Abstract
Objective
To investigate the bone mineral density (BMD) of cervical vertebrae in a population-stratified manner and correlate with that of the lumbar vertebrae.
Materials and Methods
Five hundred and ninety-eight healthy volunteers (254 males, 344 females), ranging from 20 to 64 years of age, were recruited for volumetric BMD (vBMD) measurements by quantitative computed tomography. Basic information (age, height, weight, waistline, and hipline), and vBMD of the cervical and lumbar vertebrae (C2–7 and L2–4) were recorded. Comparisons among sex, age groups and different levels of vertebrae were analyzed using analysis of variance. Linear regression was performed for relevance of different vertebral levels.
Results
The vBMD of cervical and lumbar vertebrae was higher in females than males in each age group. The vBMD of the cervical and lumbar vertebrae in males and the vBMD of lumbar vertebrae in females decreased with aging. In each age group, the vBMD of the cervical vertebrae was higher than that of the lumbar vertebrae with gradual decreases from C2 to C7 except for C3; moreover, the vBMD of C6 and C7 was significantly different from that of C2–5. Correlations of vBMD among different cervical vertebrae (females: r = 0.62–0.94; males: r = 0.63–0.94) and lumbar vertebrae (males: r = 0.93–0.98; females: r = 0.82–0.97) were statistically significant at each age group.
Conclusion
The present study provided normative data of cervical vertebrae in an age- and sex-stratified manner. Sex differences in vBMD prominently vary with age, which can be helpful to design a more comprehensive pre-operative surgical plan.
Keywords: Quantitative, Computed tomography, Bone density, Cervical, Lumbar, Vertebra, Population, Normal
INTRODUCTION
Osteoporosis is a worldwide public health issue, which has received great attention (1). In osteoporotic patients, the abnormal bone mass can affect the mechanical properties of the bone (2,3) and may lead to low back pain, disc degeneration or wedge fracture of the vertebral body (4,5,6,7,8). Moreover, the decreased bone mass of the cervical vertebrae is closely related to loosening of the spinal surgical implant screws and the artificial inter-vertebral disc substitutes (9,10).
Currently, clinical and laboratory-based measurements of the lumbar vertebrae from dual-energy X-ray absorptiometry (DEXA) or quantitative computed tomography (QCT) are applied to investigate the bony status throughout the body (11,12,13). However, the anatomy, function, and mechanisms of injury and load transfer between the cervical spine and lumbar spine differ significantly (14). The cervical spine is a complex and distinct articular system in the body due to its weight bearing requirements, 6 degrees freedom in movement (15), and function of providing passage for neural and vascular structures (16). The dense structure of the cervical vertebrae is attributed to high dynamic forces from mobility and decreased size of cervical bodies (17). Higher bone mineral density (BMD) within the cervical than lumbar spine could be explained on the basis of the unique anatomic characteristics of the cervical spine, phylogenetic and kinematic factors. Firstly, the cervical spine is exposed to high dynamic forces because of their mobility and small size (17). Secondly, the cervical vertebrae is a phylogenetic reminiscence of quadruped gait, and is exposed to much higher stress because of the supporting of the head (17). Thirdly, coupled with the unique muscular anatomy, the cervical spinal column accommodates complex motions from various muscles and in various directions (18).
The cervical vertebra has specific characteristics, hence, our knowledge of the lumbar vertebrae cannot directly extend to the cervical vertebrae. Yoganandan et al. (18,19) reported that the volumetric BMD (vBMD) of the cervical vertebrae is higher than that of the lumbar vertebrae; and the vBMD of the cervical vertebrae gradually decreases from C2 to C7 level. However, the findings of their study cannot be generalized for all ages as the study population had a mean age of 24.9 years (range from 18–40 years) in females and 25 years (range from 18–41 years) in males. To the best of our knowledge, there are no age- and sex-stratified studies to investigate the normal range of BMD of the cervical vertebrae. Moreover, the correlation between the cervical and lumbar vertebrae with age, which directly impacts pre-operative surgical plan for implant instrument, remains unknown.
In addition, vertebral bodies consist of the peripheral cortical bone and the centrally located cancellous bone, which are both sensitive indicators of bone loss in aging and especially for postmenopausal women (20). Compared with DEXA, QCT shows a more comprehensive evaluation related to the disease, since it can selectively measure the cancellous bone of the vertebral body (21,22). Therefore, the purposes of this study were (1) to investigate the sex- organd age-stratified normative vBMD values of the cervical vertebrae by QCT and (2) determine the correlations with those of the lumbar vertebrae.
MATERIALS AND METHODS
Study Subjects
The subjects included in this study were participants of an ongoing study since June 2014 on degeneration of the spine and knee. The present study analyzed existing data in the spine and knee degeneration study and the study protocol was approved by the Ethics Committee of our hospital. The criteria for inclusion were healthy adults, aged 20–65 years, and resident in Beijing > 5 years. Exclusion criteria were those who were affected by any disease that may influence bone metabolism, including trauma and tumor, and those who were taking bone metabolism regulating drugs (19,20,23). All the subjects signed informed consent.
Cervical and Lumbar Vertebra Scanning by QCT
Basic information including age (years), height (cm), weight (cm), body mass index (BMI, kg/cm2), waistline (cm), and hipline (cm) were recorded before scanning. As part of the study protocol, the cervical vertebrae from C2 to C7 and lumbar vertebrae from L2 to L4 were scanned with Toshiba CT scanner (Aquilion PRIME ESX-302A, Toshiba Medical Systems Corporation, Otawara, Japan). A QCT calibration phantom (Mindways Inc., Austin, TX, USA) was placed beneath the spine and scanned simultaneously according to the standard scanning protocol by Lang et al. (24). The scanning parameters were as follows: 120 kV, 187 mAs, field-of-view 40 cm, 1 mm slice thickness, and reconstruction matrix: 512 × 512. The measurement error of this method is reportedly lower than 1.5% (23,24).
Volumetric BMD (vBMD) Measurement
After scanning, the CT dataset were translated to the QCT workstation for further analysis with the QCT Pro 5.0.3 (Mindways Inc.). The regions of interest were defined as the oval-shaped areas containing the largest areas of the trabecular bone, not including the cortical bone or basivertebral plexus (19,25). Then, the vBMD values of C2–7 and L2–4 were recorded and analyzed, respectively.
Statistical Analysis
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were grouped based on sex and age (10 years intervals) and expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to determine the statistically significant differences between different age groups. Repeated measures ANOVA was used to determine the statistically significant differences between different levels. P < 0.05 was considered as statistically significant. Linear regression analysis was also performed for both males and females and different age groups.
RESULTS
Characteristics of Subjects
Five hundred and ninety-eight healthy volunteers (254 males, mean age 40.1 ± 8.8 years; 344 females, mean age 41.4 ± 9.0 years) were recruited in this study. These included 5327 vertebrae, including 3569 cervical vertebrae and 1758 lumbar vertebrae. Some vBMD values of vertebrae were missing due to the following reasons: artifacts caused by dentures or mandibular metal implant (C2, 4; C3, 3; C4, 3); artifacts caused by the lead-based shielding vestment used to protect volunteers against radiation (C7, 9); and refusal to undergo the lumbar spine QCT examination in 12 volunteers (L2, 12; L3, 12; L4, 12). Basic information of all the subjects by sex was shown in Table 1.
Table 1. Basic Characteristics of Subjects.
| Parameters | Male Group | Female Group | P |
|---|---|---|---|
| Sample size | 254 | 344 | |
| Age (years) | 40.1 ± 8.8 | 41.4 ± 9.0 | 0.082 |
| Height (cm) | 170.7 ± 16.3 | 160.1 ± 7.9 | < 0.001 |
| Weight (kg) | 77.1 ± 13.3 | 62.1 ± 10.4 | < 0.001 |
| BMI (kg/cm2) | 26.2 ± 3.5 | 24.6 ± 9.0 | 0.007 |
| Waistline (cm) | 90.4 ± 10.4 | 80.6 ± 11.0 | < 0.001 |
| Hipline (cm) | 100.71 ± 8.9 | 97.2 ± 6.9 | < 0.001 |
BMI = body mass index
Age-Stratified Study of vBMD Values for Males and Females
The mean vBMD values and SDs per age group for each vertebral level were shown in Table 2 for males and Table 3 for females. Additionally, charts of Tables were also shown in Figures 1 and 2 for males and females.
Table 2. Comparison of Age and vBMD Measurement for Males.
| Age | Number | C2 | C3 | C4 | C5 | C6 | C7 | L2 | L3 | L4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 20–29 | 33 | 288 ± 50bcdefghi | 274 ± 40aefghi | 279 ± 35aefghi | 277 ± 35aefghi | 245 ± 34abcdfghi | 208 ± 33abcdeghi | 167 ± 24abcdefhi | 157 ± 21abcdefg | 156 ± 21abcdefg |
| 30–39 | 89 | 286 ± 60bcdefghi | 267 ± 52acdefghi | 271 ± 54abdefghi | 261 ± 49abcefghi | 237 ± 44abcdfghi | 210 ± 43abcdeghi | 162 ± 31abcdefhi | 156 ± 28*abcdefg | 156 ± 30*abcdefg |
| 40–49 | 88 | 273 ± 61bcdefghi | 253 ± 47*†acdefghi | 257 ± 46*abefghi | 256 ± 42*aefghi | 232 ± 40abcdfghi | 198 ± 40abcdeghi | 137 ± 25*†abcdefhi | 130 ± 24*†abcdefgi | 132 ± 26*†abcdefgh |
| 50–59 | 44 | 261 ± 48*†bcdefghi | 244 ± 42*†aefghi | 247 ± 48*†aefghi | 249 ± 45*aefghi | 222 ± 41*abcdfghi | 193 ± 44†abcdeghi | 120 ± 25*†‡abcdefhi | 115 ± 27*†‡abcdefg | 115 ± 29*†‡abcdefg |
ap < 0.05 vs. vBMD values of C2; bp < 0.05 vs. vBMD values of C3; cp < 0.05 vs. vBMD values of C4; dp < 0.05 vs. vBMD values of C5; ep < 0.05 vs. vBMD values of C6; fp < 0.05 vs. vBMD values of C7; gp < 0.05 vs. vBMD values of L1; hp < 0.05 vs. vBMD values of L2; ip < 0.05 vs. vBMD values of L3. *p < 0.05 vs. 20–29 age group, †p < 0.05 vs. 30–39 age group, ‡p < 0.05 vs. 40–49 age group. vBMD = volumetric bone mineral density
Table 3. Comparison of Age and vBMD Measurement for Females.
| Age | Number | C2 | C3 | C4 | C5 | C6 | C7 | L2 | L3 | L4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 20–29 | 42 | 316 ± 50bdefghi | 296 ± 39acefghi | 312 ± 46bdefghi | 299 ± 44acefghi | 262 ± 35abcdfghi | 234 ± 34abcdeghi | 177 ± 21abcdefhi | 170 ± 19abcdefg | 171 ± 22abcdefg |
| 30–39 | 95 | 337 ± 76bcdefghi | 315 ± 60acefghi | 329 ± 65abdefghi | 314 ± 64acefghi | 274 ± 55abcdfghi | 244 ± 48abcdeghi | 171 ± 29abcdefhi | 165 ± 28abcdefgi | 167 ± 28abcdefgh |
| 40–49 | 135 | 339 ± 79bcdefghi | 318 ± 64*acdefghi | 328 ± 65abefghi | 323 ± 64*abefghi | 285 ± 57*abcdfghi | 248 ± 51abcdeghi | 168 ± 30abcdefhi | 159 ± 29*abcdefg | 159 ± 28*†abcdefg |
| 50–59 | 72 | 280 ± 70*†‡bcdefghi | 264 ± 62*†‡acefghi | 271 ± 69*†‡abefghi | 267 ± 68*†‡aefghi | 230 ± 59*†‡abcdfghi | 208 ± 54*†‡abcdeghi | 132 ± 34*†‡abcdefhi | 123 ± 34*†‡abcdefg | 125 ± 34*†‡abcdefg |
ap < 0.05 vs. vBMD values of C2; bp < 0.05 vs. vBMD values of C3; cp < 0.05 vs. vBMD values of C4; dp < 0.05 vs. vBMD values of C5; ep < 0.05 vs. vBMD values of C6; fp < 0.05 vs. vBMD values of C7; gp < 0.05 vs. vBMD values of L1; hp < 0.05 vs. vBMD values of L2; ip < 0.05 vs. vBMD values of L3. *p < 0.05 vs. 20–29 age group, †p < 0.05 vs. 30–39 age group, ‡p < 0.05 vs. 40–49 age group. vBMD = volumetric bone mineral density
Fig. 1. Outline of vBMD data between groups for males assessed by QCT.
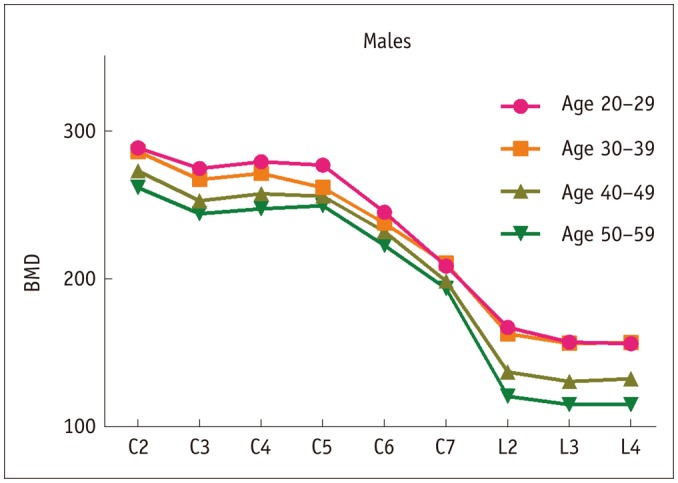
BMD = bone mineral density, QCT = quantitative computed tomography, vBMD = volumetric BMD
Fig. 2. Outline of vBMD data between groups for females assessed by QCT.
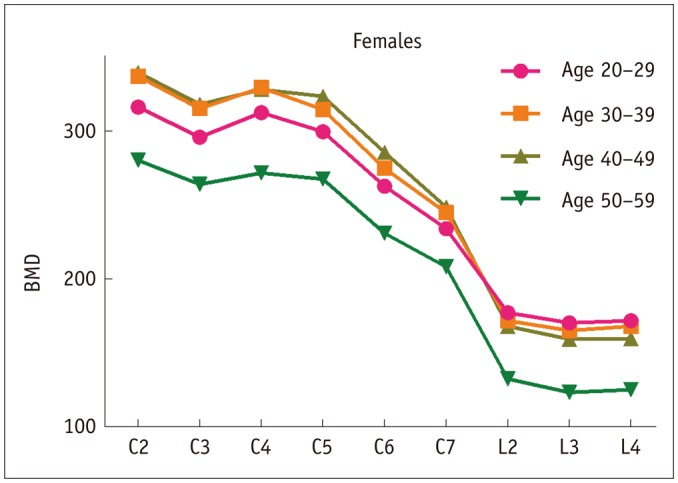
BMD = bone mineral density, QCT = quantitative computed tomography, vBMD = volumetric BMD
In population-based analysis for males, mean vBMD values were the highest in the 20–29 years age group for the cervical (C2–6) and lumbar (L2–4) vertebrae except the C7 vertebrae, which peaked in the 30–39 years age group. Generally, the vBMD values decreased with aging, while the vBMD values of L2–4 remained unchanged until 30–39 years of age and decreased more significantly than those of the cervical vertebrae in the 40–49 and 50–59 years age groups. Interestingly, most of the data showed statistically significant differences in every other age group. For the analysis of differences among vertebrae, the vBMD values of C2 was the highest, followed by C4 and C5, in order, in different age groups. Moreover, the vBMD values of C3 were between those of C2 and C4, and the vBMD values of C5–6 were reduced from C5 and significantly different from those of C2–4 vertebrae. However, the vBMD values of cervical vertebrae were higher than those of the lumbar vertebrae. In addition, the differences of vBMD values between L2–4 vertebrae were not statistically significant among different age groups.
In females, the vBMD values of cervical vertebrae increased with aging but decreased in the 50–59 years age group; conversely, the vBMD values of lumbar vertebrae peaked in the 20–29 years age group and then decreased with aging. For the analysis of difference among vertebrae, the vBMD values of C2 was the highest followed by C4 in all age groups. The shifting trends of C3, C5–7 and L2–4 were consistent with those of males; in addition, the vBMD values of cervical vertebrae were significantly higher than those of lumbar vertebrae, similar to males.
The vBMD values of vertebrae in females were significantly higher than those in males at each age group for both the cervical and lumbar vertebrae.
Age-Stratified Study of Correlations between Each Level of Vertebrae for Males and Females
The vBMD correlation coefficients between different vertebrae at each age group were shown in Table 4 for males and Table 5 for females. In both males and females, good correlations were observed among lumbar vertebrae (males: r = 0.93–0.98; females: r = 0.82–0.97) at each age group, as well as the cervical vertebrae (females: r = 0.62–0.94; males: r = 0.63–0.94), but more prominent among adjacent levels (females: r = 0.825–0.943; males: r = 0.758–0.928). The correlation of the lumbar vertebrae was higher than that of the cervical vertebrae. Besides, low correlations were detected for C2 and C7 (r = 0.49) in females and for C7 and C2–6 (r = 0.35–0.54) in males. Some correlation was also found between cervical and lumbar vertebrae (males: r = 0.46–0.69; females: r = 0.46–0.85).
Table 4. Correlation between Sections for Males.
| Sections | Age Group | C2 | C3 | C4 | C5 | C6 | C7 | L2 | L3 |
|---|---|---|---|---|---|---|---|---|---|
| C3 | 20–29 | 0.825 | |||||||
| 30–39 | 0.903 | ||||||||
| 40–49 | 0.885 | ||||||||
| 50–59 | 0.855 | ||||||||
| C4 | 20–29 | 0.784 | 0.922 | ||||||
| 30–39 | 0.870 | 0.943 | |||||||
| 40–49 | 0.880 | 0.923 | |||||||
| 50–59 | 0.850 | 0.895 | |||||||
| C5 | 20–29 | 0.657 | 0.788 | 0.837 | |||||
| 30–39 | 0.862 | 0.917 | 0.911 | ||||||
| 40–49 | 0.822 | 0.865 | 0.906 | ||||||
| 50–59 | 0.787 | 0.855 | 0.857 | ||||||
| C6 | 20–29 | 0.631 | 0.721 | 0.736 | 0.884 | ||||
| 30–39 | 0.849 | 0.904 | 0.882 | 0.937 | |||||
| 40–49 | 0.772 | 0.786 | 0.859 | 0.892 | |||||
| 50–59 | 0.826 | 0.814 | 0.858 | 0.863 | |||||
| C7 | 20–29 | 0.389 | 0.384 | 0.354 | 0.451 | 0.540 | |||
| 30–39 | 0.718 | 0.774 | 0.726 | 0.797 | 0.846 | ||||
| 40–49 | 0.672 | 0.724 | 0.769 | 0.787 | 0.866 | ||||
| 50–59 | 0.800 | 0.808 | 0.765 | 0.782 | 0.868 | ||||
| L2 | 20–29 | 0.524 | 0.590 | 0.492 | 0.546 | 0.644 | 0.492 | ||
| 30–39 | 0.600 | 0.628 | 0.639 | 0.636 | 0.680 | 0.648 | |||
| 40–49 | 0.540 | 0.546 | 0.564 | 0.631 | 0.656 | 0.592 | |||
| 50–59 | 0.548 | 0.555 | 0.559 | 0.583 | 0.660 | 0.687 | |||
| L3 | 20–29 | 0.546 | 0.595 | 0.489 | 0.542 | 0.663 | 0.510 | 0.965 | |
| 30–39 | 0.588 | 0.625 | 0.617 | 0.633 | 0.674 | 0.656 | 0.976 | ||
| 40–49 | 0.509 | 0.505 | 0.515 | 0.600 | 0.619 | 0.538 | 0.955 | ||
| 50–59 | 0.532 | 0.528 | 0.524 | 0.544 | 0.652 | 0.697 | 0.968 | ||
| L4 | 20–29 | 0.590 | 0.570 | 0.465 | 0.473 | 0.590 | 0.502 | 0.930 | 0.948 |
| 30–39 | 0.588 | 0.623 | 0.614 | 0.629 | 0.667 | 0.681 | 0.946 | 0.970 | |
| 40–49 | 0.540 | 0.541 | 0.540 | 0.622 | 0.633 | 0.542 | 0.926 | 0.959 | |
| 50–59 | 0.562 | 0.544 | 0.537 | 0.543 | 0.655 | 0.696 | 0.931 | 0.964 |
Table 5. Correlation between Sections for Females.
| Sections | Age Group | C2 | C3 | C4 | C5 | C6 | C7 | L2 | L3 |
|---|---|---|---|---|---|---|---|---|---|
| C3 | 20–29 | 0.810 | |||||||
| 30–39 | 0.943 | ||||||||
| 40–49 | 0.871 | ||||||||
| 50–59 | 0.899 | ||||||||
| C4 | 20–29 | 0.790 | 0.901 | ||||||
| 30–39 | 0.898 | 0.919 | |||||||
| 40–49 | 0.898 | 0.919 | |||||||
| 50–59 | 0.854 | 0.927 | |||||||
| C5 | 20–29 | 0.744 | 0.796 | 0.883 | |||||
| 30–39 | 0.842 | 0.870 | 0.889 | ||||||
| 40–49 | 0.794 | 0.869 | 0.903 | ||||||
| 50–59 | 0.857 | 0.888 | 0.904 | ||||||
| C6 | 20–29 | 0.616 | 0.743 | 0.801 | 0.758 | ||||
| 30–39 | 0.794 | 0.810 | 0.852 | 0.913 | |||||
| 40–49 | 0.745 | 0.822 | 0.861 | 0.914 | |||||
| 50–59 | 0.853 | 0.913 | 0.907 | 0.919 | |||||
| C7 | 20–29 | 0.491 | 0.729 | 0.820 | 0.740 | 0.795 | |||
| 30–39 | 0.813 | 0.816 | 0.828 | 0.887 | 0.928 | ||||
| 40–49 | 0.741 | 0.809 | 0.846 | 0.860 | 0.901 | ||||
| 50–59 | 0.849 | 0.865 | 0.836 | 0.866 | 0.920 | ||||
| L2 | 20–29 | 0.732 | 0.780 | 0.827 | 0.777 | 0.711 | 0.787 | ||
| 30–39 | 0.683 | 0.708 | 0.693 | 0.708 | 0.727 | 0.762 | |||
| 40–49 | 0.635 | 0.646 | 0.670 | 0.700 | 0.730 | 0.717 | |||
| 50–59 | 0.798 | 0.811 | 0.836 | 0.850 | 0.843 | 0.850 | |||
| L3 | 20–29 | 0.679 | 0.693 | 0.742 | 0.730 | 0.540 | 0.705 | 0.896 | |
| 30–39 | 0.699 | 0.720 | 0.706 | 0.716 | 0.733 | 0.786 | 0.970 | ||
| 40–49 | 0.623 | 0.621 | 0.636 | 0.672 | 0.690 | 0.687 | 0.972 | ||
| 50–59 | 0.789 | 0.800 | 0.828 | 0.817 | 0.811 | 0.806 | 0.962 | ||
| L4 | 20–29 | 0.680 | 0.674 | 0.727 | 0.715 | 0.464 | 0.647 | 0.817 | 0.942 |
| 30–39 | 0.626 | 0.666 | 0.651 | 0.662 | 0.682 | 0.730 | 0.931 | 0.961 | |
| 40–49 | 0.618 | 0.631 | 0.643 | 0.681 | 0.697 | 0.698 | 0.953 | 0.964 | |
| 50–59 | 0.799 | 0.788 | 0.817 | 0.836 | 0.815 | 0.821 | 0.944 | 0.960 |
DISCUSSION
Differences in BMD values measured by DEXA and QCT are widely recognized (20,26,27,28). The reason is possibly related to different bone turnover between the cancellous and the cortical bone. DEXA is unable to selectively measure a specific area, which will inevitably include the cortical bone. This may affect our pre-operative evaluations on the state of bone and future surgical plans. However, QCT was more advanced in selectively measuring the volumetric trabecular bone (cancellous bone only) without any superimposition of surrounding tissues (29).
Previous studies have investigated the vBMD data of cervical vertebrae using different techniques (30,31,32). Curylo et al. (32) reported significant differences of the BMD values measured by DEXA among levels of the lower cervical vertebrae in a human cadaver study (range of age: 61 to 81 years); they also found that the BMD values were the highest for C5 and decreased both cephalically and caudally. Moreover, Anderst et al. (30) and Weishaupt et al. (31) reported that the BMD value of C5 was the highest among the cervical vertebrae. The vBMD data from our study are in general agreement with those of the previous studies, with some differences. In both, males and females, the vBMD values of the cervical vertebrae were gradually reduced from the cephalic to the caudal levels, while the lumbar vertebrae showed no difference. Additionally, the vBMD values of C2 were the highest, which was different from previous studies (30,31,32); however, the vBMD values of C5 were not the highest among cervical vertebrae, according to some reports. Yoganandan et al. (18) reported a decreasing trend in mean BMD from the neck to the low back in 57 males and the highest mean BMD of C2, which was higher than that of C5. On the other hand, in another study, Yoganandan et al. (19) measured the BMD of cervical, thoracic and lumbar vertebrae of 30 female subjects and found that the BMD of C2 (275.3 mg/mL) was slightly lower than that of C5 (280.4 mg/mL). The size of the study population and methodology differ among the reports. Our study had a larger number of subjects (n = 598) than studies by Anderst et al. (30) (n = 21) and Weishaupt et al. (31) (n = 50). Furthermore, as part of the upper cervical vertebrae, C1–2 share about 60% of the rotating and 40% of the flexion-extension movements, which might contribute to the high vBMD values of C2. Genetic, racial, environmental difference and nutritional factors might be other explanations (33,34,35,36). Besides, cervical osteoporotic fractures rarely occur in C2 (37), which indirectly corroborates our finding. Moreover, the vBMD values of the cervical vertebrae were not uniform and exhibited greater fluctuations than those of lumbar vertebrae, especially in C6 and C7, which were significantly different from those of C2–5, possibly due to the unique characteristics of cervical vertebral anatomy, phylogenetic factors and complex motions from the surrounding muscles (18,38). Miller et al. (39) reported that fractures at C7 level were more common than those at other cervical levels, which could be attributed to lowest BMD values among the cervical vertebrae.
Extensive data stratified for age and sex were obtained. Similar to the lumbar vertebrae in males, the cervical vertebrae showed a decreasing trend of bone mass with aging. However, different from the decreasing trend of the lumbar vertebrae in females, the vBMD values of the cervical vertebrae increased with aging and decreased dramatically till menopause. This phenomenon might be related to the secretion and accumulation of estrogen with aging, but the specific reasons remain unknown. The vBMD values of the cervical and lumbar vertebrae were higher in females, as compared to males at every age group. The results are in accordance with earlier studies (18,19,20,40). This findings differ from the areal BMD results by DEXA, possibly since areal BMD by DEXA are size-dependent and tend to overestimate areal BMD in patients with large bones or higher BMI, and underestimate it in patients with small bones or lower BMI (41,42). The results are consistent with the phenomenon in which the incidence of cervical fractures in females is lower than that in males (43,44,45), as the cervical vertebrae might have a lower likelihood of fracture than the lumbar vertebrae due to the higher vBMD values (46,47,48).
Correlations among the lumbar vertebrae were higher than those in the cervical vertebrae, and some correlation was detected among the cervical and lumbar vertebrae, as reported previously (18,19,20), indicating that the vBMD values of the lumbar vertebrae do not accurately predict the vBMD values in the cervical vertebrae. Therefore, it is necessary to obtain the vBMD values from its adjacent levels. In addition, with the lack of differences observed among L2–4 vBMD values at each age group, the lumbar vertebrae is more suitable for clinical and biomechanical evaluation and as the reference of bone mass throughout the body.
To our knowledge, this is the first clinical study to investigate and establish the normative data on cervical vertebrae in an age-stratified and sex-related manner. In addition, we measured the vBMD values of the cervical and lumbar vertebrae on the same day in order to guarantee the accuracy of the descriptions of correlation between the cervical and lumbar vertebrae. However, there were still some limitations. First, we excluded the 10–19 years-of-age group because this group was still in the growth stage, and not authorized for study by the Ethics Committee. Moreover, the small number of subjects in the 60–69 years-of-age group could have further limited the statistical analysis.
In conclusion, the present study comparatively determined the vBMD values of the cervical and lumbar vertebrae from 598 volunteers using QCT in an age- and sex-stratified manner. The vBMD values generally decreased in both the cervical and lumbar vertebrae with aging, except for the cervical vertebrae in females, which increased with aging and then decreased dramatically till menopause. Additionally, the vBMD value of C2 was the highest, suggesting that trabecular bony architecture was denser and more protected by the high BMD values. These prominent normative data of the cervical vertebrae from the QCT could be helpful to comprehensively evaluate the cervical spine status and design a better pre-operative surgical plan for implant instrument.
Footnotes
This work was funded by Grants from Beijing Municipal Bureau of Health of 215 program (no. 2009-02-03) and the capital health research and development of special (no. 2014-2-1122).
References
- 1.Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22:1277–1288. doi: 10.1007/s00198-011-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Amelio P, Rossi P, Isaia G, Lollino N, Castoldi F, Girardo M, et al. Bone mineral density and singh index predict bone mechanical properties of human femur. Connect Tissue Res. 2008;49:99–104. doi: 10.1080/03008200801913940. [DOI] [PubMed] [Google Scholar]
- 3.Jiang C, Giger ML, Kwak SM, Chinander MR, Martell JM, Favus MJ. Normalized BMD as a predictor of bone strength. Acad Radiol. 2000;7:33–39. doi: 10.1016/s1076-6332(00)80441-9. [DOI] [PubMed] [Google Scholar]
- 4.Devlin HB, Goldman M. Backache due to osteoporosis in an industrial population. A survey of 481 patients. Ir J Med Sci. 1966;6:141–148. doi: 10.1007/BF02943677. [DOI] [PubMed] [Google Scholar]
- 5.Ensrud KE, Blackwell TL, Cawthon PM, Bauer DC, Fink HA, Schousboe JT, et al. Degree of trauma differs for major osteoporotic fracture events in older men versus older women. J Bone Miner Res. 2016;31:204–207. doi: 10.1002/jbmr.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z, Griffith JF, Leung PC, Lee R. Effect of osteoporosis on morphology and mobility of the lumbar spine. Spine (Phila Pa 1976) 2009;34:E115–E121. doi: 10.1097/BRS.0b013e3181895aca. [DOI] [PubMed] [Google Scholar]
- 7.Fechtenbaum J, Etcheto A, Kolta S, Feydy A, Roux C, Briot K. Sagittal balance of the spine in patients with osteoporotic vertebral fractures. Osteoporos Int. 2016;27:559–567. doi: 10.1007/s00198-015-3283-y. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe M, Sakai D, Yamamoto Y, Sato M, Mochida J. Upper cervical spine injuries: age-specific clinical features. J Orthop Sci. 2010;15:485–492. doi: 10.1007/s00776-010-1493-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang XM, Yu BS, Zheng ZM, Zhang JF, Lu WW. Effect of the degree of osteoporosis on the biomechanical anchoring strength of the sacral pedicle screws: an in vitro comparison between unaugmented bicortical screws and polymethylmethacrylate augmented unicortical screws. Spine (Phila Pa 1976) 2010;35:E925–E931. doi: 10.1097/BRS.0b013e3181c5fb21. [DOI] [PubMed] [Google Scholar]
- 10.Thiele OC, Eckhardt C, Linke B, Schneider E, Lill CA. Factors affecting the stability of screws in human cortical osteoporotic bone: a cadaver study. J Bone Joint Surg Br. 2007;89:701–705. doi: 10.1302/0301-620X.89B5.18504. [DOI] [PubMed] [Google Scholar]
- 11.Ebbesen EN, Thomsen JS, Beck-Nielsen H, Nepper-Rasmussen HJ, Mosekilde L. Lumbar vertebral body compressive strength evaluated by dual-energy X-ray absorptiometry, quantitative computed tomography, and ashing. Bone. 1999;25:713–724. doi: 10.1016/s8756-3282(99)00216-1. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, et al. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res. 2011;26:2194–2203. doi: 10.1002/jbmr.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebbesen EN, Thomsen JS, Beck-Nielsen H, Nepper-Rasmussen HJ, Mosekilde L. Vertebral bone density evaluated by dual-energy X-ray absorptiometry and quantitative computed tomography in vitro. Bone. 1998;23:283–290. doi: 10.1016/s8756-3282(98)00091-x. [DOI] [PubMed] [Google Scholar]
- 14.Yoganandan N, Kumaresan S, Pintar FA. Biomechanics of the cervical spine part 2. Cervical spine soft tissue responses and biomechanical modeling. Clin Biomech (Bristol, Avon) 2001;16:1–27. doi: 10.1016/s0268-0033(00)00074-7. [DOI] [PubMed] [Google Scholar]
- 15.White AA, 3rd, Panjabi MM. The basic kinematics of the human spine. A review of past and current knowledge. Spine (Phila Pa 1976) 1978;3:12–20. doi: 10.1097/00007632-197803000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Huelke DF, Nusholtz GS. Cervical spine biomechanics: a review of the literature. J Orthop Res. 1986;4:232–245. doi: 10.1002/jor.1100040212. [DOI] [PubMed] [Google Scholar]
- 17.Grote HJ, Amling M, Vogel M, Hahn M, Pösl M, Delling G. Intervertebral variation in trabecular microarchitecture throughout the normal spine in relation to age. Bone. 1995;16:301–308. doi: 10.1016/8756-3282(94)00042-5. [DOI] [PubMed] [Google Scholar]
- 18.Yoganandan N, Pintar FA, Stemper BD, Baisden JL, Aktay R, Shender BS, et al. Trabecular bone density of male human cervical and lumbar vertebrae. Bone. 2006;39:336–344. doi: 10.1016/j.bone.2006.01.160. [DOI] [PubMed] [Google Scholar]
- 19.Yoganandan N, Pintar FA, Stemper BD, Baisden JL, Aktay R, Shender BS, et al. Bone mineral density of human female cervical and lumbar spines from quantitative computed tomography. Spine (Phila Pa 1976) 2006;31:73–76. doi: 10.1097/01.brs.0000192684.12046.93. [DOI] [PubMed] [Google Scholar]
- 20.Yu W, Qin M, Xu L, van Kuijk C, Meng X, Xing X, et al. Normal changes in spinal bone mineral density in a Chinese population: assessment by quantitative computed tomography and dual-energy X-ray absorptiometry. Osteoporos Int. 1999;9:179–187. doi: 10.1007/s001980050133. [DOI] [PubMed] [Google Scholar]
- 21.Prevrhal S, Shepherd JA, Faulkner KG, Gaither KW, Black DM, Lang TF. Comparison of DXA hip structural analysis with volumetric QCT. J Clin Densitom. 2008;11:232–236. doi: 10.1016/j.jocd.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27:119–124. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bligh M, Bidaut L, White RA, Murphy WA, Jr, Stevens DM, Cody DD. Helical multidetector row quantitative computed tomography (QCT) precision. Acad Radiol. 2009;16:150–159. doi: 10.1016/j.acra.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23:130–137. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Wang W, Xu L, Cheng X, Ma Y, Liu D, et al. Relation of visceral and subcutaneous adipose tissue to bone mineral density in chinese women. Int J Endocrinol. 2013;2013:378632. doi: 10.1155/2013/378632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickhardt PJ, Bodeen G, Brett A, Brown JK, Binkley N. Comparison of femoral neck BMD evaluation obtained using Lunar DXA and QCT with asynchronous calibration from CT colonography. J Clin Densitom. 2015;18:5–12. doi: 10.1016/j.jocd.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Emohare O, Dittmer A, Morgan RA, Switzer JA, Polly DW., Jr Osteoporosis in acute fractures of the cervical spine: the role of opportunistic CT screening. J Neurosurg Spine. 2015;23:1–7. doi: 10.3171/2014.10.SPINE14233. [DOI] [PubMed] [Google Scholar]
- 28.Setiawati R, Di Chio F, Rahardjo P, Nasuto M, Dimpudus FJ, Guglielmi G. Quantitative assessment of abdominal aortic calcifications using lateral lumbar radiograph, dual-energy X-ray absorptiometry, and quantitative computed tomography of the spine. J Clin Densitom. 2016;19:242–249. doi: 10.1016/j.jocd.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Grampp S, Jergas M, Glüer CC, Lang P, Brastow P, Genant HK. Radiologic diagnosis of osteoporosis. Current methods and perspectives. Radiol Clin North Am. 1993;31:1133–1145. [PubMed] [Google Scholar]
- 30.Anderst WJ, Thorhauer ED, Lee JY, Donaldson WF, Kang JD. Cervical spine bone mineral density as a function of vertebral level and anatomic location. Spine J. 2011;11:659–667. doi: 10.1016/j.spinee.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weishaupt D, Schweitzer ME, DiCuccio MN, Whitley PE. Relationships of cervical, thoracic, and lumbar bone mineral density by quantitative CT. J Comput Assist Tomogr. 2001;25:146–150. doi: 10.1097/00004728-200101000-00027. [DOI] [PubMed] [Google Scholar]
- 32.Curylo LJ, Lindsey RW, Doherty BJ, LeBlanc A. Segmental variations of bone mineral density in the cervical spine. Spine (Phila Pa 1976) 1996;21:319–332. doi: 10.1097/00007632-199602010-00013. [DOI] [PubMed] [Google Scholar]
- 33.Johnston CC, Jr, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, et al. Calcium supplementation and increases in bone mineral density in children. N Engl J Med. 1992;327:82–87. doi: 10.1056/NEJM199207093270204. [DOI] [PubMed] [Google Scholar]
- 34.Garn SM, Pao EM, Rihl ME. Compact bone in Chinese and Japanese. Science. 1964;143:1439–1440. doi: 10.1126/science.143.3613.1439. [DOI] [PubMed] [Google Scholar]
- 35.Wright NM, Papadea N, Willi S, Veldhuis JD, Pandey JP, Key LL, et al. Demonstration of a lack of racial difference in secretion of growth hormone despite a racial difference in bone mineral density in premenopausal women--a Clinical Research Center study. J Clin Endocrinol Metab. 1996;81:1023–1026. doi: 10.1210/jcem.81.3.8772569. [DOI] [PubMed] [Google Scholar]
- 36.Abraham AC, Agarwalla A, Yadavalli A, McAndrew C, Liu JY, Tang SY. Multiscale predictors of femoral neck in situ strength in aging women: contributions of BMD, cortical porosity, reference point indentation, and nonenzymatic glycation. J Bone Miner Res. 2015;30:2207–2214. doi: 10.1002/jbmr.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23:38–40. doi: 10.1016/0020-1383(92)90123-a. [DOI] [PubMed] [Google Scholar]
- 38.Fard SA, Patel AS, Avila MJ, Sattarov KV, Walter CM, Skoch J, et al. Anatomic considerations of the anterior upper cervical spine during decompression and instrumentation: a cadaveric based study. J Clin Neurosci. 2015;22:1810–1815. doi: 10.1016/j.jocn.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Miller CP, Brubacher JW, Biswas D, Lawrence BD, Whang PG, Grauer JN. The incidence of noncontiguous spinal fractures and other traumatic injuries associated with cervical spine fractures: a 10-year experience at an academic medical center. Spine (Phila Pa 1976) 2011;36:1532–1540. doi: 10.1097/BRS.0b013e3181f550a6. [DOI] [PubMed] [Google Scholar]
- 40.Cheng XG, Li K, Ou SX, Tang GY, Wang QQ, Wang C, et al. Heterogeneity in spinal bone mineral density among young adults from three eastern provincial capital cities in mainland China. J Clin Densitom. 2016 Apr 29; doi: 10.1016/j.jocd.2016.03.009. [Epub] [DOI] [PubMed] [Google Scholar]
- 41.Weigert J, Cann C. DXA in obese patients: are normal values really normal. J Women's Imaging. 1999;1:11–17. [Google Scholar]
- 42.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 43.Trafton PG, Boyd CA., Jr Computed tomography of thoracic and lumbar spine injuries. J Trauma. 1984;24:506–515. doi: 10.1097/00005373-198406000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Fredø HL, Bakken IJ, Lied B, Rønning P, Helseth E. Incidence of traumatic cervical spine fractures in the Norwegian population: a national registry study. Scand J Trauma Resusc Emerg Med. 2014;22:78. doi: 10.1186/s13049-014-0078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riggins RS, Kraus JF. The risk of neurologic damage with fractures of the vertebrae. J Trauma. 1977;17:126–133. doi: 10.1097/00005373-197702000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Leucht P, Fischer K, Muhr G, Mueller EJ. Epidemiology of traumatic spine fractures. Injury. 2009;40:166–172. doi: 10.1016/j.injury.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Xiang L, Liu J, Zhou Y, Ou L. Gender differences in the clinical characteristics of traumatic spinal fractures among the elderly. Arch Gerontol Geriatr. 2014;59:657–664. doi: 10.1016/j.archger.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Heidari P, Zarei MR, Rasouli MR, Vaccaro AR, Rahimi-Movaghar V. Spinal fractures resulting from traumatic injuries. Chin J Traumatol. 2010;13:3–9. [PubMed] [Google Scholar]


