Abstract
Objective
To prospectively compare technical success rate and reliable measurements of virtual touch quantification (VTQ) elastography and elastography point quantification (ElastPQ), and to correlate liver stiffness (LS) measurements obtained by the two elastography techniques.
Materials and Methods
Our study included 85 patients, 80 of whom were previously diagnosed with chronic liver disease. The technical success rate and reliable measurements of the two kinds of point shear wave elastography (pSWE) techniques were compared by χ2 analysis. LS values measured using the two techniques were compared and correlated via Wilcoxon signed-rank test, Spearman correlation coefficient, and 95% Bland-Altman limit of agreement. The intraobserver reproducibility of ElastPQ was determined by 95% Bland-Altman limit of agreement and intraclass correlation coefficient (ICC).
Results
The two pSWE techniques showed similar technical success rate (98.8% for VTQ vs. 95.3% for ElastPQ, p = 0.823) and reliable LS measurements (95.3% for VTQ vs. 90.6% for ElastPQ, p = 0.509). The mean LS measurements obtained by VTQ (1.71 ± 0.47 m/s) and ElastPQ (1.66 ± 0.41 m/s) were not significantly different (p = 0.209). The LS measurements obtained by the two techniques showed strong correlation (r = 0.820); in addition, the 95% limit of agreement of the two methods was 27.5% of the mean. Finally, the ICC of repeat ElastPQ measurements was 0.991.
Conclusion
Virtual touch quantification and ElastPQ showed similar technical success rate and reliable measurements, with strongly correlated LS measurements. However, the two methods are not interchangeable due to the large limit of agreement.
Keywords: Liver fibrosis, Liver stiffness, Ultrasound elastography, Shear wave elastography
INTRODUCTION
Liver fibrosis is a clinically significant condition that is closely involved in the pathogenesis of life-threatening conditions such as cirrhosis, portal hypertension and hepatocellular carcinoma (1,2). Although pathological diagnosis using liver biopsy is the standard method to assess the nature and severity of liver fibrosis, liver biopsy is an invasive procedure with potential complications such as hemorrhage, bile leakage and pneumothorax (3). In addition, because the sampled tissue has small volume compared to liver parenchyma, liver biopsy intrinsically involves sampling bias, as well as interobserver variation (4,5,6). Thus, many non-invasive techniques such as serum-based markers and various ultrasound or magnetic resonance (MR) based imaging techniques have been developed to assess different stages of liver fibrosis and to monitor progression of liver fibrosis (7,8,9).
Among the emerging image-based techniques, elastography is gaining popularity as a rapid, inexpensive, non-invasive and reproducible method for quantitatively measuring liver stiffness (LS) (10,11). Clinical elastography techniques include transient elastography, point shear wave elastography (pSWE), two dimensional (2D) shear wave elastography (SWE) and MR elastography (3,7,9,12,13). Although transient elastography (Fibroscan-Echosens, Paris, France) is the most widely used elastography technique, it has some limitations, such as failure of detection on ultrasound image, and high rate of failure in patients with ascites or severe obesity (14,15).
On the other hand, virtual touch quantification (VTQ; Siemens, Berlin, Germany), commonly referred to as acoustic radiation force impulse imaging, is a type of pSWE technique that induces shear acoustic waves inside a specified region of interest (ROI) and measures the resulting tissue displacement (8,11). In contrast to transient elastography, which has fixed insertion depth at ROI, VTQ enables tissue measurements inside ROI of variable insertion depths and can be incorporated as part of a standard liver ultrasonography (US) examination, thus providing an alternative to transient elastography for non-invasive and more precise assessment of liver fibrosis (8,16). Several studies indicate that VTQ has sensitivity comparable to liver biopsy, as well as excellent intra and interobserver reproducibility (10,17). Elastography point quantification (ElastPQ; Philips Medical Systems, Bothell, WA, USA), recently approved by the American Food and Drug Administration, uses same physical principles as VTQ, and shows promising results in measuring LS in hepatic fibrosis patients (14,18,19). LS measurements with pSWE techniques such as VTQ and ElastPQ require only a few seconds using conventional US probes, and therefore, can be easily performed during a regular liver US session (14,18,19).
Although both pSWE techniques are clinically available, few studies compare the performance of ElastPQ with VTQ in evaluating hepatic fibrosis in patients with chronic liver diseases (14,20). Currently, it remains unclear whether the two pSWE techniques are interchangeable. Mutual validation of the two techniques is necessary as both orgmodalities are increasingly used to monitor hepatic fibrosis in patients with chronic liver disease. Therefore, the aim of the present study was to compare the performance of VTQ and ElastPQ in terms of technical success rate and reliable measurements, and to correlate the LS measurements made by the two modalities.
MATERIALS AND METHODS
Study Population
This prospective study was approved by our Institutional Review Board, and written informed consent was obtained from all patients. Our study included 85 patients, 80 of whom were diagnosed with chronic liver diseases based on serology tests (21), and 5 patients without parenchymal liver disease. LS values obtained by VTQ were used for the assignment of hepatic fibrosis stages, in accordance with the reference values provided by a previous meta-analysis (16). Detailed characteristics of the study population are provided in Table 1.
Table 1. Patients' Characteristics and Presumed Staging of Hepatic Fibrosis Based on VTQ Meta-Analysis Values from Previous Study.
| Characteristics | n (%) |
|---|---|
| Age (years) | 63 (39–91) |
| Gender | |
| Male | 60 (70.6) |
| Female | 25 (29.4) |
| Body mass index (kg/m2) | 24.7 (16.4–39.0) |
| Etiology | |
| Chronic hepatitis C | 11 (12.9) |
| Chronic hepatitis B | 62 (72.9) |
| Chronic alcoholic hepatitis | 12 (14.1) |
| Metastasis from other organs | 6 (7.1) |
| Unknown | 1 (1.2) |
| Liver fibrosis stages | |
| F ≤ 1 | 14 (16.5) |
| F2 | 19 (22.4) |
| F3 | 19 (22.4) |
| F4 | 32 (37.6) |
Age and BMI are presented as median values and range intervals. Cut-offs used in classification of liver fibrosis was F ≥ 2 (1.34 m/s), F ≥ 3 (1.55 m/s), and F = 4 (1.80 m/s) (16). n = number of patients, VTQ = virtual touch quantification
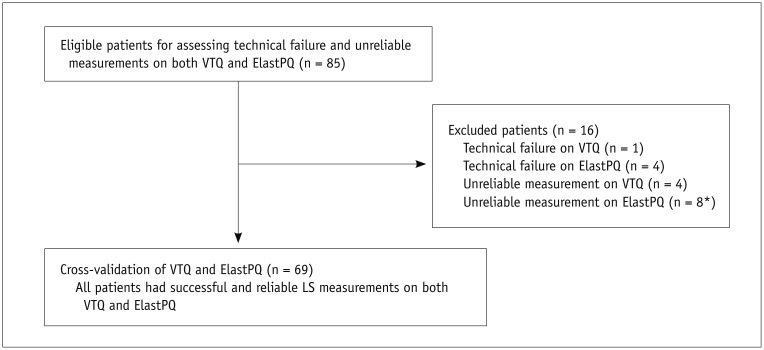
To assess the technical success rate and reliable measurements of the two pSWE techniques, the results of all 85 patients were included. Among the 85 patients, only 69 patients with successful and reliable LS measurements on both shear wave techniques were included for comparison of LS measurements (Fig. 1).
Fig. 1. Flow diagram of study population.
*One patient who had technical failure on VTQ also had unreliable measurement on ElastPQ. ElastPQ = elastography point quantification, LS = liver stiffness, VTQ = virtual touch quantification
VTQ and ElastPQ Measurements
Virtual touch quantification was performed using Siemens Acuson S2000TM ultrasound system (Siemens AG, Erlangen, Germany) and ElastPQ was performed using Philips EPIQ 7 ultrasound system (Philips Medical Systems, Bothell, WA, USA). In general, at least 10 measurements should be obtained, although some studies suggest that smaller number of measurements may have similar accuracy (22,23,24). Technical failures and unreliable results occur more frequently in patients with liver cirrhosis than in patients with normal liver (23); hence, 15 measurements were made for each instrument by one of the authors with 4 years of ultrasound elastography and 20 years of liver ultrasound experience. All patients fasted for at least 6 hours prior to the examination and were placed in the supine position, with right arm maximally abducted above the head to stretch the intercostal muscle. During acquisition, patients were asked to hold breaths at mid-respiration level so as to minimize breathing motion while avoiding deep inspiration or expiration, following a recent guideline on US elastography (11,22). A measuring box was placed in the right anterior segment of the liver through the intercostal space, with minimal scanning pressure applied by the operator.
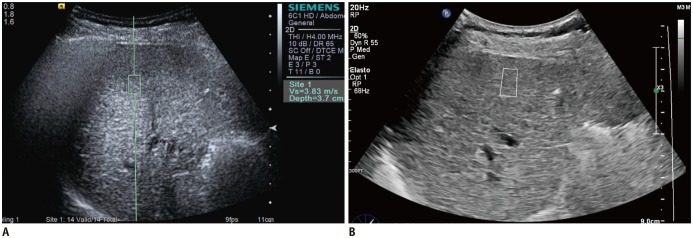
The operator performed both ElastPQ and VTQ measurements within 6 hours interval on each patient. For VTQ measurements, the operator placed a measuring box of 10/5 mm in a desired position (mostly segment V or VIII of the liver, 2–4 cm from the liver capsule), avoiding area immediately under the liver surface or vessels showing reverberation artifacts (Fig. 2A) (22). Similarly, for ElastPQ measurements, the operator placed a measuring box of 15/5 mm in a desired portion of the liver (Fig. 2B). For both pSWE techniques, the operator placed ROI box in a similar position of the liver. Mean, median and standard deviation values of LS measurements were provided by the US units, and interquartile range (IQR) was calculated using statistical software.
Fig. 2. LS measurements by two pSWE techniques.
VTQ (A) and ElastPQ (B). For both measurements, operator placed predefined measuring box in liver. ElastPQ = elastography point quantification, LS = liver stiffness, pSWE = point shear wave elastography, VTQ = virtual touch quantification
To evaluate intraobserver reproducibility of LS measurements using ElastPQ, LS measurements were repeated within 24 hours interval for the 14 patients whose study date was within the final 4 weeks of the study period.
Technical Failure and Reliable Measurements
Technical failure of pSWE methods was defined as failure to acquire 10 valid measurements after at least 15 trials. Reliable measurement of LS was defined as measurement in which the IQR/median LS of 15 measurements was < 30% (7,23).
Statistical Analysis
Liver stiffness measurements of the two pSWE methods were initially tested for normality using the Shapiro-Wilk test. To determine the skewness of the LS measurements, histograms were obtained for VTQ and ElastPQ measurements. Skewness and standard error of skewness was automatically provided by the SPSS software. χ2 analysis was used to determine whether technical success and reliable measurements of the two SWE methods were significantly different. Wilcoxon signed-rank test was used to compare the LS measurements obtained by VTQ and ElastPQ. Spearman correlation coefficient was then obtained to determine correlation between the two pSWE techniques (24). Bland-Altman analysis was used to evaluate the agreement between VTQ and ElastPQ (25). The agreement between the two methods was further investigated by calculating the intraclass correlation coefficient (ICC) from mean VTQ and ElastPQ LS measurements.
The intraobserver reproducibility of LS measurements using ElastPQ was evaluated by calculating ICC in 14 patients who underwent two ElastPQ sessions. As in previous studies, ICC > 0.75 was considered as good agreement (26). To estimate the magnitude of change in LS measurements that can be confidently detected in a single individual, the 95% limit of agreement between the repeat ElastPQ measurements was obtained following the Bland-Altman method and was expressed as the percentage of the mean (25).
All statistical analyses were performed using commercially available SPSS software, version 22.0 (IBM Corp., Released 2013, IBM SPSS Statistics for Windows, Version 22.0, Armonk, NY, USA).
RESULTS
The most common etiology of chronic liver diseases was chronic hepatitis B (72.9%), followed by chronic hepatitis C (12.9%) and chronic alcoholic hepatitis (14.1%). The presumed distribution of liver fibrosis calculated from VTQ meta-analysis showed that liver cirrhosis (F4) was the most common fibrosis stage (37.6%), followed by severe (F3) and moderate (F2) liver fibrosis (22.4% for both stages), and lastly, mild (F1) or no (F0) liver fibrosis (16.5%) (Table 1) (16).
Technical Success Rate and Reliable Measurements
Among the 85 patients who underwent pSWE imaging with both techniques, VTQ failed to provide LS values in 1 patient, and ElastPQ in 4 patients. There was no significant difference in the technical success rate of VTQ (98.8% [84 of 85]) and ElastPQ (95.3% [81 of 85]) (p = 0.823). There were 4 unreliable measurements on VTQ and 8 unreliable measurements on ElastPQ. One patient who had technical failure on VTQ also had unreliable measurement on ElastPQ. All 4 patients who had technical failure on ElastPQ had reliable measurements on VTQ. There was no significant difference between the reliable measurements of VTQ (95.3% [81 of 85]) and ElastPQ (90.6% [77 of 85]) (p = 0.509).
Correlation of LS Values Obtained by Means of VTQ Elastography and ElastPQ Elastography
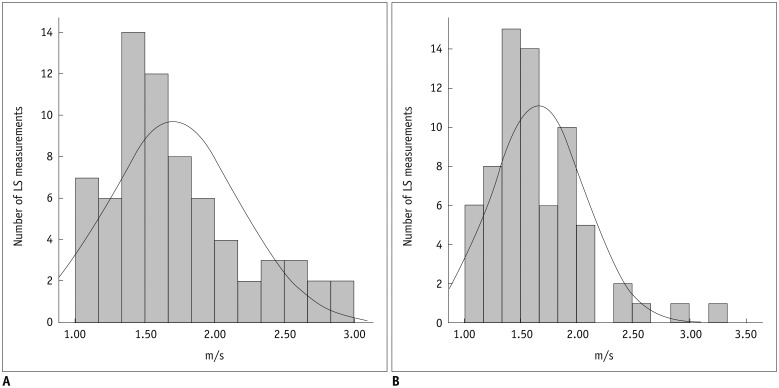
Of the 85 patients, only 69 patients with successful and reliable VTQ and ElastPQ LS measurements were included in the further correlation analysis of LS measurements. Shapiro-Wilk test showed that VTQ and ElastPQ mean LS measurements were not normally distributed (p < 0.001). Visual inspection of the histograms indicated right-skewness of LS measurements obtained by VTQ and ElastPQ (Fig. 3). Skewness/standard error of skewness was 3.10 for VTQ vs. 4.96 for ElastPQ. The difference between mean, median and IQR/median of VTQ and ElastPQ measurements was statistically insignificant (Wilcoxon signed-rank test) (Table 2). A strong correlation (r = 0.820) was observed between LS values obtained by VTQ and ElastPQ (p < 0.001). 95% Bland-Altman limit of agreement between LS measurements by VTQ and ElastPQ was 27.5% of the mean (Fig. 4).
Fig. 3. Histogram showing distribution of mean LS measurements by two pSWE techniques.
VTQ (A) and ElastPQ (B). Skewness/standard error of skewness was 3.10 for VTQ vs. 4.96 for ElastPQ. Black line = normal distribution fit. ElastPQ = elastography point quantification, LS = liver stiffness, pSWE = point shear wave elastography, VTQ = virtual touch quantification
Table 2. Mean, Median, and Median/IQR Values of Two pSWE Methods Were Compared by Wilcoxon Signed-Rank Test.
| VTQ | ElastPQ | P | |
|---|---|---|---|
| Mean | 1.71 ± 0.47 | 1.66 ± 0.41 | 0.209 |
| Median | 1.69 ± 0.47 | 1.65 ± 0.43 | 0.471 |
| IQR/median | 0.145 ± 0.062 | 0.156 ± 0.058 | 0.263 |
ElastPQ = elastography point quantification, IQR = interquartile range, pSWE = point shear wave elastography, VTQ = virtual touch quantification
Fig. 4. Bland-Altman plot shows agreement in VTQ and ElastPQ LS measurements.
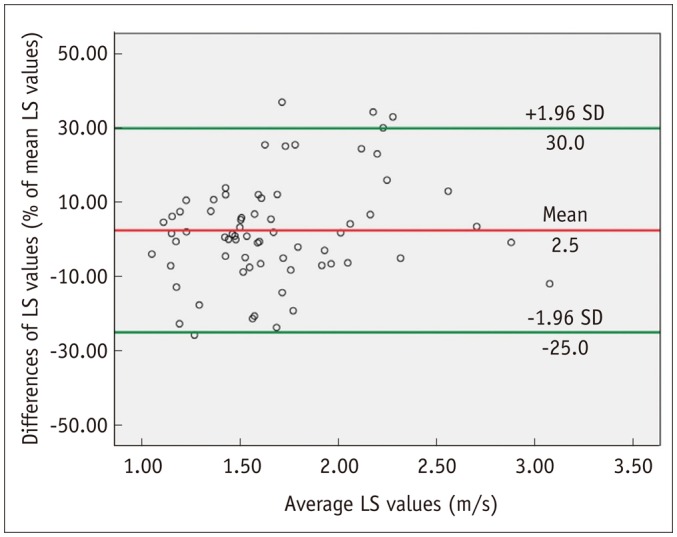
X-axis shows means of repeated LS measurements, and y-axis shows difference between VTQ and ElastPQ LS measurements. Red line = mean difference, Green line = 95% limits of agreement. ElastPQ = elastography point quantification, LS = liver stiffness, VTQ = virtual touch quantification
Intraobserver Reproducibility of ElastPQ Measurements
The intraobserver reproducibility of ElastPQ measurements was calculated by comparing repeat LS values obtained from 14 patients. The ICC of 14 ElastPQ measurements was 0.991 (95% confidence interval: 0.997, 0.973), indicating very good reproducibility. 95% Bland-Altman limit of agreement of the repeat ElastPQ measurements was 8.3% of the mean (Fig. 5).
Fig. 5. Bland-Altman plot shows intraobserver agreement of ElastPQ measurement.
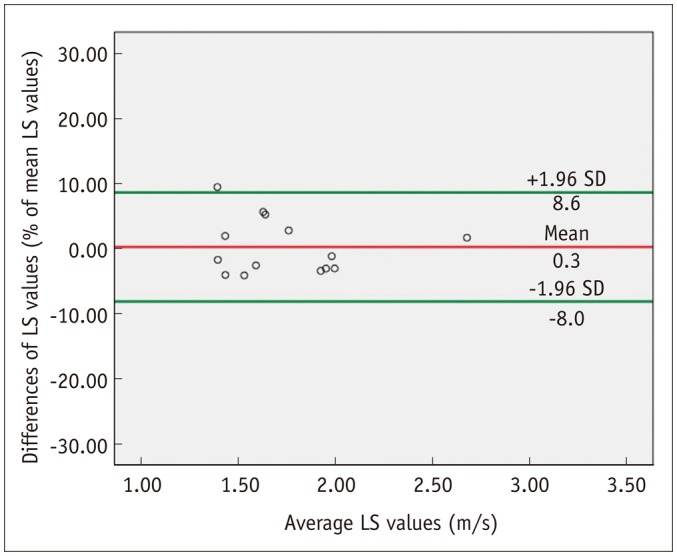
X-axis shows means of repeated LS measurements, and y-axis shows difference between repeat ElastPQ LS measurements. Red line = mean difference, Green line = 95% limits of agreement. ElastPQ = elastography point quantification, LS = liver stiffness
DISCUSSION
In this prospective study, we systemically compared the technical success rate, reliable measurements, and LS values obtained by VTQ and ElastPQ. To the best of our knowledge, our study is the first to prospectively compare the two pSWE methods in an Asian population. The 98.8% technical success rate for VTQ was not significantly different from 95.3% technical success rate for ElastPQ (p = 0.823). In addition, the two pSWE techniques showed similar reliable LS measurements: 95.3% for VTQ vs. 90.6% for ElastPQ (p = 0.509). Reliable LS measurements may be underestimated because low LS measurement with IQR/median > 0.3 may still be reliable measurements (27). The two pSWE techniques generate and measure shear wave based on similar physical principles; hence, these results are as expected.
In our study, the mean LS measurements obtained by VTQ (1.71 ± 0.47 m/s) and ElastPQ (1.66 ± 0.41 m/s) did not show any significant difference (p = 0.209). However, a previous study by Sporea et al. (20), which compared the performance of VTQ and ElastPQ, showed statistically significant difference in LS measurements by the two techniques (p < 0.05). Furthermore, the degree of correlation between the two pSWE techniques in the present study (r = 0.820) was higher than the values reported by Sporea et al. (20) (r = 0.407–0.582). These discrepancies could be explained as follows. Firstly, the study population in the present study consists mostly of patients pre-diagnosed with chronic liver diseases, while half of the study population in the previous study were healthy volunteers. Therefore, while Sporea et al. (20) assumed normal distribution of LS measurements when comparing the two modalities, the LS measurements in our study were right-skewed (Fig. 3). In addition, the US equipment and version of ElastPQ used in the two studies were different. In our study, the most recent version of ElastPQ was used (EPIQ 7), as compared to an older version of ElastPQ (iU22, Philips Medical Systems, Bothell, WA, USA) in the previous study.
Several studies have already shown that the most widely used VTQ is a sensitive and reliable technique to assess liver fibrosis (10). ElastPQ, a new pSWE technique, showed similar technical success rates, reliable LS measurements and mean LS values compared to the well-established VTQ elastography; thus, ElastPQ technique is expected to provide similar diagnostic performance in the evaluation of liver fibrosis.
In our study, the technical success rate of ElastPQ was comparable to the results of the previously study (20). In addition, the intraobserver reproducibility of ElastPQ was excellent (ICC = 0.991), exceeding the previously reported intraobserver reproducibility of VTQ LS measurements (ICC = 0.945) (10). During the clinical management of patients with chronic liver disease, such as chronic viral hepatitis B, C, or nonalcoholic steatohepatitis, monitoring liver fibrosis progression or regression is as important as diagnosing liver fibrosis. In terms of monitoring the response to antiviral treatment, pSWE techniques have advantages over repeated biopsy, whose drawbacks include poor patient compliance and invasiveness (22). Although liver biopsy is irreplaceable in some clinical contexts, pSWE is predicted to narrow the patient groups that require biopsy (16,28). Our study results illustrate that ElastPQ has high technical success rate, reliable LS measurements and intraobserver reproducibility. Therefore, ElastPQ, as well as VTQ, can be used to monitor disease progression or treatment response to antiviral agents (22).
In the current study, we prospectively correlated LS values measured at VTQ and ElastPQ, and found that the LS values measured at both pSWE examinations showed excellent correlation (r = 0.820). However, despite similar LS values of the two pSWE techniques, we believe VTQ and ElastPQ techniques cannot be used interchangeably, as the limit of agreement (27.5%) of the two pSWE techniques in our study is smaller than the difference in the cut-off values of the fibrosis stages provided by the meta-analysis of VTQ LS measurements (16). According to the meta-analysis of VTQ LS measurements for the staging of liver fibrosis, the cut-off for F2 fibrosis is 1.34 m/s, F3 fibrosis 1.55 m/s, and F4 fibrosis 1.80 m/s (16). It is likely that the difference in strength and number of push pulses to generate shearwaves of the two pSWE systems, or intrinsic inhomogeneous distribution of hepatic fibrosis in chronic liver disease, and the substantial tissue inhomogeneity and architectural distortion in cirrhotic livers may result in variation of shear wave velocity measurements by the two pSWE modalities (7,29).
In this study, we were unable to compare the diagnostic performance of ElastPQ and VTQ techniques for the staging of liver fibrosis. However, according to many previous studies, VTQ provides good diagnostic accuracy for the noninvasive staging of liver fibrosis (10,16,28,30,31). Recently, Ma et al. (14) showed that ElastPQ is a valid technology for liver fibrosis staging in chronic hepatitis B patients; the area under the receiver operating characteristics for significant fibrosis and cirrhosis were 0.94 and 0.89, respectively. Another preliminary study involving 102 patients with chronic hepatitis C showed that the accuracy of ElastPQ for staging liver fibrosis is similar to that of transient elastography (32). Our study results indicated significant correlation between the LS values of both techniques and no significant difference in mean and median LS values between VTQ and ElastPQ; hence, ElastPQ possibly provides similar diagnostic performance to the well-established VTQ elastography.
The current study had several limitations. Since there was no pathological staging of liver fibrosis, we could not evaluate the diagnostic performance of pSWE techniques in staging of liver fibrosis. In addition, we could not calculate interobserver reproducibility for ElastPQ measurements because one operator performed all LS measurements. Finally, although there are other clinically available SWE techniques such as 2D SWE technique, we merely compared two vendors' pSWE techniques.
In conclusion, ElastPQ provided comparable technical success rate and reliable LS measurements to VTQ elastography. The LS measurements made by the two modalities were strongly correlated. However, the two methods cannot be used interchangeably for hepatic fibrosis staging due to the large limit of agreement.
Acknowledgments
We would like to thank Ms. Mi Ae Shin and Vijay Shamdasani, Ph.D. for their technical support in doing this study.
Footnotes
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2A10066037).
References
- 1.Wong GL, Espinosa WZ, Wong VW. Personalized management of cirrhosis by non-invasive tests of liver fibrosis. Clin Mol Hepatol. 2015;21:200–211. doi: 10.3350/cmh.2015.21.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459–479. doi: 10.1016/j.cld.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Castera L. Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:291–303. doi: 10.1016/j.bpg.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 5.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–117. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 6.Wong GL. Prediction of fibrosis progression in chronic viral hepatitis. Clin Mol Hepatol. 2014;20:228–236. doi: 10.3350/cmh.2014.20.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772–782. doi: 10.1148/radiol.14132000. [DOI] [PubMed] [Google Scholar]
- 8.Jeong WK, Lim HK, Lee HK, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography. 2014;33:149–160. doi: 10.14366/usg.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JE, Lee JM, Lee KB, Yoon JH, Shin CI, Han JK, et al. Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis B viral infection using magnetic resonance elastography. Korean J Radiol. 2014;15:210–217. doi: 10.3348/kjr.2014.15.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kircheis G, Sagir A, Vogt C, Vom Dahl S, Kubitz R, Häussinger D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol. 2012;18:1077–1084. doi: 10.3748/wjg.v18.i10.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, et al. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44:214–219. doi: 10.1097/MCG.0b013e3181b4af1f. [DOI] [PubMed] [Google Scholar]
- 14.Ma JJ, Ding H, Mao F, Sun HC, Xu C, Wang WP. Assessment of liver fibrosis with elastography point quantification technique in chronic hepatitis B virus patients: a comparison with liver pathological results. J Gastroenterol Hepatol. 2014;29:814–819. doi: 10.1111/jgh.12479. [DOI] [PubMed] [Google Scholar]
- 15.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 17.Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol. 2012;38:1103–1108. doi: 10.1016/j.ultrasmedbio.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Ling W, Lu Q, Quan J, Ma L, Luo Y. Assessment of impact factors on shear wave based liver stiffness measurement. Eur J Radiol. 2013;82:335–341. doi: 10.1016/j.ejrad.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Sanchez W, Callstrom MR, Gorman B, Lewis JT, Sanderson SO, et al. Assessment of liver viscoelasticity by using shear waves induced by ultrasound radiation force. Radiology. 2013;266:964–970. doi: 10.1148/radiol.12120837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sporea I, Bota S, Grădinaru-Tas¸cău O, S¸irli R, Popescu A. Comparative study between two point shear wave elastographic techniques: acoustic radiation force impulse (ARFI) elastography and ElastPQ. Med Ultrason. 2014;16:309–314. doi: 10.11152/mu.201.3.2066.164.isp1. [DOI] [PubMed] [Google Scholar]
- 21.Musana KA, Yale SH, Abdulkarim AS. Tests of liver injury. Clin Med Res. 2004;2:129–131. doi: 10.3121/cmr.2.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 3: liver. Ultrasound Med Biol. 2015;41:1161–1179. doi: 10.1016/j.ultrasmedbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 24.Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003;227:617–622. doi: 10.1148/radiol.2273011499. [DOI] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 26.Kim SY, Lee SS, Byun JH, Park SH, Kim JK, Park B, et al. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010;255:815–823. doi: 10.1148/radiol.10091706. [DOI] [PubMed] [Google Scholar]
- 27.Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–1191. doi: 10.1002/hep.25993. [DOI] [PubMed] [Google Scholar]
- 28.Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. doi: 10.1371/journal.pone.0044930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125–2133. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Parthasarathy S, Goyal P, McCarthy RJ, Larson AC, Miller FH. Magnetic resonance elastography and acoustic radiation force impulse for staging hepatic fibrosis: a meta-analysis. Abdom Imaging. 2015;40:818–834. doi: 10.1007/s00261-014-0137-6. [DOI] [PubMed] [Google Scholar]
- 31.Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040–3053. doi: 10.1007/s00330-013-2927-6. [DOI] [PubMed] [Google Scholar]
- 32.Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Dal Bello B, Filice G, et al. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787–4796. doi: 10.3748/wjg.v20.i16.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]