated soft tissue lesion enhancement (Fig. 15) (1).
Fig. 14. Schematic showing spread of infectious changes after selective nerve root block (1) and therapeutic or diagnostic facet joint injection (2).
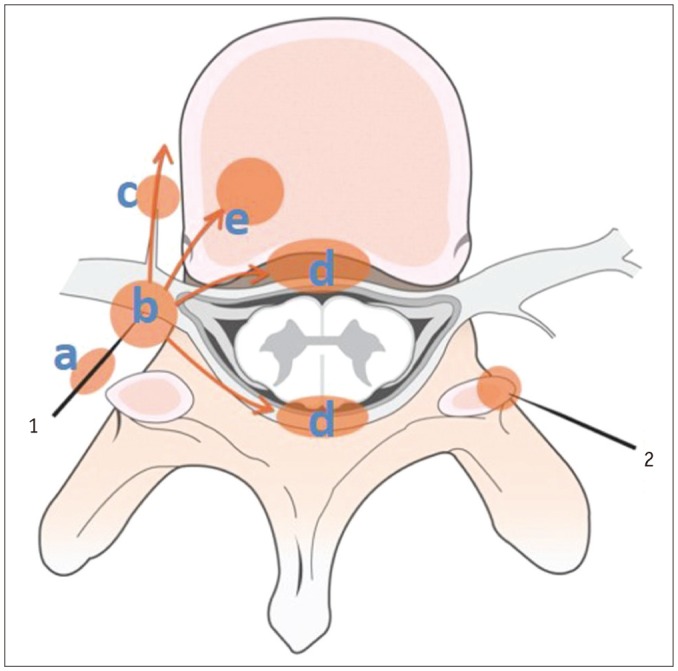
Diffuse inflammatory changes can be seen along needle track (a) mainly in back muscles. These inflammatory changes can also occur at injection sites (b), around nerve root ganglions, and spread into paraspinal soft tissues (c) and into adjacent epidural space (d). More rarely, inflammation may begin at posterolateral aspect (e) of vertebral body adjacent to injection site.
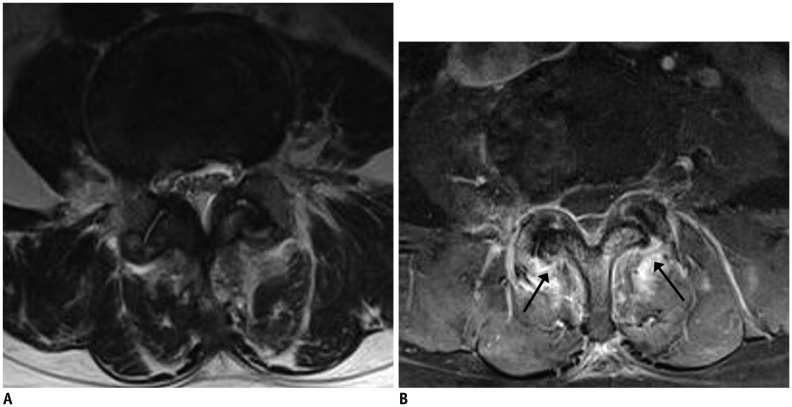
Fig. 15. Pyogenic facet joint infection caused by procedure.
62-year-old female patient presented with lower back pain after facet joint prolotherapy at L3–4 level. Axial T2-weighted imaging raised suspicion of osteoarthritis of bilateral L3–4 facet joints (A). Soft tissue edema around facet joints with bone marrow edema (arrows) and linear thin epidural enhancement were observed on fat-suppressed, contrast-enhanced axial T1-weighted imaging (B).
Although epidural phlegmon/abscesses may occur due to direct extension from spondylodiscitis or facetitis, they are more commonly associated with hematogenous spread or iatrogenic inoculation resulting from an invasive spinal procedure (1,55,56). Epidural inflammation caused by iatrogenic inoculation may be located anteriorly or posteriorly, depending on the approach used (Fig. 16). Furthermore, extension of facetitis into the central canal may initially lead to a posterior epidural process, unlike the anterior epidural spread observed in the setting of spondylodiscitis (57,58,59). On contrast-enhanced T1WI, epidural lesions in the epidural plexus engorgement/phlegmon stage may enhance homogeneously or heterogeneously, and in the mature stage of a centrally pus-filled abscess, they enhance peripherally (60,61).
Fig. 16. Pyogenic spondylodiscitis caused by procedure.
68-year-old male patient presented with lower back pain and fever 3 days after selective nerve root block. Fat-suppressed, contrast-enhanced axial T1-weighted imaging (T1WI) (A) showed mild inflammatory changes in right paraspinal soft tissue (arrow) and back muscle area (arrowheads) corresponding to needle track. Fat-suppressed, contrast-enhanced sagittal T1WI (B) showed faint epidural enhancement (arrows) and linear subligamentous enhancement (arrowheads). MRI (not shown) obtained 10 days later showed aggravation of infectious process.
MRI may be helpful for the early detection of infectious changes, but it is usually not possible to reliably diagnose infection until 3 weeks after surgery (3), and therefore, detection by MRI is challenging in the setting of postoperative infection (Fig. 17) (1). In fact, MRI cannot reliably differentiate between pathology and expected postoperative evolution until at least 6 months after surgery (1). However, two parallel thin bands of enhancement in the disc space may suggest post-discectomy change rather than infection, whereas more amorphous enhancement suggests infection (62).
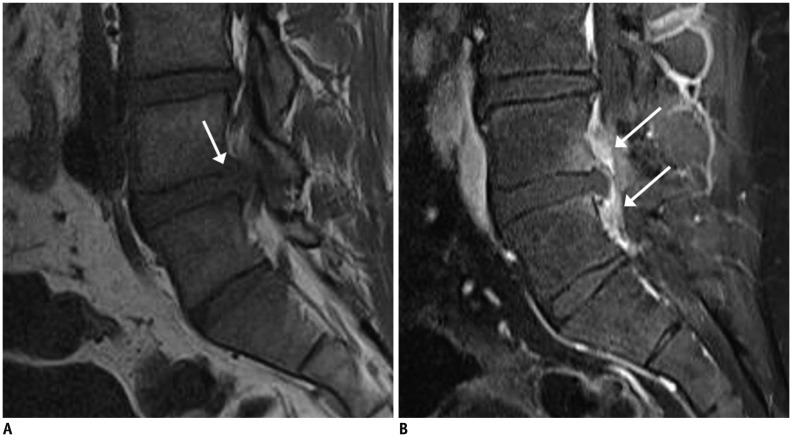
Fig. 17. Pyogenic spondylodiscitis caused by operation.
29-year-old man presented with pain and fever after percutaneous discectomy. Seven days after discectomy for disc extrusion, sagittal T1-weighted imaging (T1WI) (A) showed focal cortical discontinuity (arrow) and faint marrow signal changes. Corresponding fat-suppressed, contrast-enhanced sagittal T1WI (B) showed prominent epidural enhancement (arrows). MRI (images not shown) performed 20 days after first post-operative MRI revealed aggravation of infectious changes.
CONCLUSION
The early MR findings of infectious spondylodiscitis are non-specific and include faint bone marrow edema, paravertebral soft tissue change, and/or focal epidural enhancement, which overlap with findings of other conditions, such as Modic type 1 change, acute Schmorl's node, disc extrusion, malignancy, or acute endplate injury. It is important to recognize these findings as the earliest signs of infectious spondylitis, and a short-term follow-up MR imaging should be performed to detect any infective changes when spinal infection is clinically suspected. In a patient with these early MR findings, correlations with clinical history and laboratory test findings should be sought. In particular, careful evaluation at or around the procedure sites, such as facet joints, epidural space, muscular structures of the back, and posterolateral portion of vertebral bodies, is essential when there is a history of a procedure or surgery. To avoid delayed diagnosis, radiologists should become familiar with the atypical MR findings of early spondylodiscitis and they should be able to differentiate these findings from those of lesions with similar findings.
References
- 1.Diehn FE. Imaging of spine infection. Radiol Clin North Am. 2012;50:777–798. doi: 10.1016/j.rcl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Leone A, Dell'Atti C, Magarelli N, Colelli P, Balanika A, Casale R, et al. Imaging of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):8–19. [PubMed] [Google Scholar]
- 3.DeSanto J, Ross JS. Spine infection/inflammation. Radiol Clin North Am. 2011;49:105–127. doi: 10.1016/j.rcl.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Gürbüz MS, Berkman MZ. Spondylodiscitis occurring after diagnostic lumbar puncture: a case report. Case Rep Infect Dis. 2013;2013:843592. doi: 10.1155/2013/843592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie AR, Laing RB, Smith CC, Kaar GF, Smith FW. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209–212. doi: 10.1136/jnnp.65.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunbar JA, Sandoe JA, Rao AS, Crimmins DW, Baig W, Rankine JJ. The MRI appearances of early vertebral osteomyelitis and discitis. Clin Radiol. 2010;65:974–981. doi: 10.1016/j.crad.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Ziegelbein J, El-Khoury GY. Early spondylodiscitis presenting with single vertebral body involvement: a report of two cases. Iowa Orthop J. 2011;31:219–224. [PMC free article] [PubMed] [Google Scholar]
- 8.Ratcliffe JF. An evaluation of the intra-osseous arterial anastomoses in the human vertebral body at different ages. A microarteriographic study. J Anat. 1982;134(Pt 2):373–382. [PMC free article] [PubMed] [Google Scholar]
- 9.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11–iii24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 10.Ratcliffe JF. Anatomic basis for the pathogenesis and radiologic features of vertebral osteomyelitis and its differentiation from childhood discitis. A microarteriographic investigation. Acta Radiol Diagn (Stockh) 1985;26:137–143. doi: 10.1177/028418518502600204. [DOI] [PubMed] [Google Scholar]
- 11.Wiley AM, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg Br. 1959;41-B:796–809. doi: 10.1302/0301-620X.41B4.796. [DOI] [PubMed] [Google Scholar]
- 12.Bergman I, Wald ER, Meyer JD, Painter MJ. Epidural abscess and vertebral osteomyelitis following serial lumbar punctures. Pediatrics. 1983;72:476–480. [PubMed] [Google Scholar]
- 13.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Go JL, Rothman S, Prosper A, Silbergleit R, Lerner A. Spine infections. Neuroimaging Clin N Am. 2012;22:755–772. doi: 10.1016/j.nic.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Ratcliffe JF. The arterial anatomy of the developing human dorsal and lumbar vertebral body. A microarteriographic study. J Anat. 1981;133(Pt 4):625–638. [PMC free article] [PubMed] [Google Scholar]
- 16.Babinchak TJ, Riley DK, Rotheram EB., Jr Pyogenic vertebral osteomyelitis of the posterior elements. Clin Infect Dis. 1997;25:221–224. doi: 10.1086/514529. [DOI] [PubMed] [Google Scholar]
- 17.Turunc T, Demiroglu YZ, Uncu H, Colakoglu S, Arslan H. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect. 2007;55:158–163. doi: 10.1016/j.jinf.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Lee CM, Lee S, Bae J. Contiguous spinal metastasis mimicking infectious spondylodiscitis. J Korean Soc Radiol. 2015;73:408–412. [Google Scholar]
- 19.Sans N, Faruch M, Lapégue F, Ponsot A, Chiavassa H, Railhac JJ. Infections of the spinal column--spondylodiscitis. Diagn Interv Imaging. 2012;93:520–529. doi: 10.1016/j.diii.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Lee JY, Kim BM, Kim YM, Min SH. MRI findings on iatrogenic spinal infection following various pain management procedures. J Korean Soc Radiol. 2014;71:296–303. [Google Scholar]
- 21.Dullerud R, Nakstad PH. Side effects and complications of automated percutaneous lumbar nucleotomy. Neuroradiology. 1997;39:282–285. doi: 10.1007/s002340050410. [DOI] [PubMed] [Google Scholar]
- 22.Guyer RD, Ohnmeiss DD, Mason SL, Shelokov AP. Complications of cervical discography: findings in a large series. J Spinal Disord. 1997;10:95–101. [PubMed] [Google Scholar]
- 23.Jiménez-Mejías ME, de Dios Colmenero J, Sánchez-Lora FJ, Palomino-Nicás J, Reguera JM, García de la Heras J, et al. Postoperative spondylodiskitis: etiology, clinical findings, prognosis, and comparison with nonoperative pyogenic spondylodiskitis. Clin Infect Dis. 1999;29:339–345. doi: 10.1086/520212. [DOI] [PubMed] [Google Scholar]
- 24.Sung S, Kwon JW. Image-guided percutaneous needle biopsy for infectious spondylitis: factors affecting culture positivity. J Korean Soc Radiol. 2015;73:300–306. [Google Scholar]
- 25.Jevtic V. Vertebral infection. Eur Radiol. 2004;14(Suppl 3):E43–E52. doi: 10.1007/s00330-003-2046-x. [DOI] [PubMed] [Google Scholar]
- 26.Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol. 2004;182:1405–1410. doi: 10.2214/ajr.182.6.1821405. [DOI] [PubMed] [Google Scholar]
- 27.Thurnher MM, Bammer R. Diffusion-weighted magnetic resonance imaging of the spine and spinal cord. Semin Roentgenol. 2006;41:294–311. doi: 10.1053/j.ro.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Eastwood JD, Vollmer RT, Provenzale JM. Diffusion-weighted imaging in a patient with vertebral and epidural abscesses. AJNR Am J Neuroradiol. 2002;23:496–498. [PMC free article] [PubMed] [Google Scholar]
- 29.Moritani T, Kim J, Capizzano AA, Kirby P, Kademian J, Sato Y. Pyogenic and non-pyogenic spinal infections: emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. Br J Radiol. 2014;87:20140011. doi: 10.1259/bjr.20140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millot F, Bonnaire B, Clavel G, Deramond H, Fardellone P, Grados F. Hematogenous Staphylococcus aureus discitis in adults can start outside the vertebral body. Joint Bone Spine. 2010;77:76–77. doi: 10.1016/j.jbspin.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Tali ET. Spinal infections. Eur J Radiol. 2004;50:120–133. doi: 10.1016/j.ejrad.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Jevtic V. Magnetic resonance imaging appearances of different discovertebral lesions. Eur Radiol. 2001;11:1123–1135. doi: 10.1007/s003300000727. [DOI] [PubMed] [Google Scholar]
- 33.Hong SH, Choi JY, Lee JW, Kim NR, Choi JA, Kang HS. MR imaging assessment of the spine: infection or an imitation? Radiographics. 2009;29:599–612. doi: 10.1148/rg.292085137. [DOI] [PubMed] [Google Scholar]
- 34.Tyrrell PN, Cassar-Pullicino VN, McCall IW. Spinal infection. Eur Radiol. 1999;9:1066–1077. doi: 10.1007/s003300050793. [DOI] [PubMed] [Google Scholar]
- 35.Bron JL, de Vries MK, Snieders MN, van der Horst-Bruinsma IE, van Royen BJ. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol. 2009;28:883–892. doi: 10.1007/s10067-009-1151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battikha JG, Garcia JF, Wettstein P. Aspects of atypical degenerative lesions of vertebrae. Skeletal Radiol. 1981;6:103–107. doi: 10.1007/BF00347571. [DOI] [PubMed] [Google Scholar]
- 37.Kwon JW, Yoon YC, Choi SH, Jung JY, Choe BK. MR imaging for the differentiation of early infectious spondylitis and modic type I change in the lumbar spine. J Korean Soc Radiol. 2010;62:563–570. [Google Scholar]
- 38.Oztekin O, Calli C, Kitis O, Adibelli ZH, Eren CS, Apaydin M, et al. Reliability of diffusion weighted MR imaging in differentiating degenerative and infectious end plate changes. Radiol Oncol. 2010;44:97–102. doi: 10.2478/v10019-010-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AS, Blaser SI. Infectious and inflammatory processes of the spine. Radiol Clin North Am. 1991;29:809–827. [PubMed] [Google Scholar]
- 40.Cuénod CA, Laredo JD, Chevret S, Hamze B, Naouri JF, Chapaux X, et al. Acute vertebral collapse due to osteoporosis or malignancy: appearance on unenhanced and gadolinium-enhanced MR images. Radiology. 1996;199:541–549. doi: 10.1148/radiology.199.2.8668809. [DOI] [PubMed] [Google Scholar]
- 41.Palmer WE, Suri R, Kattapuram SV. Benign versus malignant vertebral collapse: value of a fracture line on MR images. Radiology (Suppl) 1999:213–229. [Google Scholar]
- 42.Yuh WT, Zachar CK, Barloon TJ, Sato Y, Sickels WJ, Hawes DR. Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. Radiology. 1989;172:215–218. doi: 10.1148/radiology.172.1.2740506. [DOI] [PubMed] [Google Scholar]
- 43.Van Lom KJ, Kellerhouse LE, Pathria MN, Moreland SI, Brown JJ, Zlatkin M, et al. Infection versus tumor in the spine: criteria for distinction with CT. Radiology. 1988;166:851–855. doi: 10.1148/radiology.166.3.3340783. [DOI] [PubMed] [Google Scholar]
- 44.Shih TT, Huang KM, Hou SM. Early diagnosis of single segment vertebral osteomyelitis--MR pattern and its characteristics. Clin Imaging. 1999;23:159–167. doi: 10.1016/s0899-7071(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 45.Wagner AL, Murtagh FR, Arrington JA, Stallworth D. Relationship of Schmorl's nodes to vertebral body endplate fractures and acute endplate disk extrusions. AJNR Am J Neuroradiol. 2000;21:276–281. [PMC free article] [PubMed] [Google Scholar]
- 46.Park P, Tran NK, Gala VC, Hoff JT, Quint DJ. The radiographic evolution of a Schmorl's node. Br J Neurosurg. 2007;21:224–227. doi: 10.1080/02688690701317169. [DOI] [PubMed] [Google Scholar]
- 47.Grivé E, Rovira A, Capellades J, Rivas A, Pedraza S. Radiologic findings in two cases of acute Schmörl's nodes. AJNR Am J Neuroradiol. 1999;20:1717–1721. [PMC free article] [PubMed] [Google Scholar]
- 48.Friedmand DP, Hills JR. Cervical epidural spinal infection: MR imaging characteristics. AJR Am J Roentgenol. 1994;163:699–704. doi: 10.2214/ajr.163.3.8079871. [DOI] [PubMed] [Google Scholar]
- 49.Küker W, Mull M, Mayfrank L, Töpper R, Thron A. Epidural spinal infection. Variability of clinical and magnetic resonance imaging findings. Spine (Phila Pa 1976) 1997;22:544–550. doi: 10.1097/00007632-199703010-00017. discussion 551. [DOI] [PubMed] [Google Scholar]
- 50.Ortiz AO, Bordia R. Injury to the vertebral endplate-disk complex associated with osteoporotic vertebral compression fractures. AJNR Am J Neuroradiol. 2011;32:115–120. doi: 10.3174/ajnr.A2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel-Wanis ME, Solyman MT, Hasan NM. Sensitivity, specificity and accuracy of magnetic resonance imaging for differentiating vertebral compression fractures caused by malignancy, osteoporosis, and infections. J Orthop Surg (Hong Kong) 2011;19:145–150. doi: 10.1177/230949901101900203. [DOI] [PubMed] [Google Scholar]
- 52.Fouquet B, Goupille P, Jattiot F, Cotty P, Lapierre F, Valat JP, et al. Discitis after lumbar disc surgery Features of "aseptic" and "septic" forms. Spine (Phila Pa 1976) 1992;17:356–358. doi: 10.1097/00007632-199203000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Narváez J, Nolla JM, Narváez JA, Martinez-Carnicero L, De Lama E, Gómez-Vaquero C, et al. Spontaneous pyogenic facet joint infection. Semin Arthritis Rheum. 2006;35:272–283. doi: 10.1016/j.semarthrit.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Weingarten TN, Hooten WM, Huntoon MA. Septic facet joint arthritis after a corticosteroid facet injection. Pain Med. 2006;7:52–56. doi: 10.1111/j.1526-4637.2006.00089.x. [DOI] [PubMed] [Google Scholar]
- 55.Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23:175–204. doi: 10.1007/pl00011954. discussion 205. [DOI] [PubMed] [Google Scholar]
- 56.Tompkins M, Panuncialman I, Lucas P, Palumbo M. Spinal epidural abscess. J Emerg Med. 2010;39:384–390. doi: 10.1016/j.jemermed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Ehara S, Khurana JS, Kattapuram SV. Pyogenic vertebral osteomyelitis of the posterior elements. Skeletal Radiol. 1989;18:175–178. doi: 10.1007/BF00360963. [DOI] [PubMed] [Google Scholar]
- 58.Heenan SD, Britton J. Septic arthritis in a lumbar facet joint: a rare cause of an epidural abscess. Neuroradiology. 1995;37:462–464. doi: 10.1007/BF00600094. [DOI] [PubMed] [Google Scholar]
- 59.Hickey NA, White PG. Septic arthritis of a lumbar facet joint causing multiple abscesses. Clin Radiol. 2000;55:481–483. doi: 10.1053/crad.2000.0091. [DOI] [PubMed] [Google Scholar]
- 60.Numaguchi Y, Rigamonti D, Rothman MI, Sato S, Mihara F, Sadato N. Spinal epidural abscess: evaluation with gadolinium-enhanced MR imaging. Radiographics. 1993;13:545–559. doi: 10.1148/radiographics.13.3.8316663. discussion 559-560. [DOI] [PubMed] [Google Scholar]
- 61.Sandhu FS, Dillon WP. Spinal epidural abscess: evaluation with contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12:1087–1093. [PMC free article] [PubMed] [Google Scholar]
- 62.Ross JS, Zepp R, Modic MT. The postoperative lumbar spine: enhanced MR evaluation of the intervertebral disk. AJNR Am J Neuroradiol. 1996;17:323–331. [PMC free article] [PubMed] [Google Scholar]