Abstract
That rotavirus infection can cause neurological symptoms in young children has been well established. However, it is surprising why rotavirus infection has been overlooked as a cause of neonatal seizures for many years, despite significant research interest in neonatal rotavirus infection. Neonates are the age group most vulnerable to seizures, which are typically attributed to a wide range of causes. By contrast, because rotavirus infection is usually asymptomatic, it has been difficult to identify an association between this virus and neonatal seizures. The conventional wisdom has been that, although neonates are commonly infected with rotavirus, neurological complications are rare in this age. However, recent studies using diffusion-weighted imaging (DWI) have suggested a connection between rotavirus infection and neonatal seizures and that rotavirus infection can induce diffuse white matter injury without direct invasion of the central nervous system. The clinical features of white matter injury in rotavirus-infected neonates include the onset of seizures at days 4–6 of life in apparently healthy term infants. The recent findings seem to contradict the conventional wisdom. However, white matter injury might not be a completely new aspect of rotavirus infection in neonates, considering the forgotten clinical entity of neonatal seizures, 'fifth day fits'. With increased use of DWI in neonatal seizures, we are just starting to understand connection between viral infection and white matter injury in neonates. In this review, we discuss the historical aspects of rotavirus infection and neonatal seizures. We also present the clinical features of white matter injury in neonatal rotavirus infection.
Keywords: Rotavirus, White matter, Injuries, Seizures, Newborn infant
Introduction
Cerebral white matter injury (WMI) is recognized as the most common form of injury to the developing brain of neonates, especially survivors of preterm birth1). Although multiple factors are involved in brain injury, both infection and inflammation are well-established risks factors for WMI in the preterm population2,3,4). The major period of vulnerability for WMI occurs prior to the onset of myelination (23–32 gestational weeks)5). Preterm neonates are at high risk of severe and/or multiple bacterial infections between birth and hospital discharge6). For these reasons, several studies have sought to demonstrate a relationship between infection/inflammation and WMI in preterm neonates as well as to elucidate the underlying mechanisms4,7). However, infection/inflammation-associated brain injury does not occur exclusively in preterm neonates but is also seen in term neonates. Chorioamnionitis is an important risk factor for cerebral palsy in both groups8). The risk of cerebral palsy is increased by 2 to 12 fold in term neonates with antenatal choriomanionitis8,9,10). Nevertheless, the principal anatomic substrate of infection/inflammation-associated cerebral injury in term neonates is not known.
In this regard, recent reports of neonatal seizures associated with rotavirus infection in term neonates suggest several new and surprising perspectives11,12,13). First, infection/inflammation might induce WMI in term-neonates as well as preterm neonates. Second, WMI might be caused not only by bacterial but also by viral infections. Third, the developing brain can be damaged by afebrile infections outside the central nervous system (CNS). Although further studies are needed to establish the relationship between rotavirus infection and WMI in term neonates, these recent reports11,12,13) underscore the need for a paradigm shift recognizing the following: (1) that common viral infections can induce WMI in term neonates without direct viral invasion, and (2) that rotavirus is a common cause of neonatal seizures in term neonates.
In this paper, we review the history of rotavirus-associated neurological symptoms in newborns. We then discuss the clinical characteristics and prognosis of term neonates with rotavirus-associated WMI and compare both with the features of 2 other viral infections, parechovirus and enterovirus. Finally, we suggest further directions of research into rotavirus-associated WMI in the developing brain.
History of rotavirus as a cause of neonatal seizures
Rotavirus is the most common cause of gastroenteritis-associated seizures in infants and young children14). Although neonates are vulnerable to seizures as well as rotavirus infection, the connection between the two in this age group has been overlooked for many years. Only a few studies have examined the association between rotavirus infection and neurological symptoms in newborns15,16,17,18). Rotavirus infection has been suggested as possible cause of 'fifth day fits' in 1990s by Herrmann et al.15). They15) observed an epidemic occurrence of 21 cases of 'fifth day fits'. After a coincidental detection of rotavirus in the stools of several cases of fifth day fits, they tested the stools of 19 fifth-day fitters and 30 matched controls15). Rotavirus was detected in 18 of the 19 neonates (95%) with 'fifth day fits' compared with only 12 of the 30 matched controls (40%) (P<0.01)15). Before proceeding with the main topic of this review, rotavirus-associated WMI in newborns, we first need to consider the entity of 'fifth day fits'. The term was introduced in the 1980s to describe an epidemic of neonatal seizures that occurred during the 1970s19,20). 'Fifth day fits' are defined as the onset of seizures between the fourth and sixth days of life in otherwise apparently healthy full-term infants20). The seizures last an average of 24 hours20). The newborns are neurologically normal at the onset of the convulsion but become drowsy and hypotonic for several days thereafter19). Although the long-term prognosis was not defined in the original reports, these patients were assessed as normal at the time of their discharge from the hospital21). Acute zinc deficiency and feeding type were suggested as causes of fifth day fits, but neither was convincingly confirmed22,23). Environmental factors were also proposed as the etiology because the prevalence of this syndrome was periodically variable. Interestingly, rotavirus infections in humans were first detected in the 1970s. In newborns, they became an important issue following an outbreak in a neonatal care unit in mid-1970s24,25,26). The contemporary, epidemic-like occurrence of 'fifth day fits' and rotavirus infection in newborns lent support to the suggestion of Herrmann et al.15) However, the association between rotavirus and 'fifth day fits' has not been replicated.
Around the same time with Herrmann et al.15), an association between rotavirus infection and bradycardia-apnea episodes has been reported.16) The incidence of bradycardia-apnea episodes was higher in neonates with than without rotavirus infection (33% vs. 8%, P<0.05)16). Bradycardia-apnea episodes were significantly more common in term than in preterm newborns16). However, unfortunately, they16) did not report when symptom onset occurred nor did they address the numerous central and peripheral causes that may induce apnea in newborns.
A few years later, a study in South Africa17) yielded results that conflicted with the previous 2 studies15,16). Retrospective and prospective analyses found no association between rotavirus infection and the neurological symptoms of newborns17). However, the demographic characteristics of the patients were unclear, and significant selection bias was suspected17). In the following decade, rotavirus was ignored as a cause of neonatal seizures. However, recent studies11,12,13,18) that included neuroimaging again suggested rotavirus as a cause of neonatal seizures or WMI.
Clinical characteristics of rotavirus-associated WMI in newborns
Recent studies11,12,13,18) of WMI in neonatal seizures and the potential involvement of rotavirus infection are summarized in Table 1. Verboon-Maciolek et al.18) firstly suggested rotavirus as a cause of cerebral injury in newborns based on the cranial ultrasonography and magnetic resonance imaging (MRI) findings of five preterm and three term infants who developed seizures or apnea during rotavirus infection. Clinical signs during the first week of life were noted in 2 of the 3 full-term infants18). In preterm infants, the symptoms became evident during the late preterm period (34–36 weeks of postgestation)18). Cranial ultrasonography demonstrated mild to severe periventricular echogenicity coinciding with illness related to rotavirus infection and subsequent cystic evolution in 6 patients18). Among these 6 patients, diffuse high signal intensity attributable to a cystic lesion in the white matter was seen on MRI in four preterm newborns18). The other 2 term infants showed focal diffusion-weighted MRI changes at disease onset and focal white matter cysts on repeat MRI18). Systemic infection was proven in only 2 patients based on a positive polymerase chain reaction test of the cerebrospinal fluid (CSF) and/or blood18). Neuroimaging was normal in preterm newborns in weekly cranial ultrasonography before rotavirus infection, and no other causes for seizures/WMI (including human parechovirus, enterovirus, and herpes simplex virus) were detected18). The researchers18) therefore suggested an association between rotavirus enteritis and WMI indistinguishable from that in late onset cystic-periventricular leukomalacia (PVL). The most impressive finding of Verboon-Maciolek et al.18) was that rotavirus apparently induces diffuse WMI with cystic evolution in the developing brain without direct invasion of the CNS. The adverse neurological sequelae of cystic –PVL highlight the need for additional studies to confirm their findings.
Table 1. Studies of white matter injury in neonatal seizures with rotavirus infection.
| Author | Year | No. of patients | GA (wk) | Birth weight (g) | Apgar score (1/5 min) | Clinical presentations (cases) | Time of onset of illness, days (cGA) | MRI findings (cases) | Virology (positive cases/tested cases) | Outcomes (age, cases) |
|---|---|---|---|---|---|---|---|---|---|---|
| Verboon-Maciolek et al.18) | 1994–2010 | 8 | 30–40 | 1,830–4,000 | 6–9/8–10 | Fever (2), sepsis-like illness (2), symptoms of gastroenteritis (6), lethargy (2), rash (0), seizures (7), apnea (1) | 6–42 (34–42) | Periventricular and subcortical cysts on MRI (6), restricted diffusion changes on periventricular white matter (2) | Rotavirus in stool (8/8) and CSF/blood (2/?) Enterovirus, parechovirus, herpes simplex virus, and adenovirus (0/8) |
Normal (8–12 mo, 3), infantile spasms (3–4 mo, 2), learning disability (5 yr, 1) |
| Lee et al.12) | 2008–2010 | 13 | 37–39 | 3,000±300 | 7–9/9–10 | Fever (0), rash (0), diarrhea (4), seizures (13) | 4–6 (37–39) | Restricted diffusion changes on periventricular white matter (13), cystic evolution in 4 of 10 repeated MRI | Rotavirus in stool (13/13) | Normal (6–18 mo, 6), speech delay (8–30 mo, 3), motor and speech delay (16 mo, 1) |
| Yeom et al.11) | 2009–2014 | 18 | 36–39 | 2,500–3,600 | 8–9/9–10 | Fever (1), diarrhea (1), abdominal distension (1), rash (0), seizures (16), apnea (2) | 4–7 (36–40) | Restricted diffusion changes on periventricular white matter (18), cystic evolution in 5 of 8 repeated MRI | Rotavirus in stool (17/18) Rotavirus and parechovirus in blood (0/15) and CSF (0/4) Enterovirus in CSF (0/12) |
? |
| Oh et al.13) | 2011–2013 | 30 | 36–40 | ? | ?/9–10 | Fever (0), rash (0), poor feeding(2), diarrhea (4), seizures (30) | 2–7 (?) | Restricted diffusion changes on periventricular white matter (30), abnormal findings in 6 of 22 repeated MRI | Rotavirus in stool (30/30), CSF (0/25), and serum (0/20) Enterovirus in stool (5/30) and CSF (1/25) No detection of parechovirus in any specimens |
? |
GA, gestational age; cGA, corrected gestational age; MRI, magnetic resonance imaging; CSF, cerebrospinal fluid.
Three recent reports from Korea11,12,13) lend support to the role of rotavirus infection in WMI. The main findings of these three studies11,12,13) were similar and can be summarized as follows: (1) Neonates presenting with seizures characterized by a distinctive diffusion-weighted imaging (DWI) pattern had a high positive rate of rotavirus infection. (2) Patients with this DWI pattern were apparently healthy term infants who presented with similar clinical features and suffered seizures between days 4 and 6 of life. (3) Gastroenteritis symptoms were not observed in most cases. (4) Rotavirus was detected only in stool specimens, not in blood and CSF specimens. (5) The presence of other viral pathogens, especially human parechovirus and enterovirus, was not proven. (6) There was no evidence of CSF pleocytosis. (7) Cystic evolution and neurological sequelae were observed in some of the patients. The pattern of WMI was evident on DWI, which showed extensive and symmetrical restricted diffusion in the periventricular white matter and along entire fiber tracts, including the corpus callosum (Fig. 1)11). This pattern was also seen in the full-term infants evaluated by Verboon-Maciolek et al.18). Most findings of Verboon-Maciolek et al.18) were similar to those reported from Korea11,12,13), but there were also several differences between the studies. First, preterm newborns were excluded from the three Korean studies11,12,13), perhaps because it is difficult to perform MRI in preterm neonates at the time of illness for reasons such as the clinical condition of the patients or technical limitations at the evaluating centers. According to Verboon-Maciolek et al.18), outcomes were worse in preterm than in full-term neonates, but this remains to be confirmed. Second, the sample size was larger in each of the three studies from Korea11,12,13) than in the study of Verboon-Maciolek et al.18). A 5-year single center retrospective study from Korea by Yeom et al.11) showed that rotavirus infection was identified in 17 neonates with the aforementioned DWI pattern. Other 2 studies12,13) from same single tertiary center reported that total 32 infants with this DWI pattern had rotavirus in their stool specimens for 5 consecutive years. By contrast, Verboon-Maciolek et al.18) reported on only eight patients from four hospitals for 6 years. The difference in patient number may be explained by the different patient criteria for testing rotavirus and the sensitivity of DWI in the detection of acute edema. All eight patients in the study of Verboon-Maciolek et al.18) had symptoms compatible with rotavirus gastroenteritis. Thus, rotavirus test seemed to perform in patients with gastroenteritis symptoms. However, in all three Korean studies11,12,13), a screening test for rotavirus was routinely performed and almost all patients were found to be asymptomatic for rotavirus infection. Thus, if only patients with gastroenteritis symptoms had been tested for rotavirus, a relationship between the two would not have been found11,12,13). Verboon-Maciolek et al.18) used ultrasound in their evaluations of preterm neonates. However, the sensitivity of DWI to the acute phase of edema is superior to that of ultrasound and conventional MRI, especially in the immature brain27). Conventional MRI techniques are insensitive to acute edema in the newborn brain due to the lack of myelination in neonates28). DWI with corresponding apparent diffusion coefficient maps can detect acute edema earlier and more clearly28). According to Yeom et al.11), if DWI had not been performed, the unique pattern of WMI might not have been recognized because only subtle signal changes were detected in most of their patients evaluated by conventional MRI techniques (Fig. 1).
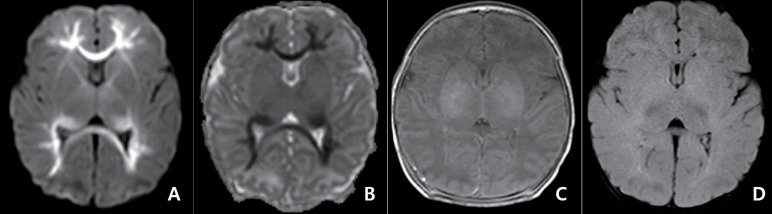
Fig. 1. Magnetic resonance imaging obtained 3 days after the onset of seizures in a term-newborns with seizures in 6th day and rotavirus infection. Diffusion-weighted imaging (A) and apparent diffusion coefficient map (B) show restricted diffusion in the periventricular white matter, corpus callosum, and optic radiation with symmetry. These findings were not apparent in T1-weighted spine echo sequence (C) and T2-weighted fluid-attenuated inversion recovery images (D) (unpublished data from Gyeongsang National University Hospital).
Although the long-term neurological sequelae have yet to be defined, rotavirus-associated WMI may not always be benign. Cystic evolution was observed in 27%–75% of patients with rotavirus-associated WMI (Fig. 2)11,12,13,18). Verboon-Maciolek et al.18) reported adverse outcomes, including epilepsy and cerebral palsy, in four of their 8 patients. Lee et al.12) detected mild neurological deficits, including speech or motor delay, in four of the 13 patients included in their study. The factors associated with poor prognosis have yet to be identified; however, they may include rotavirus infection or coinfection with enterovirus in preterm infants13,18). The clinical features of the patients with rotavirus-associated WMI who were described in recent reports11,12,13,18) are surprisingly similar to those of patients with the largely forgotten clinical entity of 'fifth day fits'. In an examination at median 4.6 years, developmental abnormalities were observed in more than half of the patients with 'fifth day fits'21).
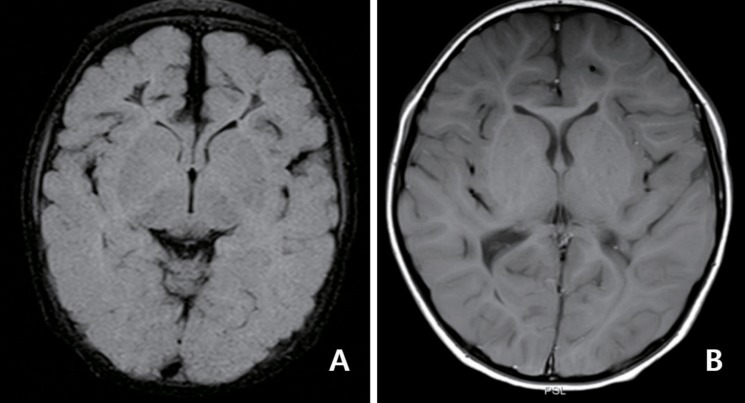
Fig. 2. Serial follow-up magnetic resonance imaging. (A) Cystic changes was observed in white matter in the frontal lobe at 2.5 months after seizure onset. (B) Note the decreased cerebral white matter volume at 2 years after onset of symptoms. Panels show T2-weighted fluid-attenuated inversion recovery image (A) and T1-weighted spine echo sequence (B) from a same patient who suffered neonatal seizures with rotavirus infection (unpublished data from Gyeongsang National University of Hospital).
Comparisons with other viral infections
The pattern of WMI seen in rotavirus infections is not unique and is, in fact, strikingly similar to that seen in neonatal enterovirus and parechovirus encephalitis11,29,30,31,32,33,34). In all 3 infections, the major findings on cerebral imaging are diffuse high signal intensity in the white matter on T2-weighted sequences and restricted diffusion in the periventricular white matter, corpus callosum, and deep white matter. However, the signal changes seen on conventional MRI sequence are more subtle in the WMI of rotavirus infections than in the WMI related to enterovirus and parechovirus infections30,34). A predilection for cystic evolution in the frontal lobes is another common feature11,12,18,30,31), as is the timing of WMI susceptibility following viral infection. Despite the broad range in the onset of these viral illnesses, the timing of neurological involvement is 34–42 weeks when corrected for gestational age11,12,13,18,30,31). Thus, it seems that the white matter is susceptible to viral infection during the late preterm to full-term period and the final common pathway to virus-induced WMI may not differ among rotavirus, enterovirus, and parechovirus.
However, there are differences in the clinical characteristics of the patients infected with the different viruses. As mentioned above, rotavirus-associated WMI characteristically occurs around the fifth day of life in full-term neonates. By contrast, the onset of illness is more heterogeneous in full-term neonates with enterovirus and parechovirus infections: 2–13 days and 1–49 days, respectively30,31,32,35). The most notable difference between infections with rotavirus vs. the other viruses is the clinical presentation of the respective patients. Sepsis-like illness, including fever and rash, is observed in most cases of enteroviral and parechviral meningoencephalitis30,31,32), whereas neither fever/rash nor symptoms of gastroenteritis are seen in most neonatal patients with rotavirus-associated WMI11,13). In cases of rotavirus infection, virus was not detected in the CSF11,12,13), whereas it is usually detected in the CSF of neonates infected with enterovirus and parechovirus30,31,33,34,35). In addition, CSF pleocytosis was not observed in rotavirus infection but was present in some cases of enterovirus infection11,30). Thus, among the viruses, the detailed pathway to WMI may differ. The similarities and differences in WMI induced by the three viruses are summarized in Table 2.
Table 2. Comparison of clinical characteristics of white matter injury, rotavirus versus enterovirus and human parechovirus.
| Clinical feature | Rotavirus | Enterovirus | Parechovirus |
|---|---|---|---|
| Onset of illness | |||
| cGA (wk) | 34–42 | 34–42 | 34–42 |
| Days in term neonates | 4–7 | 2–13 | 1–49 |
| Season | Fall to spring | Summer to fall | Summer to fall |
| Symptoms | |||
| Seizures | Almost | Almost | Almost |
| Fever | Rare | Common | Common |
| Rash | Absence | Common | Common |
| Gastroenteritis | Rare | Rare | Rare |
| CSF pleocytosis | Absence | Common | Rare |
| MRI findings | |||
| T2-weighted sequence | No or subtle changes in signal intensity on WM | Diffuse high signal intensity in the WM | Diffuse high signal intensity in the WM |
| Diffusion-weighted imaging | Extensive and symmetric areas of restricted diffusion in the periventricular WM, corpus callosum, or thalamus | Restricted diffusion in periventricular WM or corpus callosum | Symmetric areas of restricted diffusion in the periventricular WM, corpus callosum, or thalamus |
cGA, corrected gestational age; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; WM, white matter.
Possible mechanisms of WMI associated with virus infection
The activation of brain microglia is the principal initiating event in systemic infection/inflammation leading to WMI36). The activated microglia release compounds such as reactive oxygen and nitrogen species and cytokines, all of which are toxic to the premyelinating oligodendrocytes (pre-OLs) in white matter36). Pre-OLs are highly vulnerable to free radical attack and to glutamate-induced excitotoxicity36). This is the mechanism underlying pre-OL injury in systemic infection/inflammation, and it results in the acute loss of pre-OL, pre-OL maturation arrest, and/or abnormal myelination36).
In WMI caused by enterovirus and parechovirus, the virus seem to penetrate the brain30,31). The single-stranded ribonucleic acid of enterovirus and parechovirus may activate the microglia via the activation of intracellular Toll-like receptors (TLR) 7 and 8, resulting in WMI37). In addition, TLR8 localizes to neurons and axons, and its activation results in the inhibition of axonal outgrowth and neuronal apoptosis without CNS inflammation38). The latter hypothesis could explain the rare finding of CSF pleocytosis in cases of WMI induced by parechovirus37). Rotavirus, by contrast, is unlikely to penetrate the brain in cases of WMI in newborns11,18). Thus, at least in these patients, WMI caused by rotavirus cannot be explained by either of the 2 aforementioned mechanisms, and how rotavirus leads to WMI in neonates remains unclear.
Future directions
Because rotavirus infection is common in neonatal care units, recent studies11,12,13,18) demonstrating rotavirus-induced WMI would be of great concern to pediatricians. According to these studies11,12,13), rotavirus is one of the most common causes of neonatal seizures. Furthermore, they11,12,13,18) suggest a new paradigm of viral infection: infection with a common virus can induce WMI in term neonates even without direct invasion. However, the causal link between rotavirus infection and WMI in neonates remains to be validated in prospective cohort studies. If rotavirus infection indeed induces WMI in neonates, the long-term prognosis of these patients determined. Despite the lack of evidence, an enterotoxin of rotavirus (NSP4) has been considered as cause of the neurological complications of rotavirus39). Thus, the role of toxin-mediated injury deserves further consideration29). Variability in NSP4 across rotavirus strains may be a key determinant of pathogenicity29). Or, a host factor may be more important than viral factors. Neonates who had suffered from antenatal subclinical chorioamnionitis may be vulnerable to WMI by postnatal rotavirus infection. Further studies are necessary to clarify the underlying mechanisms of rotavirus neurotoxicity.
Conclusion
The relationship between rotavirus infection and WMI strongly suggests the use of sequential neuroimaging in all rotavirus-infected newborns with neurological manifestations. Neonates with neurological symptoms should be tested for rotavirus infection and evaluated using MRI including DWI. Strict measures to prevent rotavirus infection in neonatal care units should be enforced. WMI seems to be a newly recognized consequence of rotavirus infection in neonates. However, it might be a new aspect of 'fifth day fits', what we knew but forgot for long time.
Footnotes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau V, Poskitt KJ, McFadden DE, Bowen-Roberts T, Synnes A, Brant R, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 3.Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 4.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. 175.e1. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitha A, Foix-L'Hélias L, Arnaud C, Marret S, Vieux R, Aujard Y, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132:e372–e380. doi: 10.1542/peds.2012-3979. [DOI] [PubMed] [Google Scholar]
- 7.Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Post-natal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. 2012;71:274–279. doi: 10.1038/pr.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 9.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–211. [PubMed] [Google Scholar]
- 10.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 11.Yeom JS, Kim YS, Seo JH, Park JS, Park ES, Lim JY, et al. Distinctive pattern of white matter injury in neonates with rotavirus infection. Neurology. 2015;84:21–27. doi: 10.1212/WNL.0000000000001107. [DOI] [PubMed] [Google Scholar]
- 12.Lee KY, Oh KW, Weon YC, Choi SH. Neonatal seizures accompanied by diffuse cerebral white matter lesions on diffusion-weighted imaging are associated with rotavirus infection. Eur J Paediatr Neurol. 2014;18:624–631. doi: 10.1016/j.ejpn.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Oh KW, Moon CH, Lee KY. Association of rotavirus with seizures accompanied by cerebral white matter injury in neonates. J Child Neurol. 2015;30:1433–1439. doi: 10.1177/0883073814568153. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd MB, Lloyd JC, Gesteland PH, Bale JF., Jr Rotavirus gastroenteritis and seizures in young children. Pediatr Neurol. 2010;42:404–408. doi: 10.1016/j.pediatrneurol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann B, Lawrenz-Wolf B, Seewald C, Selb B, Wehinger H. 5th day convulsions of the newborn infant in rotavirus infections. Monatsschr Kinderheilkd. 1993;141:120–123. [PubMed] [Google Scholar]
- 16.Riedel F, Kroener T, Stein K, Nuesslein TG, Rieger CH. Rotavirus infection and bradycardia-apnoea-episodes in the neonate. Eur J Pediatr. 1996;155:36–40. doi: 10.1007/BF02115624. [DOI] [PubMed] [Google Scholar]
- 17.de Villiers FP, Steele AD, Driessen M. Central nervous system involvement in neonatal rotavirus infection. Ann Trop Paediatr. 2003;23:309–312. doi: 10.1179/027249303225007789. [DOI] [PubMed] [Google Scholar]
- 18.Verboon-Maciolek MA, Truttmann AC, Groenendaal F, Skranes J, Døllner H, Hunt RW, et al. Development of cystic periventricular leukomalacia in newborn infants after rotavirus infection. J Pediatr. 2012;160:165–168.e1. doi: 10.1016/j.jpeds.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 19.North KN, Storey GN, Henderson-Smart DJ. Fifth day fits in the newborn. Aust Paediatr J. 1989;25:284–287. doi: 10.1111/j.1440-1754.1989.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 20.Pryor DS, Don N, Macourt DC. Fifth day fits: a syndrome of neonatal convulsions. Arch Dis Child. 1981;56:753–758. doi: 10.1136/adc.56.10.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheffzek A, Berns-Mathio J, Scheel W, Stahl M. The developmental prognosis of children with 5-day seizures. Monatsschr Kinderheilkd. 1991;139:413–417. [PubMed] [Google Scholar]
- 22.Goldberg HJ, Sheehy EM. Fifth day fits: an acute zinc deficiency syndrome? Arch Dis Child. 1982;57:633–635. doi: 10.1136/adc.57.8.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabris C, Licata D, Stasiowska B, Lio C, Mostert M. Is type of feeding related to fifth day fits of the newborns? Unexpected outcome of a case-control study. Acta Paediatr Scand. 1988;77:162. doi: 10.1111/j.1651-2227.1988.tb10617.x. [DOI] [PubMed] [Google Scholar]
- 24.Chrystie IL, Totterdell B, Baker MJ, Scopes JW, Banatvala JE. Letter: rotavirus infections in a maternity unit. Lancet. 1975;2:79. doi: 10.1016/s0140-6736(75)90525-5. [DOI] [PubMed] [Google Scholar]
- 25.Murphy AM, Albrey MB, Crewe EB. Rotavirus infections of neonates. Lancet. 1977;2:1149–1150. doi: 10.1016/S0140-6736(77)91538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tufvesson B, Polberger S, Svanberg L, Sveger T. A prospective study of rotavirus infections in neonatal and maternity wards. Acta Paediatr Scand. 1986;75:211–215. doi: 10.1111/j.1651-2227.1986.tb10186.x. [DOI] [PubMed] [Google Scholar]
- 27.Mascalchi M, Filippi M, Floris R, Fonda C, Gasparotti R, Villari N. Diffusion-weighted MR of the brain: methodology and clinical application. Radiol Med. 2005;109:155–197. [PubMed] [Google Scholar]
- 28.Rodrigues K, Grant PE. Diffusion-weighted imaging in neonates. Neuroimaging Clin N Am. 2011;21:127–151. doi: 10.1016/j.nic.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 29.de Vries LS, Bearden D. Neurologic complications of rotavirus in neonates: More common than we thought? Neurology. 2015;84:13–14. doi: 10.1212/WNL.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 30.Verboon-Maciolek MA, Utrecht FG, Cowan F, Govaert P, van Loon AM, de Vries LS. White matter damage in neonatal enterovirus meningoencephalitis. Neurology. 2008;71:536. doi: 10.1212/01.wnl.0000324706.94229.88. [DOI] [PubMed] [Google Scholar]
- 31.Verboon-Maciolek MA, Groenendaal F, Hahn CD, Hellmann J, van Loon AM, Boivin G, et al. Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol. 2008;64:266–273. doi: 10.1002/ana.21445. [DOI] [PubMed] [Google Scholar]
- 32.Wu T, Fan XP, Wang WY, Yuan TM. Enterovirus infections are associated with white matter damage in neonates. J Paediatr Child Health. 2014;50:817–822. doi: 10.1111/jpc.12656. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Fernandez D, Siddiqui A, Tong WC, Pohl K, Jungbluth H. Extensive white matter abnormalities associated with neonatal Parechovirus (HPeV) infection. Eur J Paediatr Neurol. 2010;14:531–534. doi: 10.1016/j.ejpn.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Belcastro V, Bini P, Barachetti R, Barbarini M. Teaching neuroimages: neonatal parechovirus encephalitis: typical MRI findings. Neurology. 2014;82:e23. doi: 10.1212/WNL.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 35.Rath A, Berner R, Panning M, Krueger M, Gerecke A. Parechovirus as a cause of a sepsis-like syndrome with cerebral involvement in a 7-week old infant. Klin Padiatr. 2014;226:76–77. doi: 10.1055/s-0033-1333755. [DOI] [PubMed] [Google Scholar]
- 36.Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011;29:423–440. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpe JJ. Neonatal encephalitis and white matter injury: more than just inflammation? Ann Neurol. 2008;64:232–236. doi: 10.1002/ana.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, Haynes RL, Sidman RL, Vartanian T. TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle. 2007;6:2859–2868. doi: 10.4161/cc.6.23.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeom JS, Kim YS, Park JS, Seo JH, Park ES, Lim JY, et al. Role of Ca2+ homeostasis disruption in rotavirus-associated seizures. J Child Neurol. 2014;29:331–335. doi: 10.1177/0883073812469052. [DOI] [PubMed] [Google Scholar]