Abstract
Serotonin is important in brain functions and involved in neurological diseases. It is also drawn considerable attention in bone disease since it mainly produced by the gut. Serotonin 6 G-protein-coupled receptor (5-HT6R) is clinical targets for the treatment of neurological diseases. However, 5-HT6R as a therapeutic target in bone has not been reported. Herein, we found that 5-HT6R showed higher expression in bone, and its expression was increased during bone remodeling and osteoblast differentiation. The activation of 5-HT6R by ST1936 caused the inhibition of ALP activity and mineralization in primary osteoblast cultures, which was antagonized by SB258585, an antagonist and by the knockdown of 5-HT6R. Further investigation indicated that 5-HT6R inhibited osteoblast differentiation via Jab1 in BMP2 signaling but not PKA and ERK1/2. In vivo studies showed that the activation of 5-HT6R inhibited bone regeneration in the calvarial defect mice and also delayed bone development in newborn mice; this response was antagonized by SB258585. Therefore, our findings indicate a key role of 5-HT6R in bone formation through serotonin originating in the peripheral system, and suggest that it is a novel therapeutic target for drug development in the bone repair and bone diseases.
Serotonin is important in the regulation of virtually all brain functions, and strongly implicated in psychiatric and neurological diseases such as Alzheimer’s disease and depression1. However, most serotonin is found outside of the central nervous system and is mainly synthesized in the gut by tryptophan hydroxylase-1 (Tph-1)2. 95% of total body serotonin is released into the gut by enterochromaffin cells and enter into blood circulation, indicating that the serotonin system has various functions beyond actions of serotonin as a well-known neurotransmitter3,4.
The recent research was reported that a serotonin-mediated system originating in the gut can regulate bone mass5,6. Selective serotonin reuptake inhibitors (SSRIs) reduced bone mass in mice7 and also clinical data showed a significant increase for fractures in patients treated with SSRIs8. Yadav et al. found that serotonin production in the gut can enter into blood circulation and reduce bone formation5. Reporting in Nature medicine, Yadav et al. also reported that pharmacological inhibition of gut-derived serotonin synthesis by LP533401, a small molecule inhibitor of Tph-1 is a potential therapeutic treatment for low–bone-mass diseases9.
The physiological diversity of serotonin system is mediated by multiple serotonin receptors (5-HTRs), which have been divided into seven classes (5-HT1R through 5-HT7R) on the basis of their signaling pathway10. Among them, 5-HT6R, a G-protein-coupled receptor (GPCR) is one of the latest cloned receptor and positively coupled to adenylyl cyclase via stimulatory G (Gs) proteins and increases intracellular cyclic AMP (cAMP) production by the activation of adenylyl cyclase11. It was previously demonstrated that 5-HT6R physically and functionally interacts with various proteins including Fyn tyrosine kinase and Jun activation domain-binding protein 1 (Jab1)12,13. Over the past decades, the 5-HT6R has gained increasing attention in the brain and has become a clinical target for treating neurological diseases such as Alzheimer’s diseases, depression, and schizophrenia14,15,16. However, there is no study for 5-HT6R in the peripheral organs.
In the present study, we investigated the relative expression of 5-HT6R in bone compared to brain, and examined its role in in vitro osteoblast differentiation and in vivo bone regeneration and bone development via peripheral serotonin system. Our findings indicate that 5-HT6R has a crucial role in bone metabolism and will shed light on how peripheral serotonin system regulates bone metabolism.
Results and Discussion
In an effort to elucidate how peripheral serotonin system regulates bone formation, we first examined the relative expression of repective 5-HTRs between the bone and brain. Our results showed that 5-HT6R showed relatively higher expression in bone compared to brain (Fig. 1A). Given that 5-HT6R has been gaining great attention for treating neurological diseases in brain14,17,18, it is very important to elucidate the fundamental mechanisms responsible for peripheral serotonin-mediated bone formation via 5-HT6R.
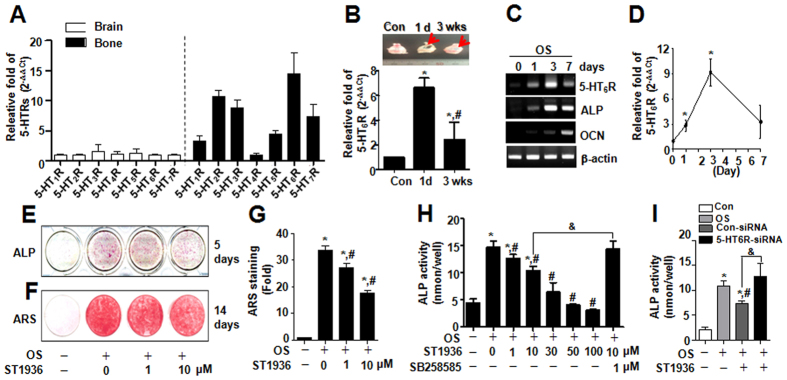
Figure 1. 5-HT6R is highly expressed in bone and its activation attenuated in vitro osteoblast differentiation in primary calvarial osteoblasts.
(A) After total RNA was isolated from brain and bone in female 10-week-old ICR mice, the expression levels of serotonin receptors were analyzed by real-time PCR and normalized to that of β-actin. (B) Calvarial defects were created in female 10-week-old ICR mice. Arrowhead indicates the defect region (upper). The mRNA level of 5-HT6R at 1 day and 3 weeks was analyzed by real-time PCR and normalized to that of β-actin (bottom). (C,D) After primary calvarial osteoblasts from 1-day-old ICR mice were cultured with osteogenic supplement medium (OS) for 0, 1, 3, and 7 days, the mRNA level analyzed by RT-PCR (C) or real-time PCR (D) and was normalized to that of β-actin. (E–G) Cells were treated with OS in the presence of 1 and 10 μM ST1936 for 5 or 14 days. Differentiation was assessed by ALP staining (E), and Alizarin Red staining (F). The intensity of Alizarin Red staining was determined by optical density (G). (H,I) ALP activity was analyzed in the presence of the indicated concentrations of ST1936 and 1 μM SB258585 for 5 days (H) or analyzed in the presence of 10 μM ST1936 for 5 days, 24 h after transfection with 5-HT6R-siRNA (I). Data shown represent the means ± SEM of three independent experiments. *p < 0.05, vs. control. #p < 0.05, vs. OS. &, p < 0.05, vs. 10 μM SB258585 or vs. 5-HT6R siRNA with 10 μM ST1936.
Following critical-sized calvarial bone defects, the expression level of 5-HT6R increased in the early stage of differentiation or remodeling (Fig. 1B) and also the 5-HT6R mRNA level was upregulated in a time-dependent manner and followed by a decrease (Fig. 1C,D), suggesting that 5-HT6R might have regulatory or tuning roles to prevent abnormal osteoblast differentiation.
Primary osteoblast cultures from newborn mouse calvaria are capable of differentiating in vitro into mature osteoblasts that is characterized by increased expression of the early marker alkaline phosphatase (ALP), later marker osteocalcin (OCN), and mineralization19. To examine the role of 5-HT6R in osteoblast differentiation and function in vitro, primary osteoblasts were cultured and treated with a recently developed selective 5-HT6R agonist, ST193620. The activation effects of 5-HT6R were determined by ALP activity and the formation of mineralized bone nodules. ALP staining (Fig. 1E) and mineralization (Fig. 1F,G) were decreased with ST1936 treatment in a concentration-dependent manner. The effects of 5-HT6R was antagonized by SB258585, a specific antagonist (Fig. 1H) and attenuated by the knockdown of endogenous 5-HT6R (Fig. 1I), indicating that 5-HT6R is involved in osteoblast differentiation for serotonin-mediated bone formation.
The bone morphogenetic protein-2 (BMP-2) signaling pathway plays an important role in osteoblast differentiation and subsequent bone formation or remodeling21. Canonical BMP-2 signaling occurs through the phosphorylation of Smad1/5/8 and induces subsequent activation of the transcription of bone-specific genes, leading to bone formation22. To determine the mechanism underlying the effect of 5-HT6R in osteoblast differentiation, the effect of 5-HT6R on BMP-2 signaling was examined in primary calvarial osteoblasts. Our data demonstrated that the treatment with ST1936 attenuated the phosphorylation of Smad1/5/8, the central molecules in BMP-2 signaling (Fig. 2A), and decreased the expression of Runx2, the downstream target gene of BMP-2 signaling (Fig. 2B). To further understand the role of 5-HT6R in osteogenic differentiation, rh-BMP-2 was treated with ST1936 in primary calvarial osteoblasts. Rh-BMP-2-induced ALP activity and mineralized nodule formation in primary osteoblasts were suppressed dramatically by ST1936 (Fig. 2C–F). Thus, these results suggested that the BMP-2 signaling pathway has an important role in 5-HT6R-mediated differentiation and function of osteoblasts.
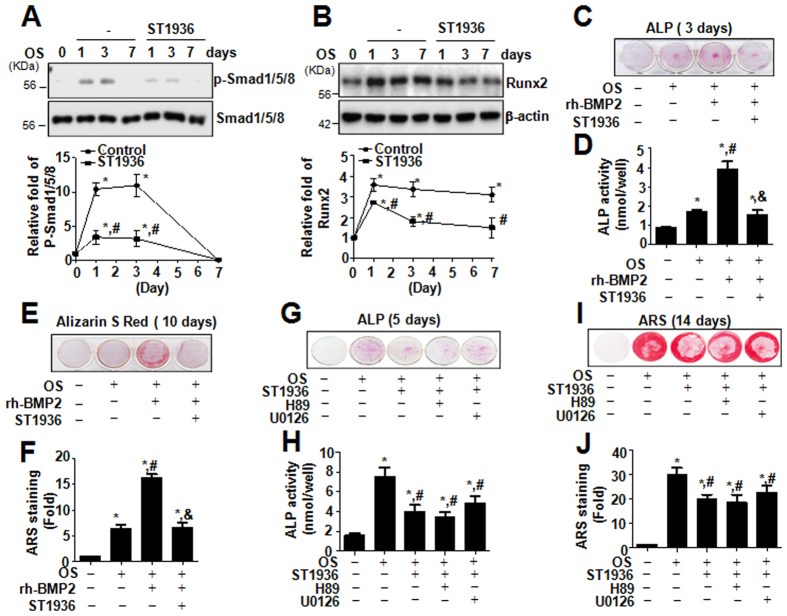
Figure 2. 5-HT6R inhibits osteoblast differentiation via the BMP-2 signaling pathway but not Gs-protein signaling.
(A,B) Primary calvarial osteoblasts from 1-day-old ICR mice were cultured with OS in the presence of 10 μM ST1936 for 0, 1, 3, and 7 days. Phospho-Smad1/5/8 and Smad1/5/8 (A), and Runx2 (B) were assessed by Western blot analysis. (C–F) Cells were cultured in OS with rh-BMP2 in the presence of ST1936 for 3 (C,D) and 10 days (E,F). ALP activity was measured via ALP staining (C) and ALP activity (D). Mineralized nodule formation was assessed by Alizarin Red staining (E), and then optical density was measured at 590 nm. (F). (G–J) Cells were pretreated with H89 (1 μM) or U0126 (1 μM) for 1 h, and then cultured in OS with ST1936 for 5 days (G,H) and 14 days (I,J). ALP activity was measured via ALP staining (G) and ALP activity (H). Mineralized nodule formation was assessed by Alizarin red staining (I) and the stains were eluted and measured at 590 nm. (J). Data shown represent the means ± SEM. *p < 0.05, vs. control. #p < 0.05, vs. OS. &, p < 0.05, vs. ST1936.
5-HT6R is a GPCR that increases cAMP by stimulating adenylyl cyclase, leading to the activation of PKA18. These GPCRs typically act through Gs proteins in response to extracellular stimuli including growth factors and neurotransmitters23. We firstly reported the cellular mechanisms of 5-HT6R-mediated Gs protein signaling via Ras-Raf-MEK-ERK1/2 pathway, providing new insights into the physiological roles of 5-HT6R in brain12,13,24. However, we, herein, observed that the differentiation of primary osteoblasts is not related to the Gs protein signaling of 5-HT6R (the PKA and ERK1/2 pathway) (Fig. 2G–J). Similar to our observation, reporting in Nature Chem Biol, Seo et al.23, and Duhr et al.25, also described that 5-HT6R controls neuronal differentiation through the CDK5 pathway in a Gs protein-independent signaling.
It was previously reported that 5-HT6R physically and functionally interacts with Jab1 and the activation of 5-HT6R releases Jab113,24. Jab1 has been reported to interact with Smad5 and cause an attenuation of BMP-dependent transcriptional responses, suggesting that Jab1 might act as an inhibitor of BMP signaling26. Also, Chen et al., reported that knockout of Jab1 increased BMP-induced phosphorylation of Smad1/5 compared with wild-type controls, but not TGF-beta induced Smad2/327. We, therefore, examined whether 5-HT6R modulates BMP2 signaling via Jab1 in primary osteoblasts. In the present study, the knockdown of endogenous Jab1 by RNA interference significantly enhanced rh-BMP-2-induced phosphorylation of Smad1/5/8, in a time-dependent manner (Fig. 3A,B), and the activation of 5-HT6R stimulated the binding of Jab1 with Smad1/5/8 (Fig. 3C). Moreover, the knockdown of Jab1 attenuated 5-HT6R agonist induced–Runx2 suppression (Fig. D) as well as osteoblast differentiation as evidenced by ALP staining (Fig. 3E) and activity (F) and differentiation markers, ALP, OCN, and bone sialoprotein (BSP) (Fig. 3G–I). These data suggest that 5-HT6R inhibits bone formation via Jab1-mediated BMP2 signaling.
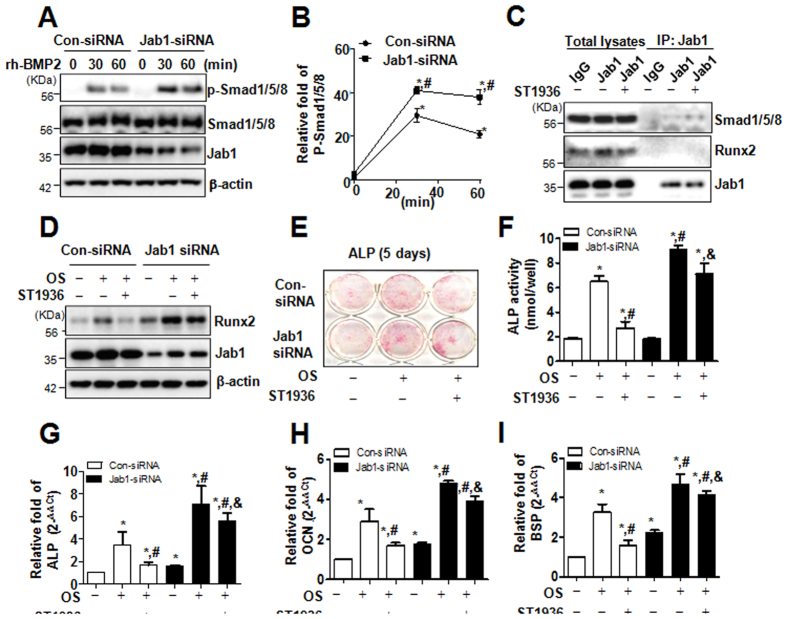
Figure 3. 5-HT6R suppresses BMP-2 signaling by Jab1 to inhibit osteoblast differentiation.
(A,B) Primary calvarial osteoblasts from 1-day-old ICR mice were transfected with negative control siRNA or Jab1 siRNA for 24 h, further incubated in serum-free medium for 24 h, and then treated with 100 ng/mL rh-BMP2 for the times indicated. The levels of p-Smad1/5/8, Smad1/5/8, Jab1, and β-actin were analyzed by Western blot (A). The line graph data are expressed as relative fold-changes from each control (B). (C) Cells were cultured in OS with ST1936 for 3 days, and were immunoprecipitated with an anti-mouse antibody and mouse anti-Jab1 antibody. The immune complexes and cell lysates were analyzed by immunoblotting with rabbit anti-Jab1, anti-Runx2, and anti-Smad1/5/8 antibodies. (D) After Cells were transfected with negative control siRNA or Jab1 siRNA for 24 h, the cells were cultured in OS with ST1936 for 3 days. Protein levels of Runx2, Jab1, and β-actin were analyzed by Western blot. (E,F) Cells were transfected with control siRNA or Jab1 siRNA for 24 h, and then cultured in OS with ST1936 for 5 days. ALP was evaluated via ALP staining (E) and ALP activity (F). (G–I) After Cells were transfected with negative control siRNA or Jab1 siRNA for 24 h, the cells were cultured in OS with 10 μM ST1936 for 7 days. The mRNA levels of ALP (G), OCN (H) and BSP (I) were analyzed by real-time PCR, and normalized to that of β-actin. Data shown represent the means ± SEM. *p < 0.05, vs. control. #p < 0.05, vs. OS. &, p < 0.05, con-siRNA vs. Jab1 siRNA with 10 μM ST1936.
The hypothesis that 5-HT6R inhibits bone formation via peripheral serotonin-mediated system was supported by the osteogenic effect of 5-HT6R in in vivo animal experiments (Fig. 4). To that end, we generated mice with surgery-generated calvarial defect, and bioabsorbable collagen sponge loaded with ST1936 (10 mg/kg) was placed into the defects (Fig. 4A). The local delivery of ST1936 into critical-sized mouse calvarial defects elicited an impaired osteogenic response (Fig. 4B–D). New bone formation visualized by 3D micro-CT images (μCT) was particularly decreased in defects treated with ST1936 (Fig. 4B) and the amount of new bone formation in the ST1936-treated group was significantly lower than that in the control group (P < 0.05) (Fig. 4C). Histological evaluation further supported the μCT findings, showing newly formed bone within the defect in controls, and no bone formation in the ST1936-treated group (Fig. 4D), indicating that 5-HT6R is required for osteoblast bone formation.
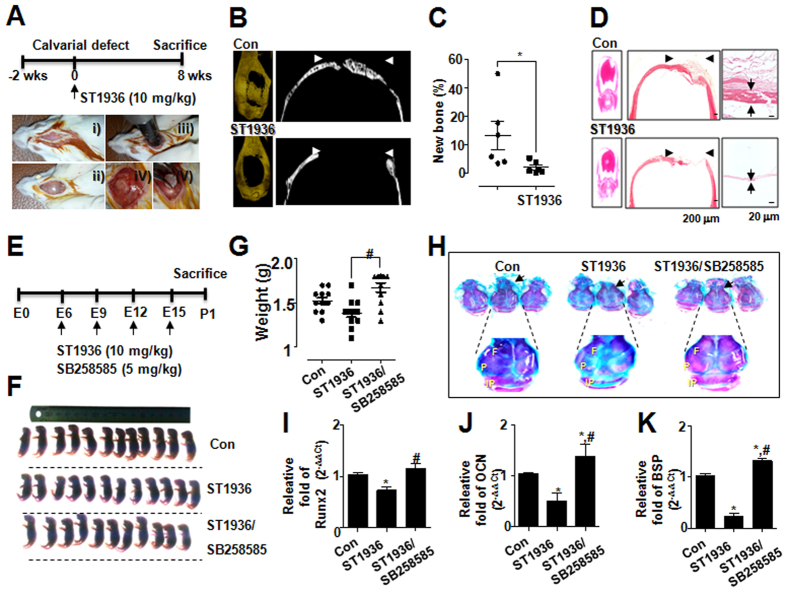
Figure 4. 5-HT6R disrupted in vivo calvarial bone regeneration and embryonic calvarial bone development.
(A) Scheme for assessing calvarial defect model. After female 8-week-old ICR mice were acclimated for 2 weeks, calvarial bone from female 10-week-old ICR mice was removed, and the calvarial defect was covered for 8 weeks with a bioabsorbable collagen sponge loaded with ST1936. (B) 3D micro CT (μCT) images from sham (control) and ST1936-treated groups were conducted to visualize and quantify calvarial bone regeneration. (C) Quantitative analysis of the relative percentage of new bone formation was performed by 3D μCT imaging and plotted as individual points. (D) H&E-stained images of the calvarial bone (left) and higher magnification images (right). Arrowhead indicates the defect region and arrows indicate width of bone tissue. Scale bars, 50 μm. (E) Scheme for assessing calvarial bone development by SR6. ST1936 (10 mg/kg, ip) or ST1936 (10 mg/kg, ip)/SB258585 (5 mg/kg, ip) were injected once per day at embryonic periods (E) 6, E9, E12, and E15. All mice had pups at E18 and postnatal 1 day (P1) mice were sacrificed. (F,G) P1 mice from each group were photographed (F) and weighed (G). (H) P1 mice were stained with Alizarin Red (red color) and Alcian Blue (blue color). The panels show calvarial bones consisting of paired frontal bones (F), paired parietal bones (P), and interparietal bone (IP). (I–K) The mRNA levels of Runx2 (I), OCN (J), BSP (K) in the calvarial bones of P1 mice were analyzed by real-time PCR and normalized to that of β-actin. Data shown represent the means ± SEM. *p < 0.05, vs. control. #p < 0.05, vs. ST1936 alone.
Calvarial sutures are major growth sites of intramembranous osteogenesis during craniofacial bone development28,29; thus, we further investigated whether 5-HT6R signaling has a critical role in intramembranous osteogenesis of calvarial bone and suture development. ST1936 or SB258585 was treated for the indicated embryonic period, and the phenotypes of bone in mice were analyzed (Fig. 4E–G). Sequential staining of mice skeletons revealed an increase in the ratio of cartilaginous (blue) to mineralized tissue (red) in the ST1936-adminstrated calvarial bones such as two frontal, two parietal, and an interparietal bone, whereas SB258585 administration reversed the effects of ST1936 (Fig. 4H). Such deformities of bones are mainly observed in calvarial sutures that are main centers for osteoblast differentiation and new bone formation postnatally29. We next examined osteoblast differentiation in calvarial bones by measuring the earliest molecular determinant of bone formation (Runx2) and late osteoblast differentiation markers (OCN and BSP)30. Our results showed that the 5-HT6R antagonist significantly reversed the 5-HT6R agonist–suppressed expression of Runx2 (Fig. 4I) and osteoblast-related genes, OCN and BSP (Fig. 4J,K). Taken together, these in vivo results demonstrated that 5-HT6R is a critical receptor to regulate bone formation in peripheral serotonin-mediated system.
In conclusion, these experiments have uncovered a novel link between peripheral serotonin system and 5-HT6R in bone formation. This is the first study to demonstrate that 5-HT6R is expressed in bone, which is crucial for in vitro osteoblast differentiation and in vivo bone development and regeneration in animal models via peripheral serotonin-mediated system. These findings suggest that 5-HT6R is a potential therapeutic target for bone repair and bone diseases, and will provide basic understanding on how peripheral serotonin-mediated system regulates bone formation.
Materials and Methods
Primary calvarial osteoblasts culture, differentiation, and transfection
Primary calvarial osteoblasts were isolated from calvariae of 1-day-old ICR mice using 0.2% collagenase-dispase enzyme solution (Sigma-Aldrich, St. Louis, MO). The cells isolated from the last four to six digests were cultured separately in α-modified minimum essential medium (α-MEM) (Gibco Laboratories, Grand Island, NY) containing 10% fetal bovine serum (FBS; Gibco Laboratories) and antibiotics (100 mg/mL penicillin G and 100 IU/mL streptomycin). After reaching a subconfluent state, the cells were removed from each flask and combined together as osteoblasts. The cells were used for all experiments at second passage, as described below. Cells were cultured in α-MEM containing 10% FBS, 5 mmol/L β-glycerophosphate, ascorbic acid (50 μg/mL), and antibiotics. For siRNA transfection, cells were transfected using Lipofectamine RNAiMAX according to the manufacturer’s specification (Invitrogen, Carlsbad, CA, USA).
Mice and ethics statement
Female 8-week-old ICR mice (Samtako, Osan, Kyoung Gi-Do, Korea) used in this study were maintained in accordance with the National Institute of Toxicological Research of the Korea Food and Drug Administration guidelines for the humane care and use of laboratory animals. All experimental procedures in the current study were approved by Kyung Hee University Animal Care Committee (approval number: KHMC-IACUC 2015-002). All experimental methods were conducted in accordance with relevant guidelines.
Calvarial bone defects
The mice were anesthetized, and a 5-mm-diameter calvarial critical-sized defect was created on each side of the calvarial bone using a dental bur attached to a slow-speed hand piece with minimal invasion of the Dura mater. The wound was closed with CollaTape (Integra LifeSciences Corporation, Carlsbad, CA) containing ST1936 (10 mg/kg, Tocris Bioscience, Bristol, United Kingdom). Animals were sacrificed 8 weeks postsurgery and the calvarial bone was carefully excised, cleaned, and fixed immediately in 10% formalin. After imaging by micro-computerized tomography (μCT), tissues were demineralized in10% EDTA for 14 days, embedded in paraffin, and sectioned at 5 μm. Sections were stained with hematoxylin and eosin (H&E).
Micro-computed tomography (μCT)
μCT was performed at the Advanced Institutes of Convergence Technology (Genoss Co., Ltd., Gyeonggi-do Korea). Micro-CT data of calvaria were acquired on a Skyscan 1173 scanner (Bruker-microCT, Kontich, Belgium). Scanning was carried out at 75 kV/106 μA for 500 ms. In total, 800 projections were collected at a resolution of 9.94 μm/pixel. Reconstruction of sections was carried out with software associated with the scanner (Nrecon), with the beam hardening correction set to 40%. Realistic 3D-Visulizatio software (Bruker-microCT, Konitch, Belgium) was used to reconstruct the CT images three-dimensionally, which were acquired on approximately 2,000 cross-sections.
Calvarial bone development and skeletal staining
Mice received intraperitoneal injection of ST1936 (10 mg/kg, Tocris Bioscience) or SB258585 (5 mg/kg, Tocris Bioscience) four times at E6, E9, E12, E15. Briefly, newborn mice were deskinned, eviscerated, and immersed in 100% ethanol for 24 h. The samples were fixed in acetone for 24 h and then stained for 24 h in a solution containing 0.1% Alizarin Red, 0.3% Alcian Blue, acetic acid, and 70% ethanol (1:1:1:17, v/v/v/v). They were then transferred to a solution of 1% KOH in 20% glycerol until clear and then stored in glycerol.
ALP activity
ALP activity was measured by spectrophotometry. Cells were homogenized in 0.5 mL of distilled water using a sonicator, and centrifuged. Aliquots of cell homogenate were incubated with 15 mm p-NPP in 0.1 m glycine-NaOH (pH 10.3) at 37 °C for 30 min. The reaction was stopped by the addition of 0.25 n NaOH. The absorbance was measured at 405 nm using an ELISA reader (Beckman Coulter).
ALP staining
Cells were washed with 1× PBS and then fixed in 4% formaldehyde for 20 min at room temperature. The cells were rinsed with distilled water, and permeabilized with 0.1% Triton X-100. Cells were incubated at 37 °C for 30 min in Naphthol-AS-BL alkaline solution mixture (Sigma-Aldrich).
Alizarin Red S staining
After 14 days of culture, cells were fixed in 70% ice-cold ethanol for 1 h and rinsed with distilled water. Cells were stained with 40 mM Alizarin Red S (pH 4.2) for 10 min with gentle agitation. The level of Alizarin Red S staining was observed under light microscopy. To quantify Alizarin Red staining, stains were eluted with 100% DMSO and measured at 590 nm.
Reverse transcriptase-polymerase chain reaction (RT-PCR) and quantitative real-time PCR
The total RNA of cells was extracted using TRIzol™ reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer’s instructions. RNA (1 μg) isolated from each sample was reverse-transcribed using oligo (dT)15 primers with AccuPower® RT PreMix (iNtRON Biotechnology, Gyeonggi-do, South Korea). Next, the generated cDNAs were amplified with AccuPower® PCR PreMix (Bioneer Corporation, Daejeon, South Korea). The primer sequences are as follows:
ALPF: 5′-ACACCTTGACTGTGGTTACTG-3′, R: 5′-CCATATAGGATGGCCGTGAAG-3′
Runx2F: 5′-ACTCTTCTGGAGCCGTTTATG-3′, R: 5′-GTGAATCTGGCCATGTTTGTG-3′
OCNF: 5′-ACACCATGAGGACCATCTTTC-3′, R:5′-CGGAGTCTGTTCACTACCTTATT-3′
BSPF: 5′-TGTTTGTAGTGGGCTTCTTCTT-3′, R: 5′-TCCATCTAGTCCCAGCTCATAG-3
5-HT1RF: 5′-TCCACTCACCTCTCACAGTAT-3′, R: 5′-CTCACACCCACACTTCCTTAG-3′
5-HT2RF: 5′-TCACCATTGCGGGAAACA-3′, R : 5′-AGGAAACCCAGCAGCATATC-3′
5-HT3RF: 5′- CTCGCTGAGACCATCTTCATT-3′, R : 5′-ATCCAGGCTATTCTGTCTAGGA-3′
5-HT4RF: 5′-GCCTTGTCACTCTTGCTATCT-3′, R: 5′-TACATTTGGGTCCTCTGACTTG-3′
5-HT5RF: 5′-CTGTGCTGACTTCTCCCATAAA-3′, R: 5′-GCTGAGAACCACATGCTAAGA-3′
5-HT6RF: 5′-CCGTATGTGACTGCATCTCTC-3′, R: 5′-ATGATAGGGTTCATGGTGCTATT-3′
5-HT7RF: 5′-GCTGAGACTGCACAACAGAA-3′, R: 5′-GTTGCCATCTCCCTCAAGATAC-3′
β-actinF: 5′-AATGTGGCTGAGGACTTTG-3′, R: 5′-GGGACTTCCTGTAACCACTTATT-3′
For mRNA quantification, total RNA was extracted using the RNAqueous® kit and the cDNA was synthesized using 1 μg of total RNA with the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using a LightCycler® 1.5 System (Roche Diagnostics GmbH, Mannheim, Germany). Thermocycling conditions consisted of an initial denaturation of 10 s at 95 °C, followed by 45 cycles of 95 °C for 10 s, 60 °C for 5 s and 72 °C for 10 s. For the calculation of relative quantification, the 2−ΔΔCT formula was used, where –ΔΔCT = (CT,target − CT,β-actin) experimental sample – (CT,target − CT,β-actin) control sample.
Western blot analysis
Cells were washed twice with ice-cold PBS, and lysed in 20 mM Tris-HCl buffer (pH 7.4) containing a protease inhibitor mixture (0.1 mM PMSF, 5 mg/mL aprotinin, 5 mg/mL pepstatin A, and 1 mg/mL chymostatin). Protein concentration was determined using Bradford reagent (Bio-Rad, Hercules, CA). Equal amounts of lysates (20 μg) resolved by sodium dodecyl-polyacrylamide gel electrophoresis (SDS–PAGE) were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA), and the membrane was blocked with 1× TBS containing 0.05% Tween 20 and 5% skim milk or 2% BSA for 1 h at room temperature. After blocking, the membranes were incubated overnight at 4 °C with the respective primary antibodies, as follows: p-ERK1/2 (1:2000, #9101S, Cell Signaling Technology, Beverly, MA), ERK1/2 (1:2000, #9102S, Cell Signaling), Runx2 (O1L7F) (1:1000, #12556S, Cell Signaling), Jab1 (1:2000, #6895, Cell Signaling), p-Smad1/5/8 (D5B10) (1:2000, #13820S, Cell Signaling), Smad1/5/8 (N-18) (1:1000, #sc-6031-R, Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (C4) (1:1000, #sc-47778, Santa Cruz Biotechnology). The membranes were washed with 1× PBS and incubated with diluted horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000, Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. After three washes, the membranes were detected using an enhanced chemiluminescence (ECL) kit (Millipore, Bedford, MA).
Co-immunoprecipitation
Cells and tissues were gently lysed with lysis buffer for 1 h on ice and then centrifuged at 15,000 g and 4 °C for 15 min, and the supernatant was collected. The soluble lysates were incubated with mouse anti-Jab1 antibody (#sc-13157, Santa Cruz Biotechnology) at 4 °C, and then with Protein A/G bead (Santa Cruz Biotechnology) and washed times. Immune complexes were eluted by boiling for 10 min at 95 °C in SDS sample buffer, followed by Western blot analysis with anti-Jab1 (1:1000, Cell Signaling), anti-Runx2 (1:1000; Cell Signaling Technology), or anti-Smad1/5/8 (1:1000; Santa Cruz Biotechnology) antibodies.
Statistical analysis
The data were analyzed using GraphPad Prism version 5 software (GraphPad Software, Inc., San Diego, CA). Data are presented as the means ± S.E.M. Statistical significance was evaluated using one-way analysis of variance (ANOVA) and the differences were assessed by the Dunnett’s test. A value of P < 0.05 was considered to indicate statistical significance.
Additional Information
How to cite this article: Yun, H.-M. et al. Peripheral serotonin-mediated system suppresses bone development and regeneration via serotonin 6 G-protein-coupled receptor. Sci. Rep. 6, 30985; doi: 10.1038/srep30985 (2016).
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (Nos 2012R1A5A2051384, 2015R1D1A1A01059240).
Footnotes
Author Contributions H.-M.Y. designed and performed most of the experiments and wrote the manuscript. K.-R.P. performed the experiments by assisting with generation and analyses of various in vivo. J.T.H. was participated in data interpretation and discussion. E.-C.K. supervised the study.
References
- Mann J. J. et al. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch Gen Psychiatry 49, 442–446 (1992). [DOI] [PubMed] [Google Scholar]
- Cote F. et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA 100, 13525–13530, 10.1073/pnas.22330561002233056100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. Serotonin in the gut: what does it do? Front Neurosci 6, 16, 10.3389/fnins.2012.00016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D. & Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414, S0016-5085(06)02436-X (2007). [DOI] [PubMed] [Google Scholar]
- Yadav V. K. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837, S0092-8674(08)01255-5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. J. Breaking into bone biology: serotonin’s secrets. Nat Med 15, 145–146, nm0209-145 (2009). [DOI] [PubMed] [Google Scholar]
- Warden S. J., Robling A. G., Sanders M. S., Bliziotes M. M. & Turner C. H. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology 146, 685–693, en.2004-1259 (2005). [DOI] [PubMed] [Google Scholar]
- Eom C. S., Lee H. K., Ye S., Park S. M. & Cho K. H. Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res 27, 1186–1195, 10.1002/jbmr.1554 (2012). [DOI] [PubMed] [Google Scholar]
- Yadav V. K. et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med 16, 308–312, nm.2098 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P. et al. DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci USA 99, 3188–3193, 10.1073/pnas.05271269999/5/3188 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R. et al. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem 66, 47–56 (1996). [DOI] [PubMed] [Google Scholar]
- Yun H. M. et al. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem 282, 5496–5505, M606215200 (2007). [DOI] [PubMed] [Google Scholar]
- Yun H. M., Baik J. H., Kang I., Jin C. & Rhim H. Physical interaction of Jab1 with human serotonin 6 G-protein-coupled receptor and their possible roles in cell survival. J Biol Chem 285, 10016–10029, M109.068759 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D., Windfeld K. & Colding-Jorgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 13, 1092–1099, S1474-4422(14)70198-X (2014). [DOI] [PubMed] [Google Scholar]
- Asaoka N. et al. Olanzapine augments the effect of selective serotonin reuptake inhibitors by suppressing GABAergic inhibition via antagonism of 5-HT receptors in the dorsal raphe nucleus. Neuropharmacology 95, 261–268, S0028-3908(15)00124-0 (2015). [DOI] [PubMed] [Google Scholar]
- Meffre J. et al. 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. Embo Mol Med 4, 1043–1056, 10.1002/emmm.201201410 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H. M., Park K. R., Kim E. C., Kim S. & Hong J. T. Serotonin 6 receptor controls alzheimer’s disease and depression. Oncotarget 6, 26716–26728, 5777 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley M. L., Marsden C. A. & Fone K. C. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord 3, 59–79 (2004). [DOI] [PubMed] [Google Scholar]
- Jonason J. H. & O’Keefe R. J. Isolation and culture of neonatal mouse calvarial osteoblasts. Methods Mol Biol 1130, 295–305, 10.1007/978-1-62703-989-5_22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccioni T. et al. ST1936 stimulates cAMP, Ca2+, ERK1/2 and Fyn kinase through a full activation of cloned human 5-HT6 receptors. Eur J Pharmacol 661, 8–14, S0014-2999(11)00437-7 (2011). [DOI] [PubMed] [Google Scholar]
- Sykaras N. & Opperman L. A. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J Oral Sci 45, 57–73 (2003). [DOI] [PubMed] [Google Scholar]
- Lian J. B. et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord 7, 1–16, 10.1007/s11154-006-9001-5 (2006). [DOI] [PubMed] [Google Scholar]
- Seo J. & Tsai L. H. Neuronal differentiation: 5-HT6R can do it alone. Nat Chem Biol 10, 488–489, nchembio.1557 (2014). [DOI] [PubMed] [Google Scholar]
- Yun H. M. & Rhim H. The serotonin-6 receptor as a novel therapeutic target. Exp Neurobiol 20, 159–168, 10.5607/en.2011.20.4.159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhr F. et al. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol 10, 590–597, nchembio.1547 (2014). [DOI] [PubMed] [Google Scholar]
- Haag J. & Aigner T. Jun activation domain-binding protein 1 binds Smad5 and inhibits bone morphogenetic protein signaling. Arthritis Rheum 54, 3878–3884, 10.1002/art.22261 (2006). [DOI] [PubMed] [Google Scholar]
- Chen D. et al. The transcriptional co-regulator Jab1 is crucial for chondrocyte differentiation in vivo. J Cell Sci 126, 234–243, jcs.113795 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman L. A. Cranial sutures as intramembranous bone growth sites. Dev Dyn 219, 472–485, (2000). [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. A., Majithia A., Booker T., Henderson J. E. & Loomis C. A. The homeoprotein engrailed 1 has pleiotropic functions in calvarial intramembranous bone formation and remodeling. Development 133, 63–74, dev.02171 (2006). [DOI] [PubMed] [Google Scholar]
- Stein G. S. et al. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 23, 4315–4329, 10.1038/sj.onc.12076761207676 (2004). [DOI] [PubMed] [Google Scholar]