Abstract
Asbestosis is the most important change noted in the lung parenchyma after environmental and occupational exposure to asbestos fibers. It is characterized by diffuse interstitial pulmonary fibrosis. In Korea, the incidence of asbestosis will continue to increase for many years to come and the government enacted the Asbestos Damage Relief Law in 2011 to provide compensation to those suffering from asbestos-related diseases. Radiologic evaluation is necessary for diagnosis of asbestosis, and radiologists play a key role in this process. Therefore, it is important for radiologists to be aware of the various imaging features of asbestosis.
Keywords: Asbestosis, Asbestos, Pulmonary, Computed tomography, Radiography, Radiology, Occupational disease
INTRODUCTION
Asbestos, once considered as a miracle mineral, being resistant to fire, heat, and corrosion, is strong, durable, flexible and inexpensive, and has been used to make a vast array of friction materials, gaskets, roofing, and fireproofing materials (1,2). There was a sharp increase in the use of asbestos in the 1970s as the Korean economy developed rapidly. However, asbestos is associated with many health problems, affecting principally the pleura and lung parenchyma, and is banned from Korea since 2009. Nonetheless, previous exposure to asbestos still causes many problems because asbestos-caused disease has a long latency period (2).
The most significant change that occurs in the lung parenchyma after asbestos exposure is lung fibrosis caused by asbestos dust, which is termed asbestosis (3). A definite dose-effect is evident between the asbestos exposure level and the severity of fibrosis (4,5). Disease usually develops approximately 20 years after initial exposure (5). Asbestosis is a principal disease of lung parenchyma exposed to asbestos, being second only to bronchogenic carcinoma in terms of frequency (6,7).
Asbestos-related diseases is expected to increase in frequency for many years to come (8,9). Based on a historical review of asbestos use and exposure in Korea, the disease is expected to peak in 2045 (10). In 2011, the Asbestos Damage Relief Law was established in Korea, and individuals who apply to the Korea Environment Corporation for asbestos damage relief are required to undergo chest computed tomography (CT) to evaluate the lung parenchyma and pleura. If compensation is to be made, CT must show changes consistent with asbestosis. In addition, it is necessary to document a history of asbestos exposure in combination with the chest CT findings, and/or to show impairment of pulmonary function. This is because pathological confirmation of disease is difficult even when asbestosis is suspected.
Therefore, radiologic evaluation of individuals exposed to asbestos plays a critical role in the assessment of asbestosis. It is important to be familiar with the radiologic characteristics of the disease because it will persist for some time. This article illustrates the imaging characteristics of early to advanced asbestosis, with particular emphasis on chest CT findings.
Pathogenesis of Asbestosis
Asbestos fibers are carried deep into the lungs, and activated macrophages attempt to ingest and remove them. However, many are retained in the lung parenchyma (11,12). The fibers induce apoptosis of macrophages and trigger inflammation. The latter effect is reduced if fibers become coated to create asbestos bodies, but most fibers in the lung parenchyma remain uncoated. Thus, asbestos fibers remain in the lung parenchyma for prolonged periods and penetrate the interstitium of the distal lung (11). The fibers induce inflammatory processes including alveolitis and inflammation of the surrounding interstitium, followed by fibrotic changes in the respiratory bronchioles that extend to adjacent alveolar tissue (13,14).
The College of American Pathologists' grading system of histologic criteria of asbestosis to describe severity and extent includes Grade I involving alveolar walls of respiratory bronchioles and the alveolar ducts; Grade II involving greater proportions of acini; Grade III involving the whole acinar structure; and Grade IV when honeycomb remodeling and large (up to 1 cm) dilated spaces are grossly visible in the lung parenchyma (15,16).
Diagnostic Criteria and Guidelines for Asbestosis
The diagnosis of asbestosis is based on the 2004 American Thoracic Society criteria and the 2014 Helsinki criteria. The former is slightly modified from the initial 1986 diagnostic criteria. Structural pathology consistent with asbestos-related disease is required. This can be shown by imaging or histology. Imaging includes chest radiography, high-resolution CT (HRCT), and possibly future imaging methods. Chest radiographs are evaluated using the International Labor Organization (ILO) classification, and must show irregular opacities and profusion score of more than 1/1. Such findings, together with a history of asbestos exposure, are adequate for a diagnosis of asbestosis. In subjects with low profusion scores on chest radiographs, CT can be helpful to diagnose asbestosis. In addition, evidence of disease causation by asbestos is required. This can be achieved by documenting occupational and environmental history, identifying a plausible latency period, demonstrating markers of asbestos exposure (usually pleural plaques), and/or recovering asbestos bodies from lung tissue. Evidence of functional impairments such as signs and symptoms and/or changes in pulmonary function test, are not required for a diagnosis, but are part of complete evaluation (17).
The 2014 Helsinki criteria require the patient to have a work history compatible with significant asbestos exposure. This may be documented via a structured interview and/or by identifying asbestos fibers in lung tissue and bronchoalveolar lavage fluid (18). For histologic diagnosis of asbestosis, the updated 2010 diagnostic criteria for asbestosis by a Committee of the College of American Pathologists and the Pulmonary Pathology Society are recommended (18,19). For radiologic diagnosis, chest radiographs revealing small opacities (ILO grade 1/0) are considered to indicate early-stage asbestosis. The use of CT imaging in diagnosis of asbestos-related diseases may be useful under the following conditions: 1) a borderline finding of lung fibrosis (ILO grade 0/1–1/0); 2) discrepancy between lung function findings of restriction and radiographs interpreted as normal; and 3) widespread pleural changes that severely hamper the radiographic visibility of lung parenchyma (18). The Helsinki criteria recommend the use of the International Classification of HRCT for Occupational and Environmental Respiratory Diseases to evaluate HRCT findings associated with asbestosis (18,20).
Asbestos-Related Malignancy
Asbestos related malignancies are lung cancer and malignant mesothelioma. Asbestos related lung cancer accounts for about 3–8% of all the lung cancers and the histologic type of cancer and its signs and symptoms in asbestos-exposed and unexposed individuals are similar (Fig. 1) (21). The exact mechanism of carcinogenesis is as yet unclear (3). However, risk of developing lung cancer is related to cumulative asbestos exposure. According to the Helsinki criteria, a cumulative exposure of 25 fibre-years is estimated to increase the risk of lung cancer 2-fold, and the clinical cases of asbestosis may occur at comparable cumulative exposures (18). In addition, asbestosis can be a marker for increased risk of lung cancer (22).
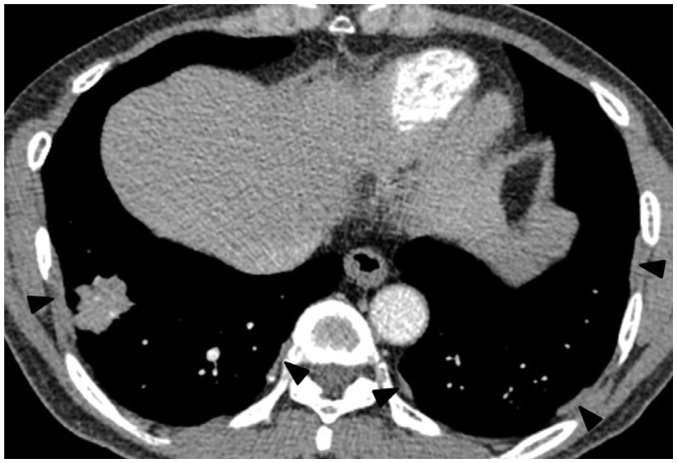
Fig. 1. 56-year-old male resident for 15 years near asbestos mine with work history at construction company for 10 years.
Chest CT shows irregular lobulated enhancing nodule in right lower lobe, which are confirmed as lung adenocarcinoma. There are multiple discrete pleural plaques (arrowheads) in both lower hemithoraces.
Malignant mesothelioma is the most common primary neoplasm of the pleura, arising from mesothelial cells. Although the necessary degree of asbestos exposure is considerably less than that of asbestosis and lung cancer, a dose dependent relationship is recognized (3,23). The attributable risk of asbestos for malignant mesothelioma is generally considered as 80 to 90%, but is around 60% in Korea. Pathologically confirmed malignant mesothelioma is internationally accepted as related to asbestos regardless of the patient's history of asbestos exposure (24). Several previous studies (25,26,27,28) report a higher mortality rate of malignant mesothelioma in workers with asbestosis, which suggests that asbestosis might have positive association between malignant mesothelioma (29).
Chest Radiographic Findings of Asbestosis
The initial radiographic characteristics of asbestosis are small, irregular or reticular opacities, predominantly in the bases of the lungs (Fig. 2), indicative of peribronchiolar and adjacent alveolar interstitial fibrosis (30). The opacities may progressively spread through the middle and upper lung zones (17). In more advanced cases, honeycombing is evident on chest radiographs (Fig. 3) (5). In addition, pleural thickening or pleural plaques may also be seen. However, there is no known radiographic finding that is pathognomonic of asbestosis. When asbestosis is evaluated via chest radiograph alone using the ILO classification, the diagnostic specificity is low in asbestos exposed individuals (7). Kipen et al. (31) show that 18% of patients with biopsy proven asbestosis have no radiographic abnormalities and 80% yield chest radiographic findings that do not correlate with their histopathologic grades. For these reasons, chest radiographs are of limited use for diagnosing asbestosis. A normal chest radiograph does not exclude the possibility of asbestosis in an asbestos-exposed individual.
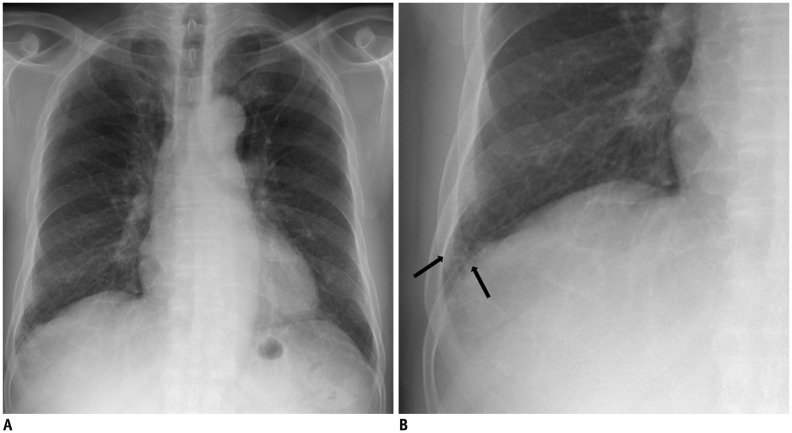
Fig. 2. 74-year-old male resident for 33 years under asbestos roof.
A. Chest PA radiograph shows reticular opacities in both lower lungs. B. Magnification of right lower lung shows reticular and small nodular opacities (arrows). PA = posteroanterior
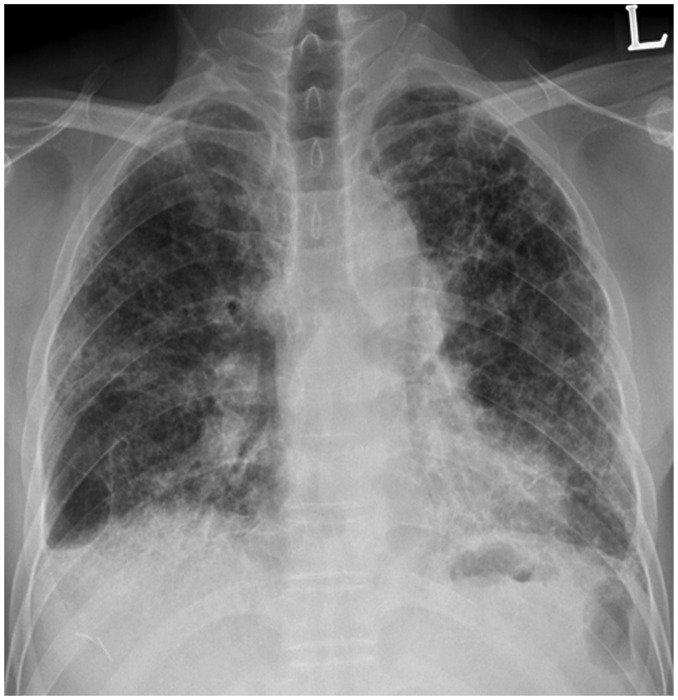
Fig. 3. 57-year-old male resident near asbestos mine since birth.
Chest PA radiograph shows reticular densities and honeycomb cysts in both lungs. PA = posteroanterior
Nonetheless, chest radiograph is important in the diagnosis of asbestosis, because it can document the presence or absence of pleural plaques as a marker of asbestos exposure, and allows the evaluation of the presence and extent of other complications such as lung cancer, pleural abnormalities, malignant mesothelioma, and round atelectasis (5).
Digital tomosynthesis is an alternative method with a low radiation dose compared to chest CT as well as better detection rate compared with chest radiograph (32,33,34,35). Digital tomosynthesis findings of asbestosis are presence of reticular opacities and/or honeycomb cysts in the bases of the lung, which are same as chest radiographs (36,37). Lee et al. (37) report that digital tomosynthesis is more sensitive than chest radiographs in the detection of asbestosis and also with detection of pleural plaques.
CT Findings of Asbestosis
Chest CT is much more sensitive than chest radiograph in evaluating asbestosis. Staples et al. (38) report that 57 of 169 asbestos exposed workers with normal chest radiographs show findings of asbestosis on CT. The chest radiographic findings are discrepant from the CT findings; only subtle changes of reticular densities are seen in chest radiographs; whereas, CT may reveal more advanced features of fibrosis such as honeycomb cysts (Fig. 4). Friedman et al. (39) show that 14 of 60 chest radiographs considered to reflect asbestosis are actually false-positives; and CT shows that the chest radiographic abnormalities are caused by emphysema, en-face plaques, or scarring from old tuberculosis.
Fig. 4. CT findings of patient of Figure 2.
Chest CT shows honeycomb cysts (arrows) in subpleural portions of both lower lobes.
Since early findings of asbestosis on CT are very subtle, it may be unsuitable to scan the patient with low dose or ultra-low dose CT scan, which increases image noise that may diminish image quality and depiction of radiologic findings of asbestosis. Tekath et al. (40) compare ultralow dose CT with standard CT for detecting asbestos related diseases. Ultra-low dose CT compares favorably with standard CT in detecting pleural plaques and diffuse pleural thickening, however interstitial pulmonary abnormalities are less frequent in ultra-low dose CT than standard CT.
Subpleural Dot-Like or Branching Opacities
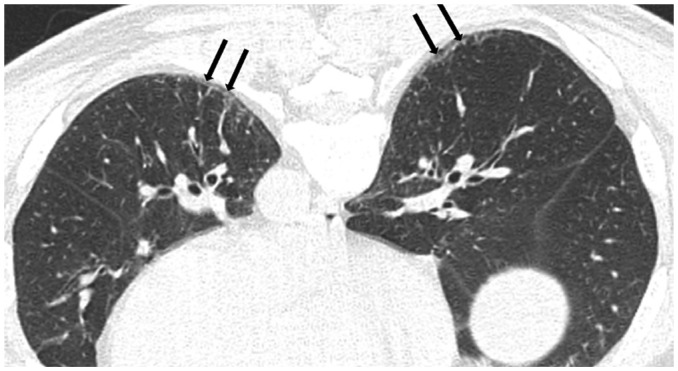
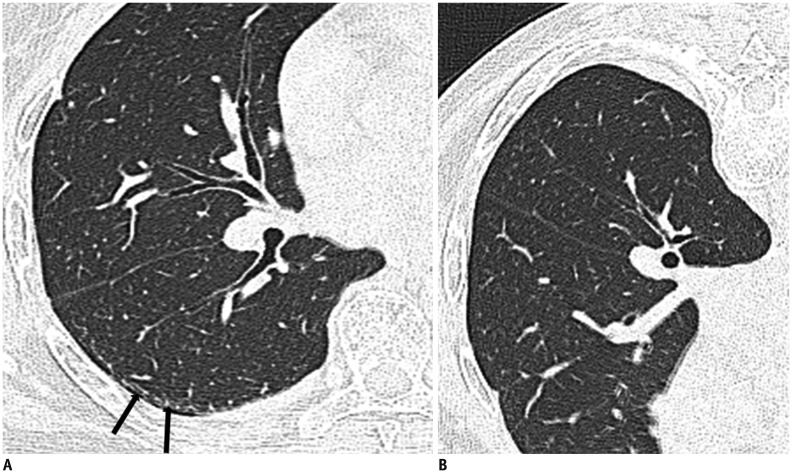
The earliest CT findings in asbestosis are subpleural dot-like or branching opacities located a few millimeters from the pleura but seldom touching the pleura, or they may appear as a fine branching structure (Fig. 5) (41,42). Some nodules are obvious whereas others present as very faint nodules of ground-glass opacity (GGO). Pathologically, subpleural dots correlate with the appearance of peribronchiolar fibrosis (41,42).
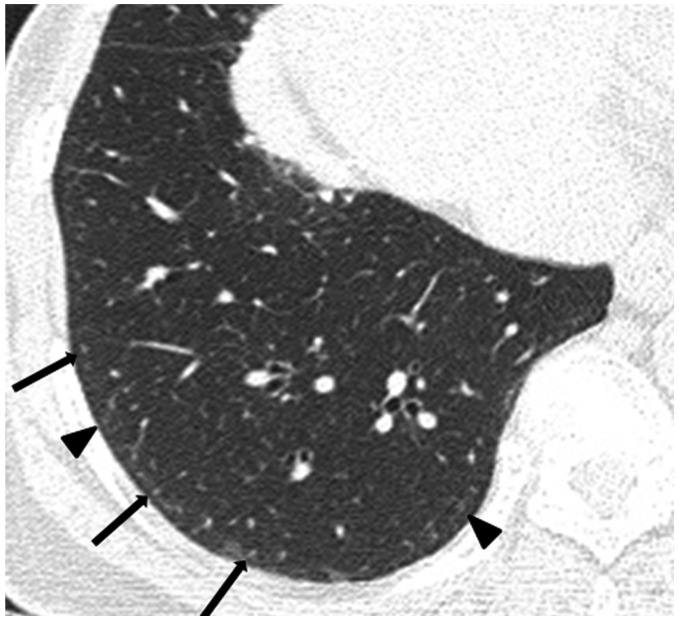
Fig. 5. 73-year-old female resident for 34 years near asbestos mine.
CT shows obvious (arrows) and faint (arrowheads) dot-like opacities in subpleural portions of lower lung.
Subpleural Curvilinear Lines
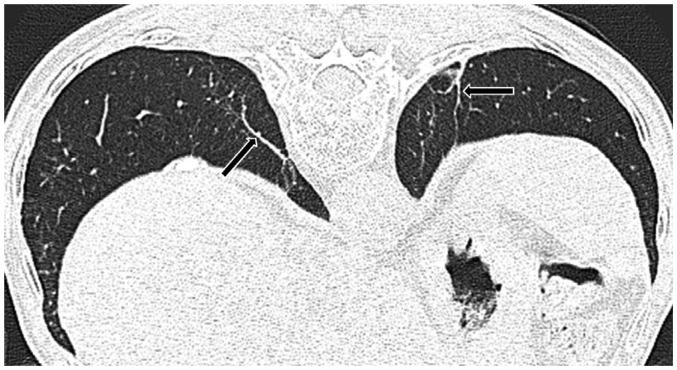
As the subpleural dot-like or branching opacities increase in number, confluence of dots creates subpleural curvilinear lines. Such lines are defined as linear areas of increased density located within 1 cm of the pleura and lying parallel to the inner chest wall on CT (Fig. 6). The development of subpleural curvilinear lines correlates with peribronchiolar fibrotic thickening combined with alveolar flattening and collapse attributable to fibrosis (41,42). Subpleural curvilinear lines can also indicate the presence of atelectasis, which has a propensity to occur adjacent to plaques (43).
Fig. 6. 60-year-old male resident near asbestos mine since birth with 3 year work history at mine.
Prone CT reveals subpleural curvilinear lines (arrows) in lower lungs.
Parenchymal Bands
Parenchymal bands are also commonly seen in asbestosis. On CT, the bands are linear, 2–5 cm in length, and contact the pleural surface (Fig. 7) (44). The bands reflect the development of fibrosis along the bronchovascular sheathes or interlobular septa, with distortion of the lung parenchyma (42). In asbestosis, these bands are often associated with areas of pleural plaques and frequently occur at the lung bases (Fig. 8) (4,45). According to Akira et al. (46), parenchymal bands occur significantly more often in patients with pleural thickening than in others.
Fig. 7. 82-year-old male resident near asbestos mine since birth.
Prone CT reveals parenchymal bands (arrows) extending through lung to contact pleural surface.
Fig. 8. 79-year-old male resident for 43 years near asbestos mine.
CT shows multiple parenchymal bands (arrows) adjacent to pleural plaques (arrowheads) in left lower lobe.
Ground Glass Opacities
Ground-glass opacity is uncommon as an isolated abnormality in asbestosis. When present, it is generally associated with other fibrotic findings (47). GGO in asbestosis reflects mild alveolar wall and interlobular thickening caused by fibrosis or edema (42).
Findings of Advanced Asbestosis
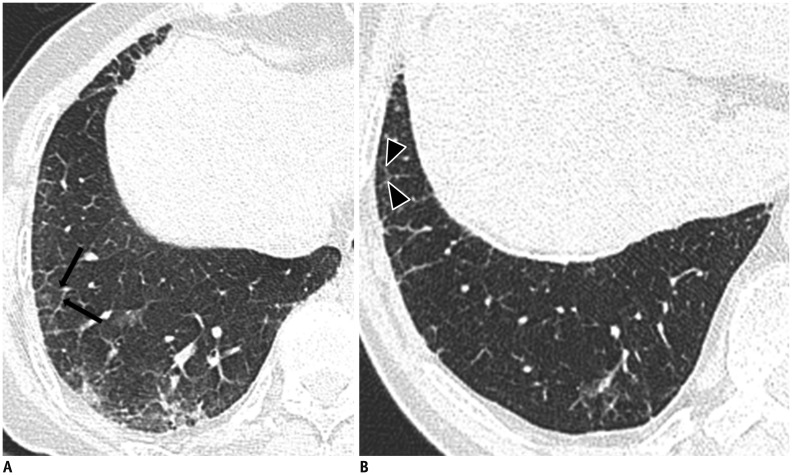
As the pulmonary fibrosis extends from the peribronchiolar lesions to involve the residual pulmonary lobules, other characteristic CT findings of pulmonary fibrosis develop. These include intralobular interstitial thickening, interlobular septal thickening (Fig. 9), traction bronchiectasis or bronchiolectasis, and honeycomb cysts (Fig. 10) (42,45,46). Intralobular interstitial thickening correlates with peribronchiolar fibrosis with subsequent involvement of the alveolar ducts and interlobular septal thickening correlates with interlobular fibrotic or edematous thickening. As findings of advanced asbestosis are similar to pulmonary fibrosis by other causes, they are difficult to distinguish.
Fig. 9. 79-year-old female resident for 60 years near asbestos mine with 5 year work history at mine.
A, B. CT shows interlobular septal thickening (arrows) (A) and intralobular interstitial thickening (arrowheads) (B) connected to peripheral pulmonary arteries.
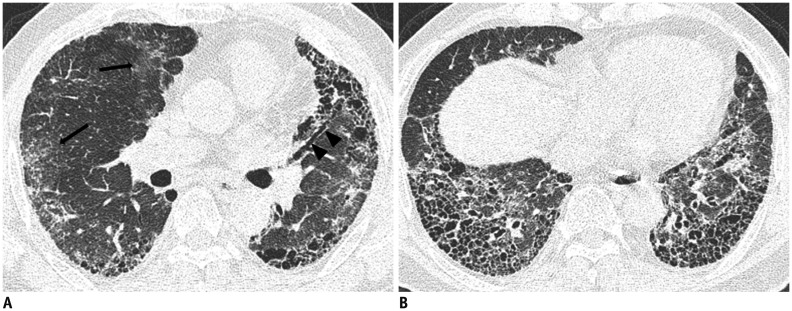
Fig. 10. 69-year-old male with 20 year work history at construction company.
A. CT shows ground-glass opacity (arrows), traction bronchiectasis (arrowheads) and honeycomb cysts. B. CT shows multilayered honeycomb cysts in both lower lungs.
These above mentioned CT abnormalities associated with asbestosis usually develop in the lower lung, posterior, and basal subpleural areas. As disease progresses, abnormalities also develop in the middle and upper lung zones. The CT abnormalities are usually bilateral and often somewhat symmetrical (41,46,48). As the posterior lung regions are typically involved early in asbestosis (49), it is important to scan asbestos-exposed individuals in the prone position to differentiate normal dependent atelectasis from early asbestosis in the posterior lung (Fig. 11) (44,45).
Fig. 11. Dependent atelectasis in patient with history of asbestos exposure.
A. On supine CT, subpleural line is visible in posterior portion of superior segment of right lower lobe (arrows). B. On prone CT, subpleural line is disappeared, indicative that thin posterior opacity is dependent atelectasis.
CT Findings of Asbestosis in Korea
Our previous study (50) indicates that the common CT findings of asbestosis in patients environmentally exposed to asbestos in Korea are centrilobular opacities, subpleural dot-like or branching opacities, interlobular septal thickening, intralobular interstitial thickening, parenchymal bands and subpleural curvilinear lines. Asbestos related lung parenchymal CT findings in the participants with environmental exposure are similar to those from occupational exposure. However, Kim et al. (51) show that occupationally exposed participants have larger extents of pleural plaques and pulmonary fibrosis than environmentally exposed participants. Subsequently, we retrospectively reviewed the chest CTs of 200 asbestosis patients compensated under the Asbestos Damage Relief Law from January 2011 to May 2013. We also reviewed 11 cases of idiopathic pulmonary fibrosis (IPF) to compare the imaging characteristics of asbestosis and IPF (Table 1).
Table 1. CT Findings in Patients with Asbestosis and IPF in Korea.
| CT Finding | Asbestosis (n = 200) | IPF (n = 11) | P |
|---|---|---|---|
| Subpleural dot-like or branching opacities | 195 (97.5%) | 0 | < 0.001 |
| Parenchymal bands | 101 (50.5%) | 0 | 0.012 |
| Parenchymal changes adjacent to pleural plaques | 79 (39.5%) | 0 | < 0.05 |
| Pleural plaques | 197 (98.5%) | 0 | < 0.001 |
| Subpleural curvilinear lines | 34 (17.0%) | 0 | |
| Intralobular interstitial thickening | 190 (95.0%) | 11 (100%) | |
| Interlobular septal thickening | 185 (92.5%) | 11 (100%) | |
| Honeycomb cysts | 35 (17.5%) | 9 (81.8%) | 0.009 |
| Ground-glass opacity | 31 (15.5%) | 11 (100%) | < 0.001 |
| Traction bronchiectasis | 87 (43.5%) | 11 (100%) | 0.004 |
IPF = idiopathic pulmonary fibrosis
In our study, subpleural dot-like or branching opacities are the most common feature of asbestosis (97.5%) but are absent in IPF (p < 0.001). The subpleural curvilinear lines are characteristic findings of asbestosis, but have relatively low frequency (17.0%) in the present study. However, such lines are absent in IPF, so the lines are very useful for the diagnosis of asbestosis. Parenchymal bands are often evident in asbestosis (50.5%) but not found in IPF (p = 0.012). Therefore, parenchymal bands are a characteristic feature of asbestosis. Parenchymal changes adjacent to pleural plaques are frequently seen in asbestosis (39.5%) (p < 0.05). Both parenchymal bands (54.4%) and round atelectasis (8.9%) are common findings. So parenchymal changes adjacent to pleural plaques are helpful in differentiating between asbestosis and IPF. Pleural plaques are observed in most cases of asbestosis (98.5%) but not IPF (p < 0.001). Therefore, pulmonary fibrosis associated with pleural plaques is helpful in the diagnosis of asbestosis. Thus, pleural plaques require careful consideration, particularly thin and small plaques.
In our study, intralobular interstitial thickening and interlobular septal thickening are common in both asbestosis and IPF. They are general findings of pulmonary fibrosis, and do not aid in differential diagnosis. Traction bronchiectasis is found in both asbestosis (43.5%) and IPF (100%) (p = 0.004). But traction bronchiectasis is more common in IPF than asbestosis, traction bronchiectasis is more indicative of IPF than asbestosis. Honeycomb cysts are much more frequently seen in IPF (81.8%) than asbestosis (17.5%) (p = 0.009). However, it is difficult to differentiate asbestosis featuring honeycomb cysts from IPF solely by findings of honeycomb cysts, and it is necessary to evaluate finding of subpleural dot-like or branching opacities in the less severely diseased lung, the history of asbestos exposure, and the presence of pleural plaques. GGO is also more frequent in IPF (100%) than asbestosis (15.5%) (p < 0.001). Although GGO is a helpful finding, it is considered a nonspecific feature of pulmonary fibrosis.
In summary, CT findings of subpleural dot-like or branching opacities, parenchymal bands, parenchymal changes adjacent to pleural plaques, and plaques are more frequently noted in asbestosis. Honeycomb cysts, GGOs, and traction bronchiectasis are more frequently noted in IPF. Although we have made an effort to differentiate asbestosis from IPF, some cases are very difficult to diagnose using CT findings alone. Appropriate history of asbestos exposure combined with CT abnormalities of the lung parenchyma and pleura must be used to ensure a reliable asbestosis diagnosis.
Differential Diagnosis between Asbestosis and IPF
A study by Akira et al. (46), emphasizes the specific combinations of CT findings strongly suggesting either asbestosis or IPF solely based on lung parenchymal findings other than associated pleural abnormalities. They show that subpleural dot-like or branching opacities, subpleural curvilinear lines, mosaic perfusions, and parenchymal bands are more common in patients with asbestosis. Honeycomb cysts, visible intralobular bronchioles and traction bronchiectasis are more common in patients with IPF; whereas, GGO, interlobular septal thickening, and emphysema are similar in both groups. They further indicate that the most important difference between asbestosis and IPF is subpleural dot-like or branching opacities in the subpleural region. Subpleural dot-like or branching opacities are found in mild as well as advanced cases of asbestosis. In advanced asbestosis, these opacities are found in less severely diseased regions of the lung (46). Copley et al. (52) compare the CT features of asbestosis with those of patients with biopsy proven nonspecific interstitial pneumonia (NSIP) and biopsy proven IPF. CT reveals coarser fibrosis in the asbestosis patients, as compared to NSIP cases; however, the fibrosis of the asbestosis is similar to that of the IPF. In study by Arakawa et al. (53) of 33 asbestos-exposed patients with pathologically confirmed pulmonary fibrosis, 15 patients are asbestosis and 18 patients include various kinds of fibrosis; subpleural curvilinear lines are the sole CT finding that differ between asbestosis and non-asbestosis cases.
CONCLUSION
The characteristic chest radiographic findings of early asbestosis are small irregular or reticular opacities, located predominantly in the lung bases and show honeycombing in advanced stages. The characteristic CT findings of asbestosis are subpleural dot-like or branching opacities, parenchymal bands, parenchymal changes adjacent to pleural plaques, subpleural curvilinear lines and plaques. In more advanced cases, honeycomb cysts, traction bronchiectasis, intralobular interstitial thickening and interlobular septal thickening are also seen. CT plays a key role in the diagnosis of asbestosis in Korea. Thus, knowledge of the characteristic CT findings of asbestosis can guide the diagnosis and management of patients. In addition, accurate diagnosis would encourage responsible asbestos-related damage judgments and lead to appropriate compensation.
Acknowledgments
The authors would like to thank Soon-Hee Jung and Jun Pyo Myong for their support to our study.
Footnotes
This research was supported by the Korea Ministry of Environment under the "The Environmental Health Action Program".
References
- 1.Cugell DW, Kamp DW. Asbestos and the pleura: a review. Chest. 2004;125:1103–1117. doi: 10.1378/chest.125.3.1103. [DOI] [PubMed] [Google Scholar]
- 2.Kim HR. Overview of asbestos issues in Korea. J Korean Med Sci. 2009;24:363–367. doi: 10.3346/jkms.2009.24.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roach HD, Davies GJ, Attanoos R, Crane M, Adams H, Phillips S. Asbestos: when the dust settles an imaging review of asbestos-related disease. Radiographics. 2002;22(Spec No):S167–S184. doi: 10.1148/radiographics.22.suppl_1.g02oc10s167. [DOI] [PubMed] [Google Scholar]
- 4.Lynch DA, Gamsu G, Ray CS, Aberle DR. Asbestos-related focal lung masses: manifestations on conventional and high-resolution CT scans. Radiology. 1988;169:603–607. doi: 10.1148/radiology.169.3.3186982. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS, Lynch DA. Imaging of nonmalignant occupational lung disease. J Thorac Imaging. 2002;17:238–260. doi: 10.1097/00005382-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Gefter WB, Epstein DM, Miller WT. Radiographic evaluation of asbestos-related chest disorders. Crit Rev Diagn Imaging. 1984;21:133–181. [PubMed] [Google Scholar]
- 7.Gefter WB, Conant EF. Issues and controversies in the plain-film diagnosis of asbestos-related disorders in the chest. J Thorac Imaging. 1988;3:11–28. [PubMed] [Google Scholar]
- 8.Clements M, Berry G, Shi J, Ware S, Yates D, Johnson A. Projected mesothelioma incidence in men in New South Wales. Occup Environ Med. 2007;64:747–752. doi: 10.1136/oem.2006.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92:587–593. doi: 10.1038/sj.bjc.6602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paek DM. Asbestos problems of Korea. Environ Law Policy. 2009;2:5–22. [Google Scholar]
- 11.Churg A, Wright JL, DePaoli L, Wiggs B. Mineralogic correlates of fibrosis in chrysotile miners and millers. Am Rev Respir Dis. 1989;139:891–896. doi: 10.1164/ajrccm/139.4.891. [DOI] [PubMed] [Google Scholar]
- 12.Churg A, Wright J, Wiggs B, Depaoli L. Mineralogic parameters related to amosite asbestos-induced fibrosis in humans. Am Rev Respir Dis. 1990;142(6 Pt 1):1331–1336. doi: 10.1164/ajrccm/142.6_Pt_1.1331. [DOI] [PubMed] [Google Scholar]
- 13.Wright JL, Cagle P, Churg A, Colby TV, Myers J. Diseases of the small airways. Am Rev Respir Dis. 1992;146:240–262. doi: 10.1164/ajrccm/146.1.240. [DOI] [PubMed] [Google Scholar]
- 14.Bégin R, Cantin A, Berthiaume Y, Boileau R, Péloquin S, Massé S. Airway function in lifetime-nonsmoking older asbestos workers. Am J Med. 1983;75:631–638. doi: 10.1016/0002-9343(83)90449-7. [DOI] [PubMed] [Google Scholar]
- 15.Craighead JE, Abraham JL, Churg A, Green FH, Kleinerman J, Pratt PC, et al. The pathology of asbestos-associated diseases of the lungs and pleural cavities: diagnostic criteria and proposed grading schema. Report of the Pneumoconiosis Committee of the College of American Pathologists and the National Institute for Occupational Safety and Health. Arch Pathol Lab Med. 1982;106:544–596. [PubMed] [Google Scholar]
- 16.Green FH, Attfield M. Pathology standards for asbestosis. Scand J Work Environ Health. 1983;9(2 Spec No):162–168. doi: 10.5271/sjweh.2429. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170:691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- 18.Wolff H, Vehmas T, Oksa P, Rantanen J, Vainio H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health. 2015;41:5–15. doi: 10.5271/sjweh.3462. [DOI] [PubMed] [Google Scholar]
- 19.Roggli VL, Gibbs AR, Attanoos R, Churg A, Popper H, Cagle P, et al. Pathology of asbestosis-an update of the diagnostic criteria: report of the asbestosis committee of the college of american pathologists and pulmonary pathology society. Arch Pathol Lab Med. 2010;134:462–480. doi: 10.5858/134.3.462. [DOI] [PubMed] [Google Scholar]
- 20.Suganuma N, Kusaka Y, Hering KG, Vehmas T, Kraus T, Arakawa H, et al. Reliability of the proposed international classification of high-resolution computed tomography for occupational and environmental respiratory diseases. J Occup Health. 2009;51:210–222. doi: 10.1539/joh.l8030. [DOI] [PubMed] [Google Scholar]
- 21.Prazakova S, Thomas PS, Sandrini A, Yates DH. Asbestos and the lung in the 21st century: an update. Clin Respir J. 2014;8:1–10. doi: 10.1111/crj.12028. [DOI] [PubMed] [Google Scholar]
- 22.Weiss W. Asbestosis: a marker for the increased risk of lung cancer among workers exposed to asbestos. Chest. 1999;115:536–549. doi: 10.1378/chest.115.2.536. [DOI] [PubMed] [Google Scholar]
- 23.Hillerdal G. Malignant mesothelioma 1982: review of 4710 published cases. Br J Dis Chest. 1983;77:321–343. [PubMed] [Google Scholar]
- 24.Kim HR, Ahn YS, Jung SH. Epidemiologic characteristics of malignant mesothelioma in Korea. J Korean Med Assoc. 2009;52:449–455. [Google Scholar]
- 25.Raffaelli I, Festa G, Costantini AS, Leva G, Gorini G. [Mortality in a cohort of asbestos cement workers in Carrara, Italy] Med Lav. 2007;98:156–163. [PubMed] [Google Scholar]
- 26.Luberto F, Amendola P, Belli S, Bruno C, Candela S, Grignoli M, et al. [Mortality study of asbestos cement workers in Emilia-Romagna] Epidemiol Prev. 2004;28:239–246. [PubMed] [Google Scholar]
- 27.Gallus S, Pacifici R, Colombo P, Scarpino V, Zuccaro P, Bosetti C, et al. Prevalence of smoking and attitude towards smoking regulation in Italy, 2004. Eur J Cancer Prev. 2006;15:77–81. doi: 10.1097/01.cej.0000180667.89087.b9. [DOI] [PubMed] [Google Scholar]
- 28.Bałczewska E. Smoking and tobacco control in Poland. Eur J Dent Educ. 2004;8(Suppl 4):42–45. doi: 10.1111/j.1399-5863.2004.00322.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Tse LA, Au RK, Yu IT, Wang XR, Lao XQ, et al. Mesothelioma and lung cancer mortality: a historical cohort study among asbestosis workers in Hong Kong. Lung Cancer. 2012;76:165–170. doi: 10.1016/j.lungcan.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Pneumoconiosis: comparison of imaging and pathologic findings. Radiographics. 2006;26:59–77. doi: 10.1148/rg.261055070. [DOI] [PubMed] [Google Scholar]
- 31.Kipen HM, Lilis R, Suzuki Y, Valciukas JA, Selikoff IJ. Pulmonary fibrosis in asbestos insulation workers with lung cancer: a radiological and histopathological evaluation. Br J Ind Med. 1987;44:96–100. doi: 10.1136/oem.44.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zachrisson S, Vikgren J, Svalkvist A, Johnsson AA, Boijsen M, Flinck A, et al. Effect of clinical experience of chest tomosynthesis on detection of pulmonary nodules. Acta Radiol. 2009;50:884–891. doi: 10.1080/02841850903085584. [DOI] [PubMed] [Google Scholar]
- 33.Vikgren J, Zachrisson S, Svalkvist A, Johnsson AA, Boijsen M, Flinck A, et al. Comparison of chest tomosynthesis and chest radiography for detection of pulmonary nodules: human observer study of clinical cases. Radiology. 2008;249:1034–1041. doi: 10.1148/radiol.2492080304. [DOI] [PubMed] [Google Scholar]
- 34.Quaia E, Baratella E, Cioffi V, Bregant P, Cernic S, Cuttin R, et al. The value of digital tomosynthesis in the diagnosis of suspected pulmonary lesions on chest radiography: analysis of diagnostic accuracy and confidence. Acad Radiol. 2010;17:1267–1274. doi: 10.1016/j.acra.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Jung HN, Chung MJ, Koo JH, Kim HC, Lee KS. Digital tomosynthesis of the chest: utility for detection of lung metastasis in patients with colorectal cancer. Clin Radiol. 2012;67:232–238. doi: 10.1016/j.crad.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 36.McLoud TC. Conventional radiography in the diagnosis of asbestos-related disease. Radiol Clin North Am. 1992;30:1177–1189. [PubMed] [Google Scholar]
- 37.Lee G, Jeong YJ, Kim KI, Song JW, Kang DM, Kim YD, et al. Comparison of chest digital tomosynthesis and chest radiography for detection of asbestos-related pleuropulmonary disease. Clin Radiol. 2013;68:376–382. doi: 10.1016/j.crad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Staples CA, Gamsu G, Ray CS, Webb WR. High resolution computed tomography and lung function in asbestos-exposed workers with normal chest radiographs. Am Rev Respir Dis. 1989;139:1502–1508. doi: 10.1164/ajrccm/139.6.1502. [DOI] [PubMed] [Google Scholar]
- 39.Friedman AC, Fiel SB, Fisher MS, Radecki PD, Lev-Toaff AS, Caroline DF. Asbestos-related pleural disease and asbestosis: a comparison of CT and chest radiography. AJR Am J Roentgenol. 1988;150:269–275. doi: 10.2214/ajr.150.2.269. [DOI] [PubMed] [Google Scholar]
- 40.Tekath M, Dutheil F, Bellini R, Roche A, Pereira B, Naughton G, et al. Comparison of the ultra-low-dose Veo algorithm with the gold standard filtered back projection for detecting pulmonary asbestos-related conditions: a clinical observational study. BMJ Open. 2014;4:e004980. doi: 10.1136/bmjopen-2014-004980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akira M, Yokoyama K, Yamamoto S, Higashihara T, Morinaga K, Kita N, et al. Early asbestosis: evaluation with high-resolution CT. Radiology. 1991;178:409–416. doi: 10.1148/radiology.178.2.1987601. [DOI] [PubMed] [Google Scholar]
- 42.Akira M, Yamamoto S, Yokoyama K, Kita N, Morinaga K, Higashihara T, et al. Asbestosis: high-resolution CT-pathologic correlation. Radiology. 1990;176:389–394. doi: 10.1148/radiology.176.2.2367652. [DOI] [PubMed] [Google Scholar]
- 43.Friedman AC, Fiel SB, Radecki PD, Lev-Toaff AS. Computed tomography of benign pleural and pulmonary parenchymal abnormalities related to asbestos exposure. Semin Ultrasound CT MR. 1990;11:393–408. [PubMed] [Google Scholar]
- 44.Aberle DR, Gamsu G, Ray CS. High-resolution CT of benign asbestos-related diseases: clinical and radiographic correlation. AJR Am J Roentgenol. 1988;151:883–891. doi: 10.2214/ajr.151.5.883. [DOI] [PubMed] [Google Scholar]
- 45.Aberle DR, Gamsu G, Ray CS, Feuerstein IM. Asbestos-related pleural and parenchymal fibrosis: detection with high-resolution CT. Radiology. 1988;166:729–734. doi: 10.1148/radiology.166.3.3340770. [DOI] [PubMed] [Google Scholar]
- 46.Akira M, Yamamoto S, Inoue Y, Sakatani M. High-resolution CT of asbestosis and idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 2003;181:163–169. doi: 10.2214/ajr.181.1.1810163. [DOI] [PubMed] [Google Scholar]
- 47.al-Jarad N, Strickland B, Pearson MC, Rubens MB, Rudd RM. High resolution computed tomographic assessment of asbestosis and cryptogenic fibrosing alveolitis: a comparative study. Thorax. 1992;47:645–650. doi: 10.1136/thx.47.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gevenois PA, de Maertelaer V, Madani A, Winant C, Sergent G, De Vuyst P. Asbestosis, pleural plaques and diffuse pleural thickening: three distinct benign responses to asbestos exposure. Eur Respir J. 1998;11:1021–1027. doi: 10.1183/09031936.98.11051021. [DOI] [PubMed] [Google Scholar]
- 49.Staples CA. Computed tomography in the evaluation of benign asbestos-related disorders. Radiol Clin North Am. 1992;30:1191–1207. [PubMed] [Google Scholar]
- 50.Lee EK, Kim JS, Kim Y, Park JS. CT Findings in people who were environmentally exposed to asbestos in Korea. J Korean Med Sci. 2015;30:1896–1901. doi: 10.3346/jkms.2015.30.12.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y, Myong JP, Lee JK, Kim JS, Kim YK, Jung SH. CT Characteristics of pleural plaques related to occupational or environmental asbestos exposure from South Korean asbestos mines. Korean J Radiol. 2015;16:1142–1152. doi: 10.3348/kjr.2015.16.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Copley SJ, Wells AU, Sivakumaran P, Rubens MB, Lee YC, Desai SR, et al. Asbestosis and idiopathic pulmonary fibrosis: comparison of thin-section CT features. Radiology. 2003;229:731–736. doi: 10.1148/radiol.2293020668. [DOI] [PubMed] [Google Scholar]
- 53.Arakawa H, Kishimoto T, Ashizawa K, Kato K, Okamoto K, Honma K, et al. Asbestosis and other pulmonary fibrosis in asbestos-exposed workers: high-resolution CT features with pathological correlations. Eur Radiol. 2016;26:1485–1492. doi: 10.1007/s00330-015-3973-z. [DOI] [PubMed] [Google Scholar]