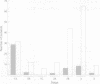
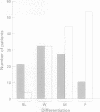
Abstract
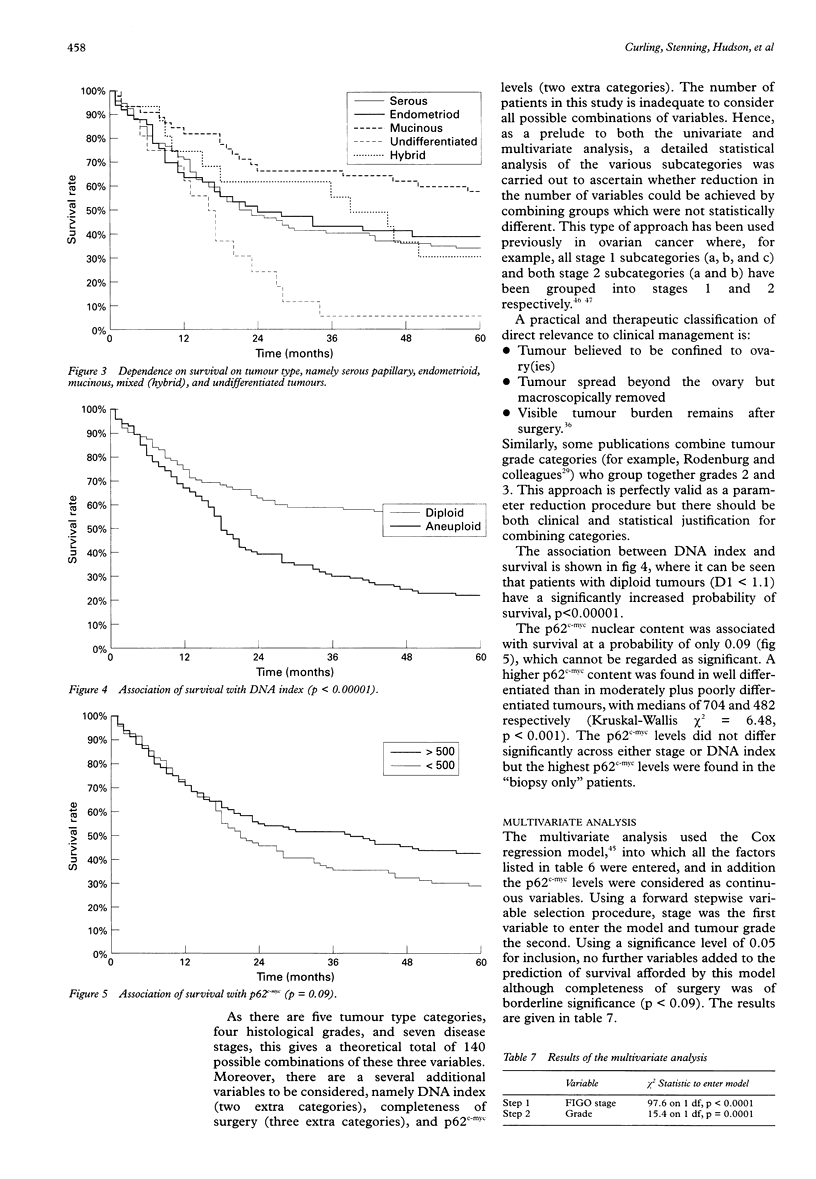
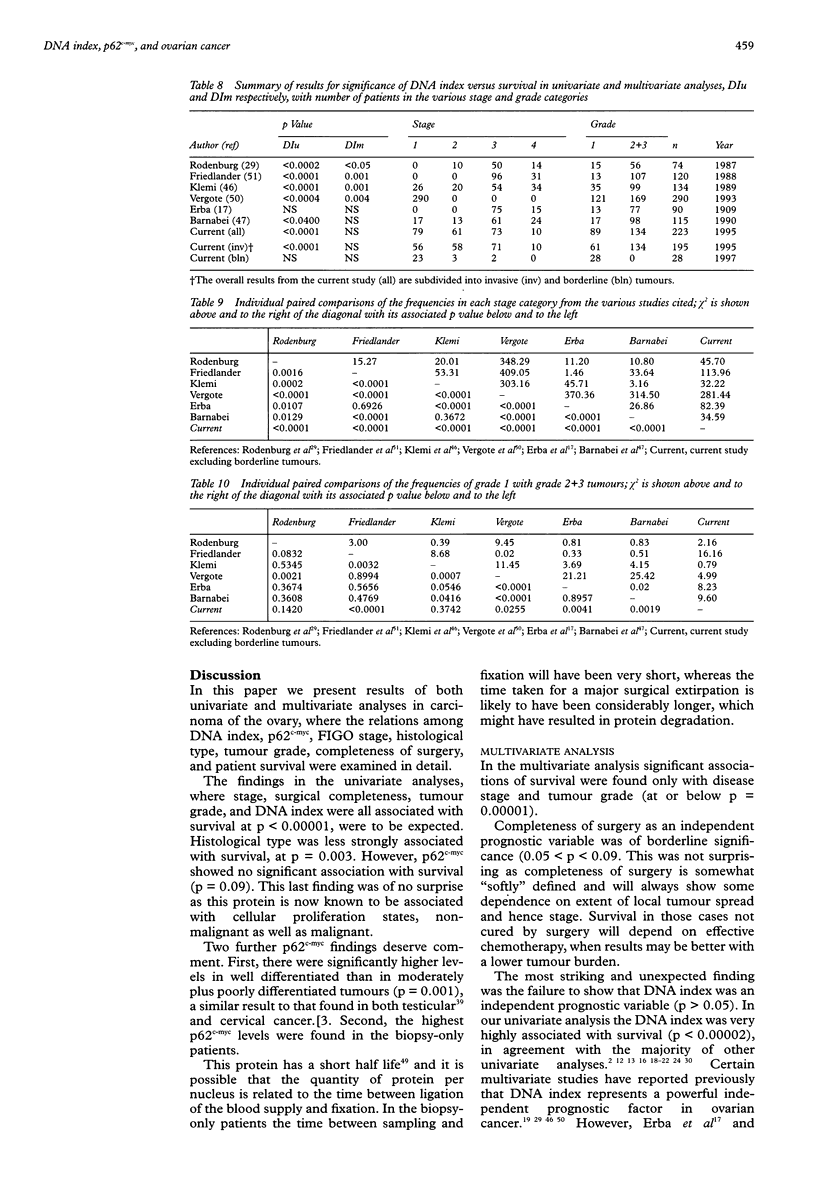
AIM: To determine if either DNA index or p62c-myc is an independent prognostic variable in ovarian cancer. METHODS: Multivariate and univariate analyses of the relation between DNA index, p62c-myc, FIGO stage, histological type, tumour grade, completeness of surgery, and patient survival in ovarian cancer were examined. RESULTS: Multivariate analysis showed significant association of survival only with stage and grade. There was no relation between survival and DNA index. CONCLUSIONS: DNA index is not an independent prognostic variable in ovarian cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B., Kay R. Prognostic significance of modal DNA value and other factors in malignant tumours, based on 1465 cases. Br J Cancer. 1979 Aug;40(2):210–221. doi: 10.1038/bjc.1979.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin N. B. Modal DNA value and chromosome number in ovarian neoplasia. A clinical and histopathologic assessment. Cancer. 1971 May;27(5):1064–1073. doi: 10.1002/1097-0142(197105)27:5<1064::aid-cncr2820270510>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Barnabei V. M., Miller D. S., Bauer K. D., Murad T. M., Rademaker A. W., Lurain J. R. Flow cytometric evaluation of epithelial ovarian cancer. Am J Obstet Gynecol. 1990 Jun;162(6):1584–1592. doi: 10.1016/0002-9378(90)90924-v. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Berchuck A., Boente M. P., Kerns B. J., Kinney R. B., Soper J. T., Clarke-Pearson D. L., Bast R. C., Jr, Bacus S. S. Ploidy analysis of epithelial ovarian cancers using image cytometry. Gynecol Oncol. 1992 Jan;44(1):61–65. doi: 10.1016/0090-8258(92)90013-9. [DOI] [PubMed] [Google Scholar]
- Blumenfeld D., Braly P. S., Ben-Ezra J., Klevecz R. R. Tumor DNA content as a prognostic feature in advanced epithelial ovarian carcinoma. Gynecol Oncol. 1987 Jul;27(3):389–402. doi: 10.1016/0090-8258(87)90264-2. [DOI] [PubMed] [Google Scholar]
- Brescia R. J., Barakat R. A., Beller U., Frederickson G., Suhrland M. J., Dubin N., Demopoulos R. I. The prognostic significance of nuclear DNA content in malignant epithelial tumors of the ovary. Cancer. 1990 Jan 1;65(1):141–147. doi: 10.1002/1097-0142(19900101)65:1<141::aid-cncr2820650128>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Chadha S., Cornelisse C. J., Schaberg A. Flow cytometric DNA ploidy analysis of ovarian granulosa cell tumors. Gynecol Oncol. 1990 Feb;36(2):240–245. doi: 10.1016/0090-8258(90)90181-j. [DOI] [PubMed] [Google Scholar]
- Christov K., Vassilev N. Flow cytometric analysis of DNA and cell proliferation in ovarian tumors. Cancer. 1988 Jan 1;61(1):121–125. doi: 10.1002/1097-0142(19880101)61:1<121::aid-cncr2820610121>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Coulson P. B., Thornthwaite J. T., Woolley T. W., Sugarbaker E. V., Seckinger D. Prognostic indicators including DNA histogram type, receptor content, and staging related to human breast cancer patient survival. Cancer Res. 1984 Sep;44(9):4187–4196. [PubMed] [Google Scholar]
- Erba E., Ubezio P., Pepe S., Vaghi M., Marsoni S., Torri W., Mangioni C., Landoni F., D'Incalci M. Flow cytometric analysis of DNA content in human ovarian cancers. Br J Cancer. 1989 Jul;60(1):45–50. doi: 10.1038/bjc.1989.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S., Arbuck S. G., Chin J. L., Greco W. R., Pavelic Z. P., Slocum H. K., Mittelman A., Piver S. M., Pontes E. J., Rustum Y. M. Prognostic applications of DNA flow cytometry for human solid tumors. Ann N Y Acad Sci. 1986;468:276–290. doi: 10.1111/j.1749-6632.1986.tb42046.x. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S., Slocum H. K., Rustum Y. M., Arbuck S. G., Pavelic Z. P., Petrelli N., Huben R. P., Pontes E. J., Greco W. R. Flow cytometric analysis of DNA aneuploidy in primary and metastatic human solid tumors. Cytometry. 1984 Jan;5(1):71–80. doi: 10.1002/cyto.990050111. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Swanson C., Russell P. Prediction of long-term survival by flow cytometric analysis of cellular DNA content in patients with advanced ovarian cancer. J Clin Oncol. 1988 Feb;6(2):282–290. doi: 10.1200/JCO.1988.6.2.282. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W. Clinical and biological significance of aneuploidy in human tumours. J Clin Pathol. 1984 Sep;37(9):961–974. doi: 10.1136/jcp.37.9.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W., Russell P., Coates A. S., Tattersall M. H. Influence of cellular DNA content on survival in advanced ovarian cancer. Cancer Res. 1984 Jan;44(1):397–400. [PubMed] [Google Scholar]
- Friedlander M. L., Taylor I. W., Russell P., Musgrove E. A., Hedley D. H., Tattersall M. H. Ploidy as a prognostic factor in ovarian cancer. Int J Gynecol Pathol. 1983;2(1):55–63. doi: 10.1097/00004347-198301000-00005. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Taylor I. W., Russell P., Tattersall M. H. Cellular DNA content--a stable feature in epithelial ovarian cancer. Br J Cancer. 1984 Feb;49(2):173–179. doi: 10.1038/bjc.1984.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert F. Chromosome aberrations and oncogenes. Nature. 1983 Jun 9;303(5917):475–475. doi: 10.1038/303475a0. [DOI] [PubMed] [Google Scholar]
- Haapasalo H., Atkin N. B., Collan Y., Pesonen E., Paljärvi L. Tumour ploidy, morphometry, histological grading and clinical features in ovarian carcinoma: mutual relations. Anal Cell Pathol. 1991 Sep;3(5):261–271. [PubMed] [Google Scholar]
- Hamaguchi K., Nishimura H., Miyoshi T., Miyahara K., Tateno N., Yakushiji M., Yokoyama M. M. Flow cytometric analysis of cellular DNA content in ovarian cancer. Gynecol Oncol. 1990 May;37(2):219–223. doi: 10.1016/0090-8258(90)90336-j. [DOI] [PubMed] [Google Scholar]
- Hedley D. W. Flow cytometry using paraffin-embedded tissue: five years on. Cytometry. 1989 May;10(3):229–241. doi: 10.1002/cyto.990100302. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hendy-Ibbs P., Cox H., Evan G. I., Watson J. V. Flow cytometric quantitation of DNA and c-myc oncoprotein in archival biopsies of uterine cervix neoplasia. Br J Cancer. 1987 Mar;55(3):275–282. doi: 10.1038/bjc.1987.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiddemann W., Schumann J., Andreef M., Barlogie B., Herman C. J., Leif R. C., Mayall B. H., Murphy R. F., Sandberg A. A. Convention on nomenclature for DNA cytometry. Committee on Nomenclature, Society for Analytical Cytology. Cancer Genet Cytogenet. 1984 Oct;13(2):181–183. doi: 10.1016/0165-4608(84)90059-1. [DOI] [PubMed] [Google Scholar]
- Hudson C. N., Potsides P., Curling O. M. An audit of surgical treatment of ovarian cancer in a metropolitan health region. Association of Obstetricians and Gynaecologists of the North East Thames Region. J R Soc Med. 1991 Apr;84(4):206–209. doi: 10.1177/014107689108400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen O. E., Skaarland E. Ploidy assessment of benign and malignant ovarian tumors by flow cytometry. A clinicopathologic study. Cancer. 1987 Jul 1;60(1):82–87. doi: 10.1002/1097-0142(19870701)60:1<82::aid-cncr2820600114>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Johnson T. S., Williamson K. D., Cramer M. M., Peters L. J. Flow cytometric analysis of head and neck carcinoma DNA index and S-fraction from paraffin-embedded sections: comparison with malignancy grading. Cytometry. 1985 Sep;6(5):461–470. doi: 10.1002/cyto.990060511. [DOI] [PubMed] [Google Scholar]
- Kaern J., Trope C., Kjorstad K. E., Abeler V., Pettersen E. O. Cellular DNA content as a new prognostic tool in patients with borderline tumors of the ovary. Gynecol Oncol. 1990 Sep;38(3):452–457. doi: 10.1016/0090-8258(90)90090-8. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Punnonen R., Mattila J., Lehtinen M., Koivula T. Prognostic significance of DNA index, multiploidy, and S-phase fraction in ovarian cancer. Cancer. 1988 Jan 15;61(2):334–339. doi: 10.1002/1097-0142(19880115)61:2<334::aid-cncr2820610224>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Klemi P. J., Joensuu H., Mäenpä J., Kiilholma P. Influence of cellular DNA content on survival in ovarian carcinoma. Obstet Gynecol. 1989 Aug;74(2):200–204. [PubMed] [Google Scholar]
- Kühn W., Kaufmann M., Feichter G. E., Rummel H. H., Schmid H., Heberling D. DNA flow cytometry, clinical and morphological parameters as prognostic factors for advanced malignant and borderline ovarian tumors. Gynecol Oncol. 1989 Jun;33(3):360–367. doi: 10.1016/0090-8258(89)90528-3. [DOI] [PubMed] [Google Scholar]
- Lage J. M., Weinberg D. S., Huettner P. C., Mark S. D. Flow cytometric analysis of nuclear DNA content in ovarian tumors. Association of ploidy with tumor type, histologic grade, and clinical stage. Cancer. 1992 Jun 1;69(11):2668–2675. doi: 10.1002/1097-0142(19920601)69:11<2668::aid-cncr2820691108>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Hopwood L., Volk D., Wilson J. F. Cytofluorometric analysis of the DNA content in ovarian carcinoma and its relationship to patient survival. Cancer. 1989 Jun 15;63(12):2456–2460. doi: 10.1002/1097-0142(19890615)63:12<2456::aid-cncr2820631216>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Houghten R. A., Walker L. E., Reisfeld R. A., Wilson I. A., Hogle J. M., Lerner R. A. Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4949–4953. doi: 10.1073/pnas.80.16.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg B. C., Arps H., Franke U., Thiedemann C., Rehpenning W., Stegner H. E., Lietz H., Schröder S., Dietel M. DNA cytophotometry and prognosis in ovarian tumors of borderline malignancy. A clinicomorphologic study of 80 cases. Cancer. 1992 May 15;69(10):2510–2514. doi: 10.1002/1097-0142(19920515)69:10<2510::aid-cncr2820691021>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Watson J. V., Lamond A., Forster A., Stinson M. A., Evan G., Fischer W., Atherton E., Sheppard R., Rabbitts T. H. Metabolism of c-myc gene products: c-myc mRNA and protein expression in the cell cycle. EMBO J. 1985 Aug;4(8):2009–2015. doi: 10.1002/j.1460-2075.1985.tb03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg C. J., Cornelisse C. J., Heintz P. A., Hermans J., Fleuren G. J. Tumor ploidy as a major prognostic factor in advanced ovarian cancer. Cancer. 1987 Jan 15;59(2):317–323. doi: 10.1002/1097-0142(19870115)59:2<317::aid-cncr2820590225>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sahni K., Tribukait B., Einhorn N. Flow cytometric measurement of ploidy and proliferation in effusions of ovarian carcinoma and their possible prognostic significance. Gynecol Oncol. 1989 Nov;35(2):240–245. doi: 10.1016/0090-8258(89)90052-8. [DOI] [PubMed] [Google Scholar]
- Schueler J. A., Cornelisse C. J., Hermans J., Trimbos J. B., van der Burg M. E., Fleuren G. J. Prognostic factors in well-differentiated early-stage epithelial ovarian cancer. Cancer. 1993 Feb 1;71(3):787–795. doi: 10.1002/1097-0142(19930201)71:3<787::aid-cncr2820710322>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Seidman J. D., Norris H. J., Griffin J. L., Hitchcock C. L. DNA flow cytometric analysis of serous ovarian tumors of low malignant potential. Cancer. 1993 Jun 15;71(12):3947–3951. doi: 10.1002/1097-0142(19930615)71:12<3947::aid-cncr2820711225>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sikora K., Chan S., Evan G., Gabra H., Markham N., Stewart J., Watson J. c-myc oncogene expression in colorectal cancer. Cancer. 1987 Apr 1;59(7):1289–1295. doi: 10.1002/1097-0142(19870401)59:7<1289::aid-cncr2820590710>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Sikora K., Evan G., Stewart J., Watson J. V. Detection of the c-myc oncogene product in testicular cancer. Br J Cancer. 1985 Aug;52(2):171–176. doi: 10.1038/bjc.1985.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Vergote I. B., Kaern J., Abeler V. M., Pettersen E. O., De Vos L. N., Tropé C. G. Analysis of prognostic factors in stage I epithelial ovarian carcinoma: importance of degree of differentiation and deoxyribonucleic acid ploidy in predicting relapse. Am J Obstet Gynecol. 1993 Jul;169(1):40–52. doi: 10.1016/0002-9378(93)90129-7. [DOI] [PubMed] [Google Scholar]
- Volm M., Brüggemann A., Günther M., Kleine W., Pfleiderer A., Vogt-Schaden M. Prognostic relevance of ploidy, proliferation, and resistance-predictive tests in ovarian carcinoma. Cancer Res. 1985 Oct;45(10):5180–5185. [PubMed] [Google Scholar]
- Volm M., Mattern J., Sonka J., Vogt-Schaden M., Wayss K. DNA distribution in non-small-cell lung carcinomas and its relationship to clinical behavior. Cytometry. 1985 Jul;6(4):348–356. doi: 10.1002/cyto.990060412. [DOI] [PubMed] [Google Scholar]
- Watson J. V. A method for improving light collection by 600% from square cross section flow cytometry chambers. Br J Cancer. 1985 Mar;51(3):433–435. doi: 10.1038/bjc.1985.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. V., Curling O. M., Munn C. F., Hudson C. N. Oncogene expression in ovarian cancer: a pilot study of c-myc oncoprotein in serous papillary ovarian cancer. Gynecol Oncol. 1987 Oct;28(2):137–150. doi: 10.1016/0090-8258(87)90207-1. [DOI] [PubMed] [Google Scholar]
- Watson J. V. Enzyme kinetic studies in cell populations using fluorogenic substrates and flow cytometric techniques. Cytometry. 1980 Sep;1(2):143–151. doi: 10.1002/cyto.990010209. [DOI] [PubMed] [Google Scholar]
- Watson J. V., Sikora K., Evan G. I. A simultaneous flow cytometric assay for c-myc oncoprotein and DNA in nuclei from paraffin embedded material. J Immunol Methods. 1985 Oct 24;83(1):179–192. doi: 10.1016/0022-1759(85)90071-7. [DOI] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Ramsay N. Retinoblastoma and subband deletion of chromosome 13. Am J Dis Child. 1978 Feb;132(2):161–163. doi: 10.1001/archpedi.1978.02120270059012. [DOI] [PubMed] [Google Scholar]
- Zangwill B. C., Balsara G., Dunton C., Varello M., Rebane B. A., Hernandez E., Atkinson B. F. Ovarian carcinoma heterogeneity as demonstrated by DNA ploidy. Cancer. 1993 Apr 1;71(7):2261–2267. doi: 10.1002/1097-0142(19930401)71:7<2261::aid-cncr2820710716>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Zimmerman P. V., Hawson G. A., Bint M. H., Parsons P. G. Ploidy as a prognostic determinant in surgically treated lung cancer. Lancet. 1987 Sep 5;2(8558):530–533. doi: 10.1016/s0140-6736(87)92923-0. [DOI] [PubMed] [Google Scholar]