Abstract
The aim of this study was to compare the immunologic response to a prime-boost immunization strategy combining the 13-valent conjugate pneumococcal vaccine (PCV13) with the 23-valent polysaccharide pneumococcal vaccine (PPSV23) versus the PPSV23 alone in HIV-infected adults. HIV-infected adults were randomized to receive PCV13 at week 0 followed by PPSV23 at week 4 (n = 31, prime-boost group) or PPSV23 alone at week 4 (n = 33, PPSV23-alone group). Serotype specific IgG geometric mean concentration (GMC) and functional oposonophagocytic (OPA) geometric mean titer (GMT) were compared for 12 pneumococcal serotypes shared by both vaccines at week 8 and week 28. The prime-boost vaccine group were more likely to achieve a ≥2-fold increase in IgG GMC and a GMC >1 ug/ml at week 8 (odds ratio (OR) 2.00, 95% confidence interval (CI) 1.46–2.74, p < 0.01) and week 28 (OR 1.95, 95% CI 1.40–2.70, p < 0.01). Similarly, the prime-boost vaccine group were more likely to achieve a ≥4-fold increase in GMT at week 8 (OR 1.71, 95% CI 1.22–2.39, p < 0.01) and week 28 (OR 1.6, 95% CI 1.15–2.3, p < 0.01). This study adds to evidence supporting current pneumococcal vaccination recommendations combining the conjugate and polysaccharide pneumococcal vaccines in the United States and Europe for HIV-infected individuals.
Invasive pneumococcal diseases (IPD) remains a significant cause of morbidity and mortality in HIV-infected individuals in the era of highly active antiretroviral therapy (HAART)1 (Harboe et al. 2014b). The incidence of IPD in HIV-infected individuals has been reported at up to 100 times greater than that of the general population2,3. In addition, HIV-infected individuals are at increased risk of recurrent episodes of IPD with up to one in four experiencing a recurrence during a 12 months period4,5.
The level of immunocompromise is considered the most important risk factor for IPD in HIV-infected individuals. Numerous studies have reported a decrease in incidence of IPD following the introduction of HAART, likely due to resultant immune-reconstitution6,7. However this has not been a consistent finding8,9,10.
PPSV23 has been shown to be safe and effective for the prevention of pneumococcal infection in the general adult population11 and covers the majority of pneumococcal serotypes implicated in IPD12,13. Given the burden of pneumococcal infection observed in HIV-infected individuals, PPSV23 has been recommended in consensus immunistation guidelines for HIV-infected adults in the US since 199614,15. However, clinical and immunological efficacy of PPSV23 in HIV-infected adults remains debated16.
PCV13 has recently been added to immunization recommendations for HIV-infected adults although evidence supporting this recommendation for primary prevention of pneumococcal infection is limited17,18,19. The conjugate pneumococcal vaccine (PCV) has been shown to prevent pneumococcal pneumonia in HIV-infected children20 and to prevent recurrent IPD in HIV-infected adults21.
Immunogenicity studies with PCV, or combination regimens of PCV7 and PPSV23 conducted in HIV-infected adults have yielded variable results22,23,24,25,26,27,28,29.
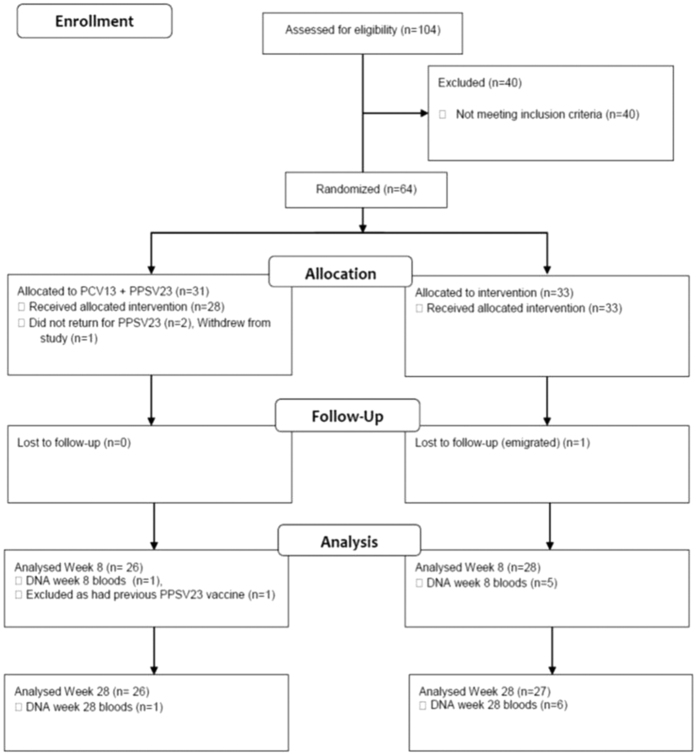
Figure 1. Study flow of vaccination with PCV13 and PPSV23 or PPSV23 alone and follow up.
This study compares immunogenicity of a prime-boost immunization strategy combining the 13-valent conjugate pneumococcal vaccine (PCV13) at week 0 followed by the 23-valent polysaccharide pneumococcal vaccine (PPSV23) (prime-boost group) at week 4 versus PPSV23 at week 4 (PPSV23-alone group) in pneumococcal vaccine naïve HIV-infected adults.
The primary objective of this study was to compare serotype specific IgG geometric mean concentration (GMC) and opsonophagocytic (OPA) responses in the prime-boost versus the PPSV23-alone group for 12 serotypes (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) contained in both vaccines at week 8 and week 28.
Secondary objectives were to compare the proportion of responses to the 12 shared pneumococcal polysaccharide serotypes (PPS) at week 8 and week 28 in the prime-boost versus the PPSV23-alone groups.
Results
Study Flow
Of 104 patients screened between April 2011 and July 2012, 64 patients (62%) were enrolled in the study. Thirty-one patients were randomized to receive PCV13 at week 0 followed by PPSV23 at week 4 (prime-boost group) and 33 were randomized to receive PPSV23 alone at week 4 (PPSV23-alone group).
60 patients completed the allocated vaccination series, 27 in the prime-boost group, 33 in the PPSV23 alone group.
In the prime-boost group two patients did not return within the time allocation to receive PPSV23, one patient withdrew consent and one patient was noted to have received PPSV23 prior to study entry and all were excluded from further analysis. Of 27 participants remaining in the prime-boost group, one patient did not attend for study bloods at week 8 and one patient did not attend for study bloods at week 28.
All 33 patients in the PPSV23-alone group received a single dose of PPSV23. Five patients did not attend for week 8 bloods and 6 patients did not attend for week 28 bloods.
Patient Characteristics
Baseline characteristic of participants are outlined in Table 1. Mean age [SD] was 37 [9] years, 92% were male, mean CD4 count was 503 [209] cells/mm3. Prior to randomization, 47% of patients were on HAART. Mean HIV RNA was 4.5log10 copies/ml.
Table 1. Baseline characteristics of patients by vaccine group.
| TOTAL (n = 60) | PPSV 23 group (n = 33) | PCV13 + PPSV23 (n = 27) | P | |
|---|---|---|---|---|
| Male n, (%) | 55 (92) | 29 (88) | 26 (96) | 0.37 |
| Age (mean) [SD] | 37 [10] | 37 [10] | 36 [10] | 0.70 |
| Race n, (%) | ||||
| Caucasian | 48 (80) | 25 (76) | 23 (85) | 0.52 |
| Hispanic | 7 (12) | 4 (12) | 3 (11) | 1 |
| Black | 5 (8) | 4 (12) | 1 (4) | 0.37 |
| Risk of Acquisitions n, (%) | ||||
| MSM | 49 (82) | 25 (76) | 24 (89) | 0.32 |
| Hetero | 8 (13) | 6 (18) | 2 (7) | 0.28 |
| IDU | 3 (5) | 2 (6) | 1 (4) | 0.49 |
| CD4 + count (mean) [SD] | 503 [219] | 447 [181] | 572 [243] | 0.03 |
| On HAART n, (%) | 28 (47) | 17 (52) | 11 (40) | 0.09 |
| HIV log10 (mean) [SD] | 2.37 [2.1] | 2.16 [2] | 3 [2] | 0.45 |
| Smoker n, (%) | 20 (33) | 11 (33) | 9 (27) | 1.1 |
| Co-infection Hepatitis C (PCR positive) | 5 | 3 | 2 | 1.0 |
Abbreviations: HAART, highly active antiretroviral therapy; SD, standard deviation; MSM, Men who have sex with men; Hetero, Heterosexual, IDU, intravenous drug user.
Patients in the prime-boost group were more likely to have a higher CD 4 T cell count (p = 0.03). No other significant differences in baseline characteristics were observed between groups.
Serotype specific IgG response
At week 8, GMCs in the prime-boost group were higher versus the PPSV23-alone group for all serotypes with the exception of serotype 14. However, a statistically significant difference was reached for a single serotype only, 23F (3.20 vs. 0.52 μg/mL, p < 0.01) (Table 2).
Table 2. Geometric mean antibody concentration (μg/mL) for polysaccharide pneumococcal serotypes.
| Serotype | W 0 IgG GMCb (95% CI) | pa | W 8 IgG GMCb (95% CI) | pa | W 28 IgG GMCb (95% CI) | pa | |||
|---|---|---|---|---|---|---|---|---|---|
| PCV13 + PPSV23 n = 27 | PPSV23 n = 33 | PCV13 + PPSV23 n = 26 | PPSV23 n = 28 | PCV13 + PPSV23 n = 26 | PPSV23 n = 27 | ||||
| 1 | 0.17 (0.05–0.37) | 0.14 (0.09–0.19) | 0.59 | 0.86 (0.51–1.94) | 0.73 (0.45–1.17) | 0.45 | 0.48 (0.25–0.77) | 0.30 (0.21–0.43) | 0.05 |
| 3 | 0.38 (0.11–1.07) | 0.47 (0.26–0.87) | 0.77 | 2.16 (1.21–3.42) | 1.18 (0.60–2.30) | 0.06 | 1.21 (0.84–1.98) | 0.56 (0.33–0.95) | 0.02 |
| 4 | 0.12 (0.05–0.15) | 0.11 (0.08–0.15) | 0.77 | 0.82 (0.33–1.26) | 0.35 (0.19–0.64) | 0.08 | 0.41 (0.23–0.57) | 0.17 (0.12–0.24) | 0.01 |
| 5 | 0.18 (0.05–0.40) | 0.15 (0.10–0.24) | 0.77 | 1.68 (0.84–4.77) | 1.01 (0.49–2.09) | 0.35 | 0.83 (0.44–1.97) | 0.42 (0.21–0.81) | 0.09 |
| 6B | 0.18 (0.05–0.44) | 0.18 (0.11–0.27) | 0.84 | 1.56 (0.84–3.67) | 0.81 (0.33–1.99) | 0.20 | 0.82 (0.33–2.03) | 0.49 (0.25–0.96) | 0.18 |
| 7F | 0.51 (0.24–0.29) | 0.49 (0.33–0.71) | 0.61 | 1.97 (0.80–4.38) | 2.14 (1.29–3.54) | 0.89 | 1.34 (0.72–2.19) | 1.21 (0.80–1.86) | 0.77 |
| 9V | 0.29 (0.16–0.63) | 0.27 (0.17–0.44) | 0.7524 | 1.97 (0.90–4.38) | 1.17 (0.63–2.18) | 0.19 | 0.99 (0.52–2.09) | 0.70 (0.38–1.25) | 0.44 |
| 14 | 0.44 (0.16–0.63) | 1.04 (0.57–1.90) | 0.07 | 4.61 (1.88–20.96) | 5.71 (3.15–10.36) | 0.93 | 2.91 (1.24–8.15) | 3.44 (2.03–5.81) | 0.91 |
| 18C | 0.17 (0.05–0.27) | 0.32 (0.20–0.52) | 0.06 | 3.67 (0.85–13.36) | 1.81 (0.98–3.36) | 0.20 | 1.44 (0.42–5.39) | 1.08 (0.58–2.01) | 0.73 |
| 19A | 0.52 (0.32–0.27) | 0.66 (0.40–1.10) | 0.61 | 3.69 (1.65–10.80) | 3.48 (1.63–7.40) | 0.72 | 1.94 (0.84–4.11) | 2.11 (1.10–4.02) | 0.92 |
| 19F | 0.43 (0.24–0.64) | 0.45 (0.28–0.71) | 0.45 | 2.90 (1.64–4.7) | 1.54 (0.86–2.76) | 0.18 | 1.51 (1.05–1.99) | 0.88 (0.57–1.36) | 0.04 |
| 23F | 0.25 (0.05–0.92) | 0.17 (0.11–0.28) | 0.45 | 3.20 (0.86–14.44) | 0.55 (0.28–1.07) | <0.01 | 1.55 (0.63–4.78) | 0.42 (0.24–0.75) | 0.01 |
Abbreviations: W, week; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine, n, number of patients with valid assay results for the specified serotype.
aStudent test or Wilcoxon test.
bGMCs were calculated using all subjects with available data for the specified blood collection. GMCs expressed as micrograms per millilitre (μg/mL).
At week 28, GMC in the prime-boost group was significantly greater for 5 serotypes; 1 (0.48 vs 0.3 μ0g/ml, p = 0.05), 3 (1.21–0.56 g/mμl, p = 0.02), 4 (0.17 vs. 0.42 μg/ml, p = 0.02), 19F (1.51 vs. 0.88 μg/ml, p = 0.04), 23F (1.54 vs. 0.42 μg/ml., p = 0.013).
Two-fold IgG serotype specific response
At week 8, number of participants in the PPSV23-alone group with a twofold increase in serotype specific IgG levels to 0, 1–3, 4–6, 7–9 and 10–12 pneumococcal polysaccharide serotypes (PPS) was 0, 2, 5, 9 and 12 versus 0, 1, 2, 4 and 19 in the prime-boost group. Twofold IgG response was observed more frequently in the prime-boost group versus the PPSV23 alone group (80% versus 70% respectively, OR 2.00, 95% CI 1.38–2.92, p < 0.01).
At week 28, the number of participants in the PPSV23-alone group with a two-fold increase in serotype specific IgG levels to 0, 1–3, 4–6, 7–9 and 10–12 PPS was 0, 7, 8, 10 and 4 versus 0, 2, 4, 10 and 10 in the prime-boost group.
Twofold IgG response was observed more frequently in the prime-boost versus the PPSV23-alone group (70% versus 52% respectively, OR 2.19, 95% CI 1.59–3.03; p < 0.01).
Two fold serotype specific IgG vaccine response and IgG > 1 ug/ml
A second more stringent criteria for vaccine response was applied where twofold increase in serotype specific IgG antibody levels and IgG concentrations >1 μg/ml was required.
Applying the more stringent criteria, at week 8 number of participants with two-fold IgG response and IgG >1 ug/ml to 0, 1–3, 4–6, 7–9 and 10–12 PPS were 0, 8, 8, 8, and 3 in the PPSV23-alone group versus 1, 3, 6, 6, and 10 in the prime-boost group. A greater frequency of response was observed in the prime-boost group versus the PPSV23-alone group (63% versus 46%, OR 2.00, 95% CI 1.46–2.74, p < 0.01).
At week 28, the frequency of responses to 0, 1–3, 4–6, 7–9 and 10–12 PPS were 2, 17, 5, 5, and 0 in the PPSV23-alone group versus 1, 7, 10, 6, and 2 in the prime-boost group. A greater frequency of response was observed in the prime-boost group versus the PPSV23-alone group (43% versus 27%, OR 1.95, 95% CI 1.40–2.70, p < 0.01).
OPA GMT response
At week 8, OPA GMT was not significantly different in the prime-boost versus the PPSV23 alone group for 12 shared study serotypes. The greatest difference between serotypes observed was for serotype 23F where GMT was higher in the prime-boost group, 330.9 μg/ml versus 31.56 µg/ml in the unprimed group, although this was of borderline significance (p = 0.06) (Table 3).
Table 3. Geometric mean antibody titres (ug/ml) for pneumococcal OPA.
| Serotype | W0 GMT (95% CI) | pa | Week 8 GMT (95% CI) | pa | Week 28 GMT (95% CI) | pa | |||
|---|---|---|---|---|---|---|---|---|---|
| PCV13 + PPSV23 n = 27 | PPSV23 n = 33 | PCV13 + PPSV23 n = 26 | PPSV23 n = 28 | PCV13 + PPSV23 n = 26 | PPSV23 n = 27 | ||||
| 1 | 4.98 (4.07–6.08) | 4.80 (4.02–5.73) | 0.89 | 27.35 (13.97–53.54) | 33.42 (18.11–61.66) | 0.85 | 12.20 (7.31–20.37) | 12.34 (8.51–17.91) | 0.29 |
| 3 | 5.28 (3.63–7.69) | 5.28 (3.80–7.32) | 0.91 | 19.53 (11.78–32.37) | 13.36 (7.46–23.93) | 0.66 | 6.91 (4.50–10.61) | 5.95 (4.33–8.18) | 0.36 |
| 4 | 10.19 (4.48–23.17) | 6.77 (3.66–12.53) | 0.67 | 938 (397–2216) | 431 (153–1218) | 0.26 | 281 (95.08–828) | 50.05 (15.96–157) | 0.26 |
| 5 | 5.39 (4.10–7.09) | 4.42 (3.79–5.16) | 0.24 | 55.19 (27.21–112) | 24.60 (12.12–49.93) | 0.80 | 22.02 (11.73–41.34) | 10.36 (5.94–18.09) | 0.19 |
| 6A | 8.14 (3.98–16.66) | 9.89 (4.83–20.25) | 0.66 | 618 (238–1607) | 38.55 (13.44–111) | <0.01 | 209 (72.90–598) | 23.02 (7.95–66.66) | 0.05 |
| 6B | 68.12 (16.63–279) | 81.54 (22.04–302) | 0.82 | 738 (250–2178) | 717 (253–2028) | 0.40 | 522 (184–1479) | 643 (266–1553) | 0.68 |
| 7F | 11.87 (5.10–27.64) | 10.19 (4.97–20.91) | 0.81 | 1354 (809–2267) | 613 (295–1277) | 0.21 | 395 (191–816) | 142 (47.69–424) | 0.88 |
| 9V | 94.74 (26.67–337) | 73.21 (24.02–223) | 0.66 | 1324 (701–2502) | 955 (386–2363) | 0.65 | 1193 (772–1844) | 292 (95.85–892) | 0.31 |
| 14 | 190 (57.69–624.9) | 414 (222–773) | 0.24 | 2544 (1721–3762) | 1266 (827–1938) | 0.11 | 1010 (541–1886) | 375 (185 762) | 0.03 |
| 18C | 13.69 (4.84–38.74) | 7.92 (3.87–16.21) | 0.81 | 1090 (466–2551) | 295 (108–806) | 0.23 | 225 (78.75–645) | 97.45(34.98272) | 0.20 |
| 19A | 8.84 (5.44–14.38) | 17.64 (9.78–31.81) | 0.30 | 239 (127–452) | 228 (127–408) | 0.55 | 88.37 (48.72–160) | 85.80 (46.80–157) | 0.76 |
| 19F | 10.41 (6.22–17.42) | 13.24 (7.12–24.60) | 0.31 | 274 (134–560) | 157 (80.43–305) | 0.47 | 57.10 (30.79–106) | 30.66 (16.42–57.25) | 0.50 |
| 23F | 7.68 (3.90–15.10) | 7.16 (4.03–12.74) | 0.81 | 331 (128–856) | 31.65 (11.79–85.00) | 0.06 | 99.02 (37.64–261) | 18.32 (8.50–39.52) | 0.03 |
Abbreviations: W, week; PCV 13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; number of patients with valid assay results for the specified serotype.
aStudent test or Wilcoxon test b GMTs were calculated using all subjects with available data for the specified blood collection.
At week 28, OPA GMT in the prime-boost group was significantly higher for serotype 14 (1010 versus 375.4 μg/ml, p = 0.03) and serotype 23F (99.0 versus 18.3 μg/ml, p = 0.03). GMT for serotype 6A (contained in PCV13 only) was significantly higher in the prime-boost group at week 8 (p < 0.01) and week 28 (p = 0.05).
At week 8 the proportion of serotypes with a 4-fold increase in GMT was 64% in the prime-boost group versus 51% in the PPSV23-alone group (OR 1.71, 95% CI 1.22–2.39, p < 0.01).
At week 28, the proportion of serotypes with a 4-fold increase in GMT remained greater in the prime-boost group 48% vs. 36% in the PPSV23-alone group (OR 1.6, 95% CI 1.15–2.3, p < 0.01).
Discussion
Evidence supporting current Advisory Committee on Immunisation Practice (ACIP) and Infectious Diseases Society of America (IDSA) guidelines which recommend PCV13 followed by PPSV23 in HIV-infected individuals is limited17,18,19. This is the first study to investigate immunological response to PCV13 followed by PPSV23 versus PPSV23 in HIV-infected adults.
Studies evaluating antibody response to PPSV23 alone have consistently reported poor immunogenicity and lack of clinical efficacy has been observed in HIV-infected patients with CD4 cell counts <500/ml21,30,31.
We report a greater proportion of 2-fold IgG pneumococcal serotype responses in the prime-boost vaccine group compared to PPSV23-alone at week 8 and week 28 (OR 2.00, 95%CI 1.46–2.74 and 2.19, 95%CI 1.59–3.03 respectively). Frequency of serotype responses remained greater in the prime-boost group using the more stringent response definition (2-fold increase in IgG and IgG > 1 ug/ml) at week 8 and week 28 (OR 2.00, 95%CI 1.46–2.74 and 1.95, 95% CI 1.40–2.70 respectively).
Similarly, proportion of 4-fold serotype OPA responses was significantly greater in the prime-boost group versus the PPSV23-alone group at week 8 and week 28 (OR 1.71, 95%CI 1.22–2.39 and 1.6, 95% CI 1.15–2.3 respectively).
Our study findings support previously published research undertaken in HIV-infected adults by Lesprit et al. which reported a greater proportion of IgG and OPA serotype responses in the prime-boost immunization group versus PPSV23-alone group22.
IgG GMC was higher for all but one serotype in the prime-boost versus the PPSV23-alone group at week 8 however a statistically significant difference in GMC was not observed between groups. This may reflect limited power of the study due to lower than expected numbers being recruited. A study examining immunological response to a number of pneumococcal vaccine strategies, including a prime-boost immunization group similarly did not observe a significant increase in IgG GMC in the prime-boost group (n = 67)24.
In this study, IgG GMC declined more rapidly in the PPSV23-alone group versus the prime-boost vaccine group, and by week 28, IgG GMC was significantly greater in the prime-boost group for 5 serotypes (serotype 1, 3, 4, 19F, 23F) (Table 2). Other studies have reported a greater durability of antibodies with conjugate pneumococcal vaccine32.
We did not observe a significant difference in OPA GMT (widely regarded as the gold standard response measure for pneumococcal vaccine) at week 8 for the 12 shared pneumococcal serotypes between groups. At week 28 OPA GMT was significantly greater for 2 serotypes (14 and 23F).
Other studies investigating various response endpoints to pneumococcal vaccination in HIV-infected adults have reported variable immunological outcomes23,24,25,26,27,28,29.
Currently, there are no validated correlates of pneumococcal vaccine protection in adults. Historically, the most commonly accepted criteria for an adequate vaccination response to pneumococcal vaccine in adults have been either an absolute level of >1 μg/ml or a 4-fold increase in antibody concentration at a time point greater than 4 weeks following vaccination, usually in 70% of serotypes33,34.
Clinical data on the protective effects of these thresholds are limited, particularly in immunocompromised patient groups and as a result, a universal consensus does not exist. Lower thresholds have been suggested as correlates of response for the percentage of serotypes (50% versus 70%) and the fold change (2-fold versus 4-fold) required35,36 for a response to be documented.
Standardization of serological measures and reported responses, as well as further studies to identify immunologic correlates of protective response to pneumococcal vaccine in HIV-infected individuals are warranted.
Level of immunosuppression in HIV-infected individuals has been identified as one of the most important risk factors for IPD6,7. Low CD4 T cell count has also been shown to be associated with poor response to pneumococcal vaccine31,37,38. Immunization guidelines recommended pneumococcal vaccination with PCV13 irrespective of CD4 count as even an attenuated response will offer some protection in this high risk group.
Some studies suggest that individuals who are not on HAART or who have uncontrolled viraemia have diminished response to pneumococcal vaccine irrespective of CD4 count39. A recently undertaken randomized controlled trial assessing optimal timing of pneumococcal vaccine found that in HIV-infected individuals with CD4 count ≥200 cells/mm3, delaying PPSV23 until more than 6 months after initiating HAART did not improve responses and may lead to missed opportunities for immunization40.
We did not observe any adverse effects from vaccine during the course of the study. The safety of pneumococcal vaccine, both conjugate and polysaccharide has been demonstrated in other studies39,40.
Limitations
Our study has several limitations. Sample size was small and more subjects withdrew or were lost to follow-up than expected making the final study group smaller than intended. We did not recruit sufficient numbers of participants to carry out a multivariate analysis thus we did not investigate differences in vaccine responses based on factors such as gender, smoking status, CD4 count, HIV viral load or HAART.
Baseline CD4 count in the prime-boost group was greater compared to the PPSV23 alone group which may represent a confounder. Duration of serological follow up (28 weeks) is relatively short and we cannot comment on sustainability of immunological response in the cohort. The majority of participants in this study were male Caucasians and thus results cannot be extrapolated to females or the wider population. In addition, whether the increase in antibody response observed for some serotypes after the conjugate vaccine in our study will translate into improved clinical efficacy is unknown.
Conclusion
Our study suggests that combining PCV13 with PPSV23 elicits a greater magnitude of IgG and OPA immune response compared to PPSV23 alone in HIV-infected individuals with CD4 count >200 cells/mm3 over the study period.
Further studies addressing clinical end points and identifying immune correlates of vaccine protection are warranted as HIV-infected individuals will continue to be a group who will benefit greatly from protection against pneumococcal disease.
Our study adds to evidence supporting the current pneumococcal vaccination recommendations in the United States and Europe for HIV-infected individuals.
Ongoing monitoring of pneumococcal disease trends, particularly in high risk patient groups including those with HIV infection is warranted to determine the optimal pneumococcal prevention strategy in the future particularly as extended valency vaccines become available.
Methods
This study was undertaken in an ambulatory HIV clinic in the Department of Genitourinary Medicine and Infectious Diseases (GUIDE), St James’s Hospital, Dublin, Ireland.
Pneumococcal vaccine naïve HIV-infected individuals >18 years with CD4 cell counts ≥200 cells/mm3 meeting eligibility criteria outlined in the study protocol (EudraCT number 2011-000260-99) were recruited between April 2011 and July 2012. As international pneumococcal vaccine recommendations changed in 2012 to recommend PCV13 followed by PPSV23 for all HIV-infected adults recruitment to this study was discontinued and the prime boost immunisation strategy implemented as standard of care for all patients attending our clinic.
Participants were randomized by computer generated allocation sequence with equal probability of being randomized to PCV13 (Prevnar; Wyeth Pharmaceuticals) at week 0 followed by PPSV23 at week 4 (prime boost group) or PPSV23 alone (Pneumovax; Merck) at week 4 (PPSV23 group). All subjects provided written informed consent.
Vaccines were supplied in pre-filled single-dose syringes without preservatives and stored according to manufacturer guidelines at 2–8 °C. Vaccines were injected intramuscularly (0.5 mL) in the deltoid muscle with the use of a 23-gauge, 1-inch needle.
Study Objectives
The primary objective of this study was to compare serotype specific IgG geometric mean concentration (GMC) and opsonophagocytic (OPA) responses in the prime-boost versus the PPSV23-alone group for 12 serotypes (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) contained in both vaccines at week 8 and week 28.
Secondary objectives were to compare the proportion of responses to the 12 shared pneumococcal polysaccharide serotypes (PPS) at week 8 and week 28 in the prime-boost versus the PPSV23-alone groups.
Immunogenicity
IgG geometric mean concentration
Serum concentrations of anti-capsular IgG for 12 shared PPS (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) were determined using a validated enzyme-linked immunosorbent assay and expressed as micrograms per milliliter (μg/ml)41.
Briefly, test sera were pre-adsorbed with C-PS (5 μg/ml) to remove nonfunctional antibodies. A further adsorption step was included with serotype 22F polysaccharide (5 μg/ml). The lower limit of detection is 0·01 μg/ml41.
Opsonophagocytic geometric mean titer
Opsonophagocytic assays measure complement mediated killing, one of the main protective responses against S. pneumoniae infection and are widely accepted as the gold standard measure of pneumococcal vaccine response. Functional antibacterial OPA activity was measured using 13 serotype-specific validated assays and comparisons made for the 12 serotypes contained in both vaccines.
Briefly, heat-inactivated sera were serially diluted 2.5-fold in buffer. Target bacteria were added to assay plates and were incubated for 30 min at 25 °C on a shaker. Baby rabbit complement (3–4 week old, Pel-Freez, 12.5% final concentration) and differentiated HL-60 cells, were then added to each well at an approximate effector to target ratio of 200:1. Assay plates were incubated for 45 min at 37 °C on a shaker. To terminate the reaction, 80 μL of 0.9% NaCl was added to all wells, mixed, and a 10-μL aliquot were transferred to the wells of Millipore, MultiScreenHTS HV filter plates containing 200 μL of water. Liquid was filtered through the plates under vacuum, and 150 μL of HySoy medium was added to each well and filtered through. The filter plates were then incubated at 37 °C, 5% CO2 overnight and were then fixed with Destain Solution (Bio-Rad). The plates were then stained with Coomassie Blue and destained once. Colonies were imaged and enumerated on a Cellular Technology Limited (CTL) ImmunoSpot Analyzer®. The OPA antibody titer was interpolated from the reciprocal of the two serum dilutions encompassing the point of 50% reduction in the number of bacterial colonies when compared to the control wells that did not contain immune serum42,43.
IgG concentrations and opsonophagocytic titers were measured in blood samples obtained before (week 0) vaccination, at week 8 and week 28 post vaccination.
The laboratory measurements were performed blinded to case-control status.
Definitions and statistical methods
In the first instance serotype specific IgG response was defined as a 2-fold increase from baseline of serotype specific IgG antibody levels (μg/ml).
A second more stringent definition of response was applied. This was defined as a 2-fold increase in serotype specific IgG and an IgG levels >1 μg/ml.
OPA response was defined as a 4-fold or greater increase in OPA titer.
Descriptive statistics are presented as N (percentages) for categorical values, means with standard deviations (SDs) for numerical values.
IgG concentrations (reported as μg/ml) and OPA titers (reported as μg/ml) are expressed as geometric means with 95% CIs using logarithmically transformed assay results.
The Wilcoxon or Mann-Whitney U test were used compare pre and post vaccination IgG and OPA responses between groups study.
Proportion of vaccine serotype responses between vaccine groups was compared using the chi square test.
Data analyses were performed using Graphpad prism (version 6) and SPSS (version 22). No imputations were performed for missing data.
Ethical considerations
This was a single center study undertaken in an ambulatory HIV out-patient’s clinic in the Department of Genitourinary Medicine and Infectious Diseases (GUIDE), St James’s Hospital, Dublin, Ireland from April 2011-July 2012. The study protocol was approved by the St James’s Hospital/Tallaght Hospital Research Ethics Committee (approval number 10102010) and the Irish Medicines Board (approval number 2095901). This study was registered on the European Clinical Trials Database on 04/12/2011 (EudraCT number 2011-000260-99). Written informed consent was obtained from all study participants. All study methods were performed in accordance with the relevant guidelines and regulations.
Additional Information
How to cite this article: Sadlier, C. et al. Immunological efficacy of pneumococcal vaccine strategies in HIV-infected adults: a randomized clinical trial. Sci. Rep. 6, 32076; doi: 10.1038/srep32076 (2016).
Acknowledgments
The authors would like to acknowledge patients who participated in this study. The authors would like to acknowledge the Vaccine Evaluation Unit, Manchester, United Kingdom for performing serotype specific IgG analysis and the Pfizer, Vaccine Clinical Research, New York, US for undertaking the serotype specific oposonophagocytic analysis.
Footnotes
Author Contributions All named authors contributed to this submission. C.S., C.B., N.C. and J.D. were involved in study design and planning. S.O.D. and C.S. were involved in patient recruitment. C.S. and K.B. carried out statistical analysis. C.S. and C.B. drafted the manuscript. All authors reviewed and approved the final draft of the submitted manuscript.
References
- Yin Z. R. B., Waight P, Miller E., George R., Brown A. E. et al. Invasive pneumococcal disease among HIV-positive individuals, 2000–2009. AIDS 26, 87–94 (2012). [DOI] [PubMed] [Google Scholar]
- Janoff E. N., Breiman R. F., Daley C. L. & Hopewell P. C. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med 117, 314–324 (1992). [DOI] [PubMed] [Google Scholar]
- Jones N., Huebner R., Khoosal M., Crewe-Brown H. & Klugman K. The impact of HIV on Streptococcus pneumoniae bacteraemia in a South African population. Aids 12, 2177–2184 (1998). [DOI] [PubMed] [Google Scholar]
- French N. et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 355, 2106–2111 (2000). [DOI] [PubMed] [Google Scholar]
- McEllistrem M. C. et al. Recurrent invasive pneumococcal disease in individuals with human immunodeficiency virus infection. J Infect Dis 185, 1364–1368, 10.1086/339882 (2002). [DOI] [PubMed] [Google Scholar]
- Grau I. et al. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med 165, 1533–1540, 10.1001/archinte.165.13.1533 (2005). [DOI] [PubMed] [Google Scholar]
- Feikin D. R., Feldman C., Schuchat A. & Janoff E. N. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis 4, 445–455, 10.1016/s1473-3099(04)01060-6 (2004). [DOI] [PubMed] [Google Scholar]
- Barry P. M. et al. Invasive pneumococcal disease in a cohort of HIV-infected adults: incidence and risk factors, 1990–2003. Aids 20, 437–444, 10.1097/01.aids.0000206507.54901.84 (2006). [DOI] [PubMed] [Google Scholar]
- Nunes M. C. et al. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PloS one 6, e27929, 10.1371/journal.pone.0027929 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordano Q. et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 38, 1623–1628, 10.1086/420933 (2004). [DOI] [PubMed] [Google Scholar]
- Moberley S., Holden J., Tatham D. P. & Andrews R. M. Vaccines for preventing pneumococcal infection in adults. The Cochrane database of systematic reviews 1, Cd000422, 10.1002/14651858.CD000422.pub3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Bryant J., Paradiso P. R. & Siber G. R. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 30, 100–121, 10.1086/313608 (2000). [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Bryant J., Kloek C., Paradiso P. R. & Siber G. R. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis 30, 122–140, 10.1086/313609 (2000). [DOI] [PubMed] [Google Scholar]
- USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: disease-specific recommendations. USPHS/IDSA Prevention of Opportunistic Infections Working Group. Clin Infect Dis 21, Suppl 1, S32–S43 (1995). [PubMed] [Google Scholar]
- 1997 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. USPHS/IDSA Prevention of Opportunistic Infections Working Group. MMWR Recomm Rep 46, 1–46 (1997). [PubMed] [Google Scholar]
- Pedersen R. H., Lohes N., Østergaard L. & Søgaard O. S. The effectiveness of pneumococcal polysaccharide vaccination in HIV-infected adults: a systematic review. HIV Medicine 12, 323–333 (2011). [DOI] [PubMed] [Google Scholar]
- ACIP. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults With Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). JAMA 309, 334, 10.1001/jama.2012.31377 (2013). [DOI] [PubMed] [Google Scholar]
- Geretti A. M. & British H. I. V. Association guidelines for immunization of HIV-infected adults 2008. HIV Medicine 9, 795–848, 10.1111/j.1468-1293.2008.00637.x (2008). [DOI] [PubMed] [Google Scholar]
- Konkle-Parker D. Vaccination of immunocompromised individuals: IDSA clinical practice guidelines. HIV Clin 26, 1–3 (2014). [PubMed] [Google Scholar]
- Black S. et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 19, 187–195 (2000). [DOI] [PubMed] [Google Scholar]
- French N. et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 362, 812–822, 10.1056/NEJMoa0903029 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesprit P. et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS 21, 2425–2434, 10.1097/qad.0b013e3282887e91 (2007). [DOI] [PubMed] [Google Scholar]
- Lee K.-Y. et al. Pneumococcal vaccination among HIV-infected adult patients in the era of combination antiretroviral therapy. Human Vaccines & Immunotherapeutics 10, 3700–3710, 10.4161/hv.32247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feikin D. R. et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 20, 545–553 (2001). [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone N. F. et al. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. The Journal of infectious diseases 202, 1114–1125, 10.1086/656147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. L., Brandao A. P., de Cunto Brandileone M. C. & Lopes M. H. Immunogenicity and safety of pneumococcal conjugate polysaccharide and free polysaccharide vaccines alone or combined in HIV-infected adults in Brazil. Vaccine 31, 4047–4053, 10.1016/j.vaccine.2013.04.065 (2013). [DOI] [PubMed] [Google Scholar]
- Lu C. L. et al. Revaccination with 7-valent pneumococcal conjugate vaccine elicits better serologic response than 23-valent pneumococcal polysaccharide vaccine in HIV-infected adult patients who have undergone primary vaccination with 23-valent pneumococcal polysaccharide vaccine in the era of combination antiretroviral therapy. Vaccine 32, 1031–1035, 10.1016/j.vaccine.2014.01.009 (2014). [DOI] [PubMed] [Google Scholar]
- Penaranda M., Payeras A., Cambra A., Mila J. & Riera M. Conjugate and polysaccharide pneumococcal vaccines do not improve initial response of the polysaccharide vaccine in HIV-infected adults. AIDS (London, England) 24, 1226–1228, 10.1097/QAD.0b013e3283389de5 (2010). [DOI] [PubMed] [Google Scholar]
- Ohtola J. A. et al. Alterations in serotype-specific B cell responses to the 13-valent pneumococcal conjugate vaccine in aging HIV-infected adults. Vaccine 34, 451–457, http://dx.doi.org/10.1016/j.vaccine.2015.12.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barradas M. C. et al. Antibody to capsular polysaccharides of Streptococcus pneumoniae after vaccination of human immunodeficiency virus-infected subjects with 23-valent pneumococcal vaccine. J Infect Dis 165, 553–556 (1992). [DOI] [PubMed] [Google Scholar]
- Kroon F. P., van Dissel J. T., de Jong J. C., Zwinderman K. & van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 18, 3040–3049, http://dx.doi.org/10.1016/S0264-410X(00)00079-7 (2000). [DOI] [PubMed] [Google Scholar]
- Cheng A. et al. Long-term immune responses and comparative effectiveness of one or two doses of 7-valent pneumococcal conjugate vaccine (PCV7) in HIV-positive adults in the era of combination antiretroviral therapy. Journal of the International AIDS Society 19, 20631, 10.7448/ias.19.1.20631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris K. & Sorensen R. U. Assessment and clinical interpretation of polysaccharide antibody responses. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology 99, 462–464, 10.1016/s1081-1206(10)60572-8 (2007). [DOI] [PubMed] [Google Scholar]
- Beck S. C. Making sense of serotype-specific pneumococcal antibody measurements. Annals of clinical biochemistry 50, 517–519, 10.1177/0004563213500241 (2013). [DOI] [PubMed] [Google Scholar]
- Orange J. S. et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. The Journal of allergy and clinical immunology 130, S1–24, 10.1016/j.jaci.2012.07.002 (2012). [DOI] [PubMed] [Google Scholar]
- Kamchaisatian W., Wanwatsuntikul W., Sleasman J. W. & Tangsinmankong N. Validation of current joint American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology guidelines for antibody response to the 23-valent pneumococcal vaccine using a population of HIV-infected children. The Journal of allergy and clinical immunology 118, 1336–1341, 10.1016/j.jaci.2006.09.036 (2006). [DOI] [PubMed] [Google Scholar]
- Ahmed F. et al. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J Infect Dis 173, 83–90 (1996). [DOI] [PubMed] [Google Scholar]
- Kroon F. P., van Dissel J. T., de Jong J. C. & van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. Aids 8, 469–476 (1994). [DOI] [PubMed] [Google Scholar]
- Teshale E. H. et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine on pneumonia in HIV-infected adults in the United States, 1998–2003. Vaccine 26, 5830–5834, 10.1016/j.vaccine.2008.08.032 (2008). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Barradas M. C. et al. Quantitative and Qualitative Antibody Responses to Immunization With the Pneumococcal Polysaccharide Vaccine in HIV-Infected Patients After Initiation of Antiretroviral Treatment: Results From a Randomized Clinical Trial. J Infect Dis 211, 1703–1711, 10.1093/infdis/jiu819 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer P. et al. Measurement and interpretation of pneumococcal IgG levels for clinical management. Clinical and Experimental Immunology 133, 364–369, 10.1046/j.1365-2249.2003.02232.x (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. et al. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 29, 7207–7211, 10.1016/j.vaccine.2011.06.056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Steiner S. et al. Multilaboratory Evaluation of a Viability Assay for Measurement of Opsonophagocytic Antibodies Specific to the Capsular Polysaccharides of Streptococcus pneumoniae. Clinical and Vaccine Immunology 10, 1019–1024, 10.1128/cdli.10.6.1019-1024.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]