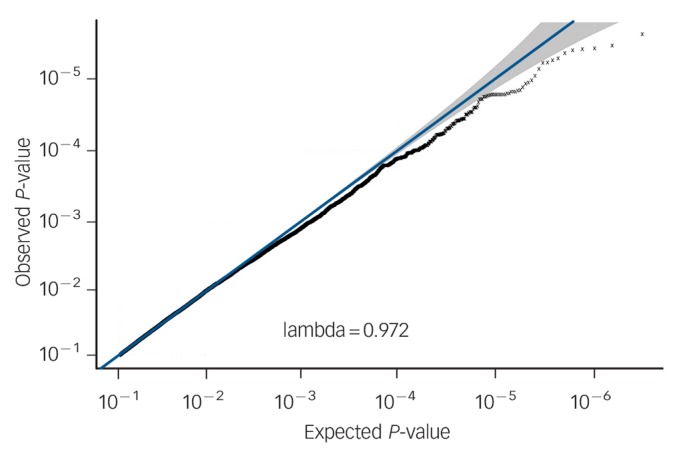
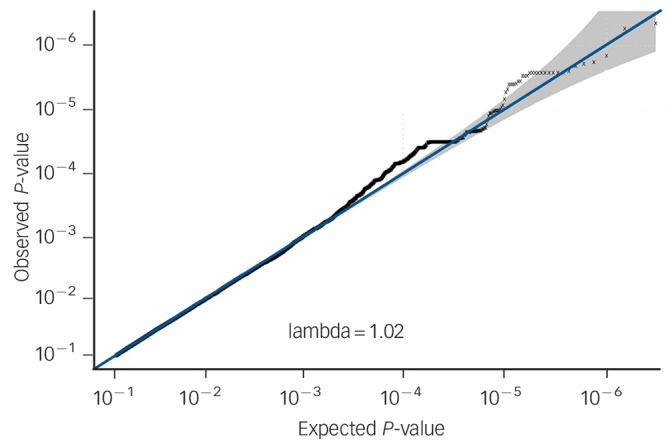
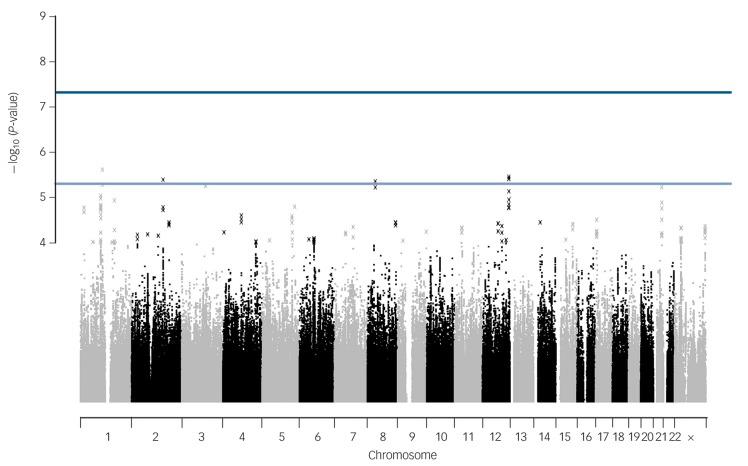
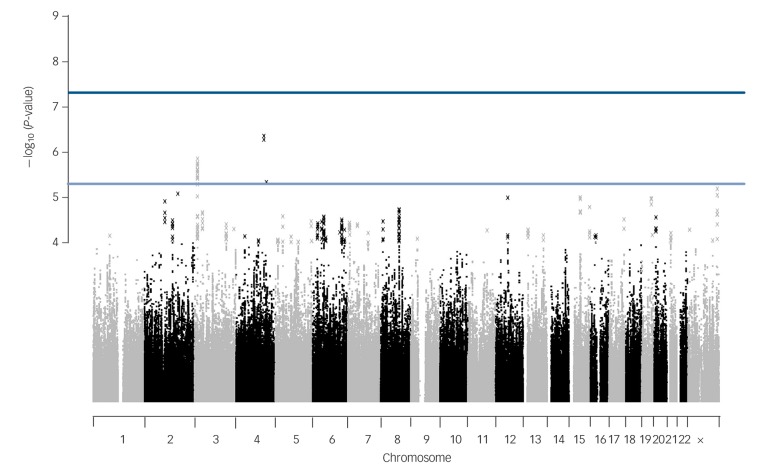
Jonathan R I Coleman
Jonathan R I Coleman
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Kathryn J Lester
Kathryn J Lester
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Robert Keers
Robert Keers
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Susanna Roberts
Susanna Roberts
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Charles Curtis
Charles Curtis
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Kristian Arendt
Kristian Arendt
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Susan Bögels
Susan Bögels
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Peter Cooper
Peter Cooper
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Cathy Creswell
Cathy Creswell
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Tim Dalgleish
Tim Dalgleish
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Catharina A Hartman
Catharina A Hartman
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Einar R Heiervang
Einar R Heiervang
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Katrin Hötzel
Katrin Hötzel
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Jennifer L Hudson
Jennifer L Hudson
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Tina In-Albon
Tina In-Albon
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Kristen Lavallee
Kristen Lavallee
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Heidi J Lyneham
Heidi J Lyneham
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Carla E Marin
Carla E Marin
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Richard Meiser-Stedman
Richard Meiser-Stedman
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Talia Morris
Talia Morris
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Maaike H Nauta
Maaike H Nauta
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Ronald M Rapee
Ronald M Rapee
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Silvia Schneider
Silvia Schneider
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Sophie C Schneider
Sophie C Schneider
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Wendy K Silverman
Wendy K Silverman
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Mikael Thastum
Mikael Thastum
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Kerstin Thirlwall
Kerstin Thirlwall
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Polly Waite
Polly Waite
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Gro Janne Wergeland
Gro Janne Wergeland
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,
Gerome Breen
Gerome Breen
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,*,
Thalia C Eley
Thalia C Eley
1Jonathan R. I. Coleman, MSc, King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), MRC Social, Genetic and Developmental Psychiatry (SGDP) Centre, UK; Kathryn J. Lester, DPhil, King's College London, IoPPN, MRC SGDP Centre, UK, and School of Psychology, University of Sussex, UK; Robert Keers, PhD, Susanna Roberts, MSc, King's College London, IoPPN, MRC SGDP Centre, UK; Charles Curtis, MSc, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Kristian Arendt, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Susan Bögels, PhD, Research Institute Child Development and Education, University of Amsterdam, The Netherlands; Peter Cooper, DPhil, School of Psychology and Clinical Language Sciences, University of Reading, UK, and Department of Psychology, Stellenbosch University, South Africa; Cathy Creswell, DClinPsy, PhD, School of Psychology and Clinical Language Sciences, University of Reading, UK; Tim Dalgleish, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Catharina A. Hartman, PhD, Department of Psychiatry, University of Groningen, University Medical Center Groningen, The Netherlands; Einar R. Heiervang, PhD, Institute of Clinical Medicine, University of Oslo, Norway; Katrin Hötzel, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Jennifer L. Hudson, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Tina In-Albon, PhD, Clinical Child and Adolescent Psychology, Universität Koblenz-Landau, Germany; Kristen Lavallee, PhD, Department of Psychology, University of Basel, Switzerland; Heidi J. Lyneham, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Carla E. Marin, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Richard Meiser-Stedman, PhD, MRC Cognition & Brain Sciences Unit, Cambridge, UK; Talia Morris, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Maaike H. Nauta, PhD, Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, The Netherlands; Ronald M. Rapee, PhD, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Silvia Schneider, PhD, Department of Psychology, Ruhr-Universität Bochum, Germany; Sophie C. Schneider, BPsych, Centre for Emotional Health, Department of Psychology, Macquarie University, Sydney, Australia; Wendy K. Silverman, PhD, Yale University, Child Study Center, New Haven, Connecticut, USA; Mikael Thastum, PhD, Department of Psychology and Behavioural Sciences, Aarhus University, Denmark; Kerstin Thirlwall, DClinPsy, Polly Waite, DClinPsy, School of Psychology and Clinical Language Sciences, University of Reading, UK; Gro Janne Wergeland, MD, Department of Child and Adolescent Psychiatry, Haukeland University Hospital, Bergen, and Anxiety Disorders Research Network, Haukeland University Hospital, Norway; Gerome Breen, PhD, King's College London, IoPPN, MRC SGDP Centre, UK, and National Institute for Health Research Biomedical Research Centre, South London and Maudsley National Health Service Trust, UK; Thalia C. Eley, PhD, King's College London, IoPPN, MRC SGDP Centre, UK
1,*,✉