Abstract
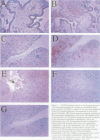
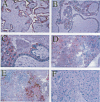
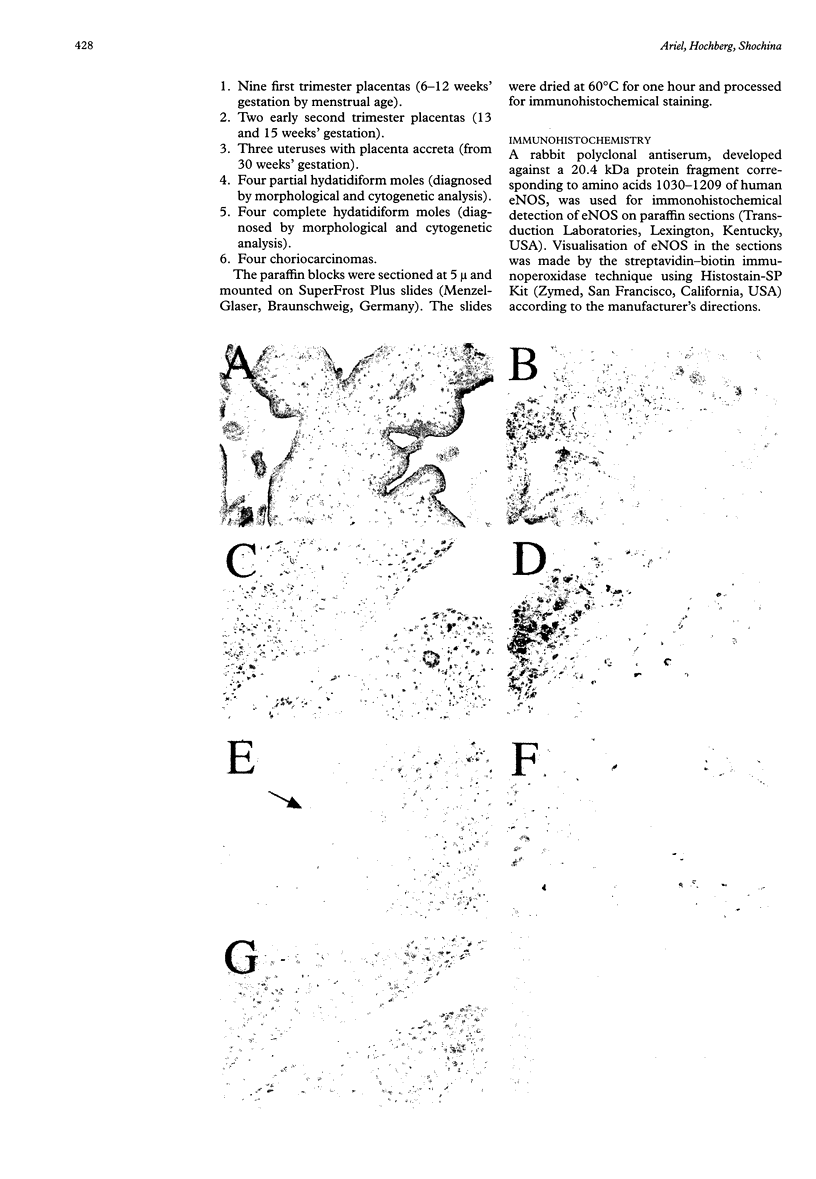
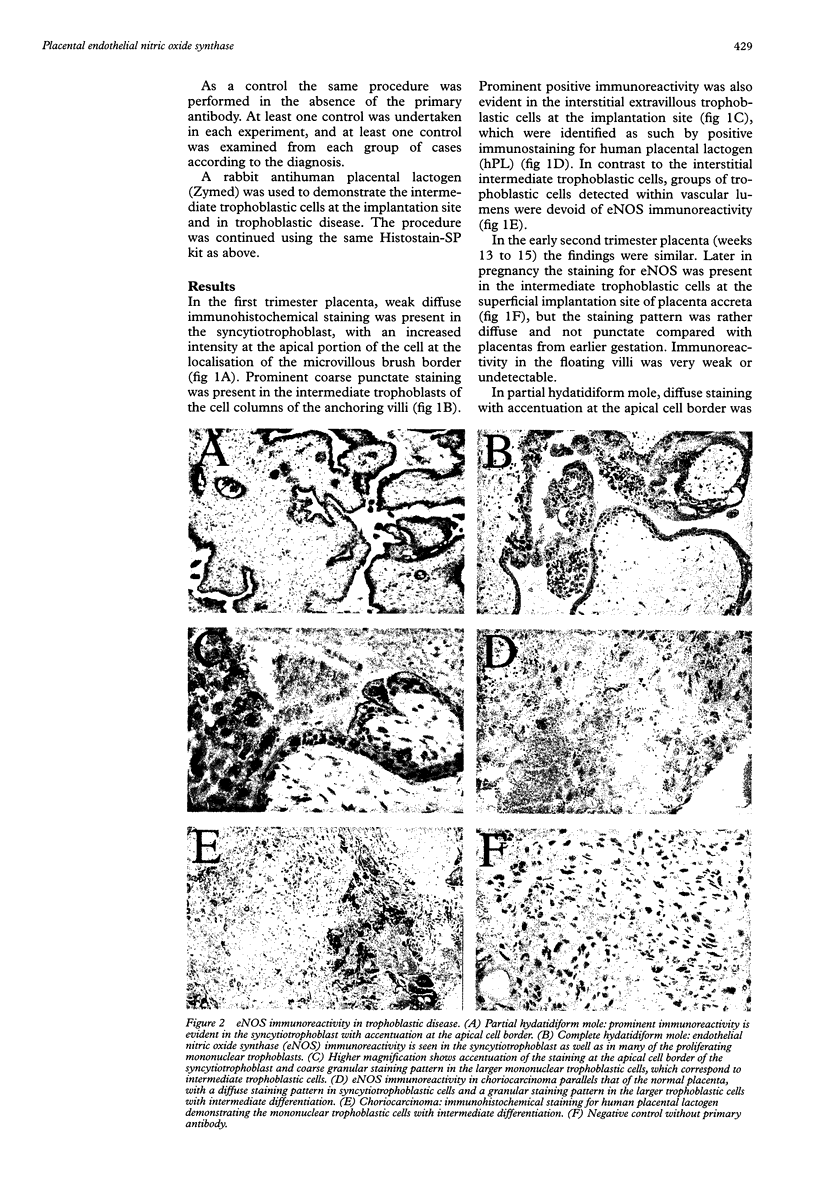
AIMS: To study the localisation of the endothelial nitric oxide synthase (eNOS) in the normal placenta, with special emphasis on the implantation site in the first trimester of pregnancy, and in the different subtypes of trophoblastic cells in gestational trophoblastic disease. METHODS: The immunoperoxidase technique with an antibody directed against eNOS was applied to paraffin sections from first and second trimester placentas, placenta accreta, partial and complete hydatidiform moles, and choriocarcinoma. Immunoperoxidase staining for human placental lactogen (hPL) was performed on parallel sections. RESULTS: Prominent immunoreactivity for eNOS was found to be present in the intermediate trophoblastic cells of the cell columns of the anchoring villi and in trophoblastic cells at the implantation site. Staining was also present in the syncytiotrophoblast, most conspicuous at the apical cell border. In trophoblastic disease, proliferating large mononuclear cells, which were strongly positive for hPL, were found to be immunoreactive for eNOS. CONCLUSIONS: eNOS immunoreactivity is strongly positive in the extravillous trophoblastic cells and to a lesser extent in the syncytiotrophoblast. In the former it may play a role in implantation and vascular invasion. Cells with differentiation to intermediate trophoblast in complete hydatidiform mole and choriocarcinoma also show high levels of eNOS, which may be associated with the haematogenous mode of spread of trophoblastic disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Snyder S. H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Buttery L. D., McCarthy A., Springall D. R., Sullivan M. H., Elder M. G., Michel T., Polak J. M. Endothelial nitric oxide synthase in the human placenta: regional distribution and proposed regulatory role at the feto-maternal interface. Placenta. 1994 Apr;15(3):257–265. doi: 10.1016/0143-4004(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Chhatwal V. J., Ngoi S. S., Chan S. T., Chia Y. W., Moochhala S. M. Aberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peritumoral normal mucosa. Carcinogenesis. 1994 Oct;15(10):2081–2085. doi: 10.1093/carcin/15.10.2081. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Vill M., McGuire P. G., Dail W. G., Davis A. K. Expression of nitric oxide synthase by syncytiotrophoblast in human placental villi. FASEB J. 1993 Oct;7(13):1269–1276. doi: 10.1096/fasebj.7.13.7691671. [DOI] [PubMed] [Google Scholar]
- Dueñas-Gonzalez A., Isales C. M., del Mar Abad-Hernandez M., Gonzalez-Sarmiento R., Sangueza O., Rodriguez-Commes J. Expression of inducible nitric oxide synthase in breast cancer correlates with metastatic disease. Mod Pathol. 1997 Jul;10(7):645–649. [PubMed] [Google Scholar]
- Eis A. L., Brockman D. E., Pollock J. S., Myatt L. Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta. 1995 Mar;16(2):113–126. doi: 10.1016/0143-4004(95)90000-4. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Hustin J., Dubois M., Schaaps J. P. The human placenta becomes haemochorial at the 13th week of pregnancy. Int J Dev Biol. 1992 Sep;36(3):451–453. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Tuttle J. V., Covington K., Merrill B. M., Wood E. R., Baylis S. A., Charles I. G. Purification and characterization of the constitutive nitric oxide synthase from human placenta. Arch Biochem Biophys. 1994 Jun;311(2):235–241. doi: 10.1006/abbi.1994.1232. [DOI] [PubMed] [Google Scholar]
- Ghabour M. S., Eis A. L., Brockman D. E., Pollock J. S., Myatt L. Immunohistochemical characterization of placental nitric oxide synthase expression in preeclampsia. Am J Obstet Gynecol. 1995 Sep;173(3 Pt 1):687–694. doi: 10.1016/0002-9378(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Stuehr D. J. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Jenkins D. C., Charles I. G., Baylis S. A., Lelchuk R., Radomski M. W., Moncada S. Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br J Cancer. 1994 Nov;70(5):847–849. doi: 10.1038/bjc.1994.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman R. J., Main C. S., Chen H. C. Intermediate trophoblast: a distinctive form of trophoblast with specific morphological, biochemical and functional features. Placenta. 1984 Jul-Aug;5(4):349–369. doi: 10.1016/s0143-4004(84)80015-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morris N. H., Eaton B. M., Sooranna S. R., Steer P. J. NO synthase activity in placental bed and tissues from normotensive pregnant women. Lancet. 1993 Sep 11;342(8872):679–680. [PubMed] [Google Scholar]
- Myatt L., Brockman D. E., Eis A. L., Pollock J. S. Immunohistochemical localization of nitric oxide synthase in the human placenta. Placenta. 1993 Sep-Oct;14(5):487–495. doi: 10.1016/s0143-4004(05)80202-4. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R., Bland J. M., Robertson W. B., Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983 Oct-Dec;4(4):397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- Pipili-Synetos E., Sakkoula E., Maragoudakis M. E. Nitric oxide is involved in the regulation of angiogenesis. Br J Pharmacol. 1993 Apr;108(4):855–857. doi: 10.1111/j.1476-5381.1993.tb13476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodesch F., Simon P., Donner C., Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992 Aug;80(2):283–285. [PubMed] [Google Scholar]
- Sneddon J. M., Vane J. R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L. L., Lawton F. G., Knowles R. G., Beesley J. E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994 Mar 1;54(5):1352–1354. [PubMed] [Google Scholar]
- Thomsen L. L., Miles D. W., Happerfield L., Bobrow L. G., Knowles R. G., Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995 Jul;72(1):41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]