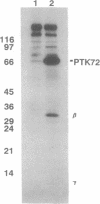
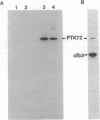
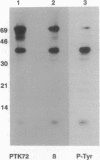
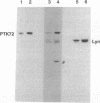
Abstract
In RBL-2H3 rat tumor mast cells, cross-linking the high-affinity IgE receptor Fc epsilon RI causes tyrosine phosphorylation of multiple proteins. These phosphoproteins include phospholipase C gamma 1, the beta and gamma subunits of the Fc epsilon RI, the Src family protein-tyrosine kinase Lyn, and a 72-kDa protein that coimmunoprecipitates from lysates of antigen-stimulated cells with antibody to the receptor beta subunit. We now present evidence that the 72-kDa Fc epsilon RI-associated protein is the protein-tyrosine kinase PTK72 that forms part of the antigen receptor complex in B lymphocytes. The identification is based on immunoreactivity with anti-PTK72 antiserum, chromatographic profiles on the affinity resin heparin/agarose, and one-dimensional phosphopeptide mapping studies. Enzymatic activity of the kinase is increased in anti-PTK72 immune complexes prepared from lysates of antigen-activated RBL-2H3 cells. The 72-kDa protein-tyrosine kinase is the principal substrate for in vitro tyrosine phosphorylation in anti-phosphotyrosine immunoprecipitates of RBL-2H3 cells. The discovery that RBL-2H3 mast cells share a receptor-activated protein-tyrosine kinase, PTK72, with B lymphocytes provides additional support for the existence of common signaling pathways initiated by multichain immune recognition receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B. Signal transduction by the SRC family of tyrosine protein kinases in hemopoietic cells. Cell Growth Differ. 1991 Aug;2(8):409–414. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Connelly P. A., Farrell C. A., Merenda J. M., Conklyn M. J., Showell H. J. Tyrosine phosphorylation is an early signaling event common to Fc receptor crosslinking in human neutrophils and rat basophilic leukemia cells (RBL-2H3). Biochem Biophys Res Commun. 1991 May 31;177(1):192–201. doi: 10.1016/0006-291x(91)91967-h. [DOI] [PubMed] [Google Scholar]
- Deanin G. G., Martinez A. M., Pfeiffer J. R., Gardner M. E., Oliver J. M. Tyrosine kinase-dependent phosphatidylinostiol turnover and functional responses in the Fc epsilon R1 signalling pathway. Biochem Biophys Res Commun. 1991 Aug 30;179(1):551–557. doi: 10.1016/0006-291x(91)91406-3. [DOI] [PubMed] [Google Scholar]
- Deanin G. G., Pfeiffer J. R., Cutts J. L., Fore M. L., Oliver J. M. Isoprenoid pathway activity is required for IgE receptor-mediated, tyrosine kinase-coupled transmembrane signaling in permeabilized RBL-2H3 rat basophilic leukemia cells. Cell Regul. 1991 Aug;2(8):627–640. doi: 10.1091/mbc.2.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. src-related tyrosine protein kinases as signaling components in hematopoietic cells. Cancer Cells. 1990 Oct;2(10):303–310. [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. B lymphocyte activation is accompanied by phosphorylation of a 72-kDa protein-tyrosine kinase. J Biol Chem. 1991 Aug 15;266(23):14846–14849. [PubMed] [Google Scholar]
- Keegan A. D., Paul W. E. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today. 1992 Feb;13(2):63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Li W., Deanin G. G., Margolis B., Schlessinger J., Oliver J. M. Fc epsilon R1-mediated tyrosine phosphorylation of multiple proteins, including phospholipase C gamma 1 and the receptor beta gamma 2 complex, in RBL-2H3 rat basophilic leukemia cells. Mol Cell Biol. 1992 Jul;12(7):3176–3182. doi: 10.1128/mcb.12.7.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992 Mar 5;356(6364):71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992 Feb;125:37–48. doi: 10.1111/j.1600-065x.1992.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Seagrave J., Stump R. F., Pfeiffer J. R., Deanin G. G. Signal transduction and cellular response in RBL-2H3 mast cells. Prog Allergy. 1988;42:185–245. [PubMed] [Google Scholar]
- Paolini R., Jouvin M. H., Kinet J. P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991 Oct 31;353(6347):855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. IgE-induced tyrosine phosphorylation of phospholipase C-gamma 1 in rat basophilic leukemia cells. J Biol Chem. 1991 Dec 25;266(36):24237–24240. [PubMed] [Google Scholar]
- Quarto R., Metzger H. The receptor for immunoglobulin E: examination for kinase activity and as a substrate for kinases. Mol Immunol. 1986 Nov;23(11):1215–1223. doi: 10.1016/0161-5890(86)90154-9. [DOI] [PubMed] [Google Scholar]
- Rivera J., Kinet J. P., Kim J., Pucillo C., Metzger H. Studies with a monoclonal antibody to the beta subunit of the receptor with high affinity for immunoglobulin E. Mol Immunol. 1988 Jul;25(7):647–661. doi: 10.1016/0161-5890(88)90100-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Yu K. T., Lyall R., Jariwala N., Zilberstein A., Haimovich J. Antigen- and ionophore-induced signal transduction in rat basophilic leukemia cells involves protein tyrosine phosphorylation. J Biol Chem. 1991 Nov 25;266(33):22564–22568. [PubMed] [Google Scholar]
- Zioncheck T. F., Harrison M. L., Geahlen R. L. Purification and characterization of a protein-tyrosine kinase from bovine thymus. J Biol Chem. 1986 Nov 25;261(33):15637–15643. [PubMed] [Google Scholar]
- Zioncheck T. F., Harrison M. L., Isaacson C. C., Geahlen R. L. Generation of an active protein-tyrosine kinase from lymphocytes by proteolysis. J Biol Chem. 1988 Dec 15;263(35):19195–19202. [PubMed] [Google Scholar]