Abstract
Longevity assurance homolog 2 of yeast LAG1 (LASS2) has been reported to act as an important tumor suppressor in the development of human cancers. However, little is known about the prognostic value of LASS2 in hepatocellular carcinoma (HCC) . In the present study, we analyzed correlation between LASS2 and TGF-β1 levels, and evaluated their prognostic values in HCC patients. We first analyzed the expression of LASS2 and TGF-β1 in two independent cohorts (test cohort: 184 HCC patients; validation cohort: 118 HCC patients) using immunohistochemistry (IHC). Kaplan-Meier survival and Cox regression analyses were executed to evaluate the prognosis of HCC. The results of IHC analysis revealed a positive correlation between the expression of LASS2 and TGF-β1. HCC Patients with low expression of LASS2 and TGF-β1 had shorter overall survival (OS) and time to recurrence (TTR) than patients with high expression of LASS2 and TGF-β1. Furthermore, combination of LASS2 and TGF-β1 was an independent and significant risk factor for OS and TTR. In conclusion, low expression of LASS2 and TGF-β1 contributes to the aggressiveness and poor prognosis of HCC, and may represent a novel prognostic biomarker for HCC patients.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors, the third major cause of cancer-related death worldwide1. Despite many improvements have been achieved in clinical treatments such as liver surgical resection, chemotherapy and transplantation, HCC patients still have high recurrence incidence and poor prognosis2,3. The prognosis of HCC patients after surgical resection remains unsatisfactory, with a 5-year survival rate ranged from 25–39%, and a 5-year recurrence rate ranged from 65–80%4,5,6. Therefore, it is critical to identify valuable factors for prognosis prediction and novel therapeutic strategies.
Homo sapiens longevity assurance homolog 2 of yeast LAG1 (LASS2), which is also known as tumor metastasis suppressor gene (TMSG1) or ceramide synthase 2 (CerS2), is the gene identified from a human liver cDNA library by our laboratory7. As one member of LASS family, including LASS1–6, LASS2 mRNA is at the highest level of all LASS members, and has the broadest tissue distribution, particularly abundant in the liver, kidney and brain in mice8. A growing amount of research has reported that LASS2 mainly acts as a tumor suppressor and is closely associated with a variety of tumor progression, including prostate9, breast10, bladder11 and liver cancer12. Our previous investigations have shown that low expression of LASS2 is associated with poor prognosis in patients with breast cancer10, and deletion of LASS2 is associated with a high risk of spontaneous or diethylnitrosamine (DEN)-induced HCC in hepatocyte-specific LASS2-knockout (KO) mice models13,14. However, no study has been performed to assess whether there are any correlations among the protein level of LASS2, clinicopathological parameters, and the survival rate in clinical HCC samples.
Transforming growth factor-β1 (TGF-β1) plays biphasic functions in tumor tumorigenesis, having a growth inhibitory effect at early stages, but at later stages enhancing the malignant conversion. The TGF-β1 signaling could cross-talk with multiple intracellular signaling in tumor cell. Generally, TGF-β1 signal is transduced from plasma membrane to the nucleus via serine/threonine kinase receptor type I (TβR-I) and type II (TβR-II) and their downstream effectors, smads. Moreover, TGF-β1 signal is also transduced via Smad-independent pathways through activation of mitogen-activated protein kinase (MAPK), nuclear factor kappa-B (NF-κB) and phosphatidylinositol 3-kinase (PI3K)15. As a multifunctional cytokine, TGF-β1 regulates important cellular processes including cell growth, differentiation, apoptosis, epithelial-mesenchymal transition (EMT), immunosuppressant and senescence16,17,18,19. Lots of evidences show that TGF-β1 functions as either a tumor suppressor or a tumor promoter mainly depending on the stage of carcinogenesis20,21,22. TGF-β1 particularly achieves its tumor suppressive effect by inhibiting cell cycle progression through G1-arrest23,24, and inducing apoptosis25. Besides, TGF-β1 can also induce senescence of epithelial cells and HCC18,26,27,28. In a previous study, we found that LASS2 was closely associated with TGF-β1 levels in DEN-induced liver tumorigenesis13. In the present study, we investigated the relationships between LASS2 and TGF-β1, evaluated their prognostic values in two independent cohorts of HCC patients, as well as their relationships with clinicopathological parameters.
Results
The relationship between LASS2 and TGF-β1 in HCC patients
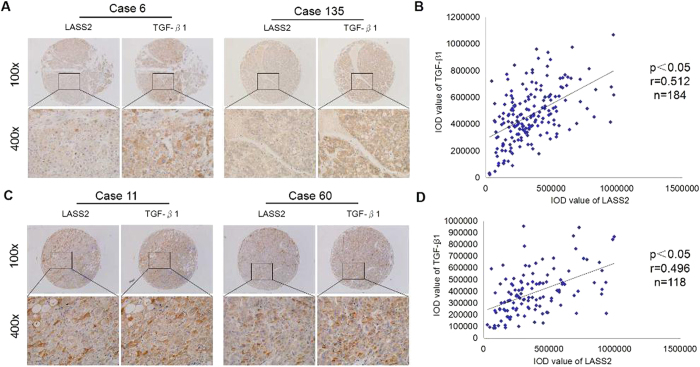
We enrolled a test cohort consisting of 184 HCC patients and a validation cohort of 118 HCC patients, who had undergone curative resection between 2008 and 2013. Using immunohistochemical staining, we examined expression of LASS2 and TGF-β1 in the two independent cohorts, and analyzed their coexpression. The observed LASS2 staining pattern in HCC tissues was both plasma membranous and cytoplasmic. The level and extent of staining varied from weak focal to extensive strong expression. For TGF-β1 staining, TGF-β1 was primarily localized in the cytoplasm of tumor cells, which were producing TGF-β1 (Fig. 1A,C). Based on analysis of integrated optical density (IOD) value, we found that a significant positive correlation between expression of LASS2 and TGF-β1 was observed in the test cohort (Pearson’s correlation, r = 0.512, n = 184, p < 0.05, Fig. 1B). Similarly, the positive correlation between LASS2 and TGF-β1 was also observed in the validation cohort (Pearson’s correlation, r = 0.496, n = 118, p < 0.05, Fig. 1D).
Figure 1. The relationship between LASS2 and TGF-β1 in HCC patients.
(A). Two representative cases of LASS2 and TGF-β1 expression in the test cohort. (B). Relevance of LASS2 and TGF-β1 in the test cohort. (Pearson’s correlation, r = 0.512, n = 184, p < 0.05). (C). Two representative cases of LASS2 and TGF-β1 expression in the validation cohort. (D). Relevance of LASS2 and TGF-β1 in the validation cohort. (Pearson’s correlation, r = 0.496, n = 118, p < 0.05).
Low expression of LASS2 and TGF-β1 significantly correlated with progression and poor prognosis of HCC in the test cohort
Among the 184 HCC patients of test cohort, 64 (34.8%) and 100 (54.3%) cases had high expression of LASS2 and TGF-β1, respectively. We performed further analyses to determine the clinicopathological significance of LASS2 and TGF-β1 in HCC. The expression level of LASS2 was negatively correlated with the tumor size, tumor differentiation, and TNM stage (Table 1). In addition, we also found that TGF-β1 expression was correlated with age and TNM stage (Table 1).
Table 1. Relationships of LASS2 and TGFβ1 expression level with the clinicopathological features in HCC patients from test and validation cohorts.
| Characteristics | Test |
p-value | Validation |
p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LASS2 |
p-value | TGF-β1 |
LASS2 |
p-value | TGF-β1 |
|||||||
| Low (n = 109) | High (n = 75) | Low (n = 69) | High (n = 115) | Low (n = 78) | High (n = 40) | Low (n = 65) | High (n = 53) | |||||
| Sex | 0.862 | 0.585 | 0.129 | 0.549 | ||||||||
| male | 94 | 64 | 58 | 100 | 73 | 34 | 58 | 49 | ||||
| female | 15 | 11 | 11 | 15 | 5 | 6 | 7 | 4 | ||||
| Age | 0.173 | 0.009* | 0.810 | 0.705 | ||||||||
| ≤50 | 62 | 35 | 45 | 52 | 33 | 16 | 28 | 21 | ||||
| >50 | 47 | 40 | 24 | 63 | 45 | 24 | 37 | 32 | ||||
| HBsAg | 0.673 | 0.767 | 0.460 | |||||||||
| negative | 15 | 12 | 9 | 18 | 0.628 | 12 | 7 | 9 | 10 | |||
| positive | 94 | 63 | 60 | 97 | 66 | 33 | 56 | 43 | ||||
| Serum AFP | 0.637 | 0.282 | 0.927 | 0.560 | ||||||||
| ≤20 ng/ml | 37 | 28 | 21 | 44 | 26 | 13 | 20 | 19 | ||||
| >20 ng/ml | 72 | 47 | 48 | 71 | 52 | 27 | 45 | 34 | ||||
| Liver cirrhosis | 0.127 | 0.966 | 0.337 | 0.898 | ||||||||
| no | 36 | 17 | 20 | 33 | 28 | 18 | 25 | 21 | ||||
| yes | 73 | 58 | 49 | 82 | 50 | 22 | 40 | 32 | ||||
| TNM stage | 0.545 | 0.193 | 0.030* | 0.030* | ||||||||
| I | 32 | 27 | 21 | 38 | 24 | 18 | 18 | 24 | ||||
| II | 61 | 40 | 35 | 66 | 39 | 21 | 34 | 26 | ||||
| III-IV | 16 | 8 | 13 | 11 | 15 | 1 | 13 | 3 | ||||
| Child-pugh | 0.301 | 0.366 | 0.965 | 0.395 | ||||||||
| A | 102 | 67 | 65 | 104 | 70 | 36 | 57 | 49 | ||||
| B | 7 | 8 | 4 | 11 | 8 | 4 | 8 | 4 | ||||
| Tumor size | 0.003* | 0.109 | 0.614 | 0.131 | ||||||||
| ≤3 cm | 16 | 25 | 11 | 30 | 20 | 12 | 14 | 18 | ||||
| >3 cm | 93 | 50 | 58 | 85 | 58 | 28 | 51 | 35 | ||||
| Tumor number (multiple vs single) | 0.635 | 0.460 | 0.074 | 0.101 | ||||||||
| single | 84 | 60 | 52 | 92 | 57 | 35 | 47 | 45 | ||||
| multiple | 25 | 15 | 17 | 23 | 21 | 5 | 18 | 8 | ||||
| Tumor differentiation | 0.033* | 0.538 | 0.487 | 0.978 | ||||||||
| well | 6 | 13 | 5 | 14 | 5 | 4 | 5 | 4 | ||||
| moderate | 102 | 61 | 63 | 100 | 73 | 36 | 60 | 49 | ||||
| poor | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | ||||
| Microvascular invasion | 0.512 | 0.691 | 0.087 | 0.083 | ||||||||
| no | 37 | 29 | 26 | 40 | 30 | 22 | 24 | 28 | ||||
| yes | 72 | 46 | 43 | 75 | 48 | 18 | 41 | 25 | ||||
Statistical analyses were done by the Chi-square (χ2) test.
*p-value smaller than 0.05.
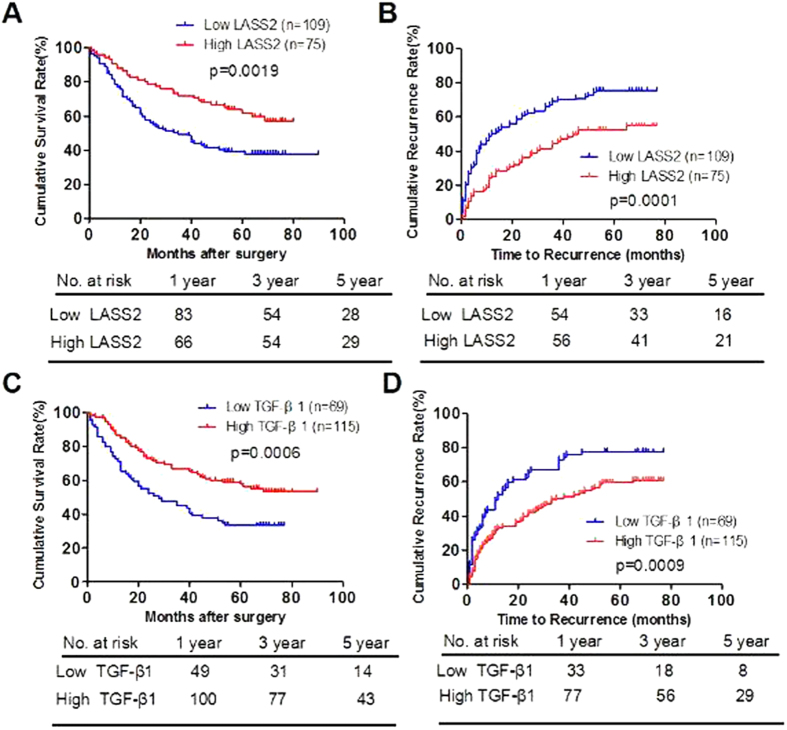
We determine the prognostic value of LASS2 and TGF-β1 in the test cohort of HCC patients. OS and TTR were evaluated using the Kaplan-Meier method. Our results showed that the median OS time was 46.5 (95% CI: 39.7–53.3) months for HCC patients with low expression of LASS2 and 58.4 (95% CI: 51.9–64.9) months for HCC patients with high expression of LASS2 (p = 0.0019, Fig. 2A). The median TTR was 28.9 (95% CI: 23.2–34.5) months for patients with HCC who had low expression of LASS2 and 45.9 (95% CI: 38.9–53.0) months for patients with HCC who had high expression of LASS2 (p = 0.0001, Fig. 2B). Accordingly, we found that HCC patients with low expression of TGF-β1 had shorter OS (38.1 versus 60.5 months, p = 0.0006, Fig. 2C) and TTR (26.5 versus 41.4 moths, p = 0.0009, Fig. 2D).
Figure 2. Low expression of LASS2 and TGF-β1 significantly correlates with poor prognosis of hepatocelluar carcinoma in the test cohort.
(A,B). Kaplan-Meier analysis of the correlation between LASS2 expression and OS (A) or TTR (B) in the test cohort. (C,D). Kaplan-Meier analysis of the correlation between TGF-β1 expression and OS (C) or TTR (D) in the test cohort. Log-rank tests were used to determine statistical significance.
The combination of LASS2 and TGF-β1 exhibited the improved prognostic value for HCC patients in the test cohort
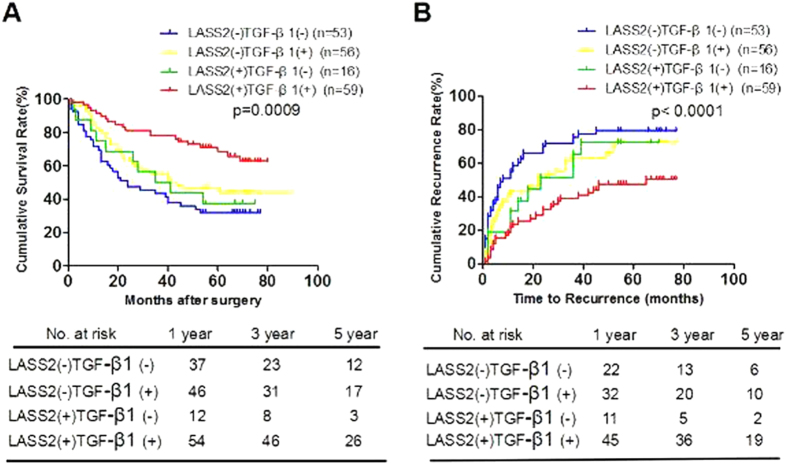
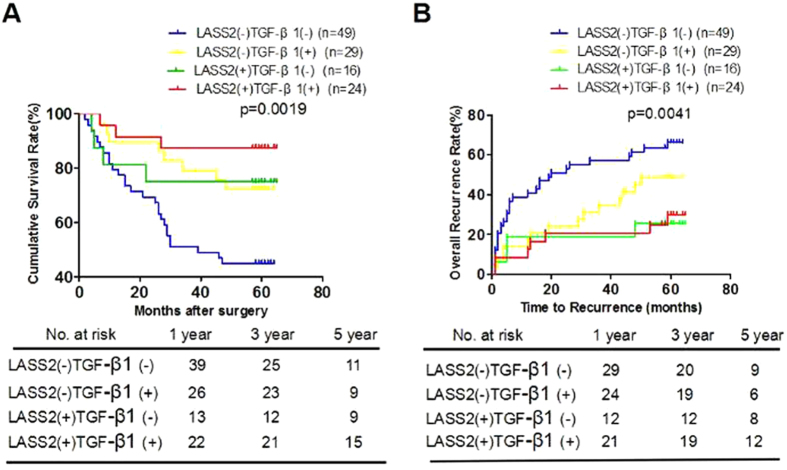
To analyze the prognostic value of combining LASS2 and TGF-β1 levels for HCC in the test cohort, we divided patients into the following four groups, such as: low LASS2 low TGF-β1 group, high LASS2 low TGF-β1 group, low LASS2 high TGF-β1 group, and high LASS2 high TGF-β1 group. Using the Kaplan–Meier method, low LASS2 low TGF-β1 group had the shortest OS (median months: 36.6, 95% CI: 28.5–44.8) and TTR (median months: 23.9, 95% CI: 16.1–31.7), whereas high LASS2 high TGF-β1 group had the longest OS (median months: 62.4, 95% CI: 55.5–69.2) and TTR (median months: 48.9, 95% CI: 41.0–56.7) (Fig. 3). Collecting, the results indicated that the combination of low LASS2 expression and low TGF-β1 expression in HCC tissues appeared to be predictive of the poorest prognosis.
Figure 3. The combination of LASS2 and TGF-β1 exhibited the improved prognostic value for HCC patients in the test cohort.
(A,B). Kaplan-Meier analysis of the correlation between LASS2/TGF-β1 and OS (A) or TTR (B) in the test cohort. GI, LASS2−/TGF-β1−(n = 53); GII, LASS2−/TGF-β1 + (n = 56); GIII, LASS2+/TGF-β1-(n = 16); GIV, LASS2+/TGF-β1 + (n = 59).
Univariate and multivariate analyses of prognostic variables for HCC patients in the test cohort
To evaluate whether the expression levels of LASS2 and TGF-β1 were independent prognostic value for HCC patients in the test cohort, univariate and multivariate analyses using a Cox regression model were applied. As shown in Table 2, LASS2 level, TGF-β1 level, and the LASS2/TGF-β1 combination was responsible for the OS and TTR of HCC patients. In addition to the above factors, serum AFP, TNM stage, child-pugh, tumor size, tumor number, microvascular invasion, and tumor differentiation were also correlated with OS and/or TTR (Table 2). Factors showed significance in univariate analysis were then subjected to multivariate Cox proportional hazards analysis. Despite neither LASS2 nor TGF-β1 alone were independent prognostic markers for OS and TTR, the LASS2/TGF-β1 combination was an independent prognostic marker for both OS and TTR in the test cohort. These results indicated that the LASS2/TGF-β1 combination had a potent prognostic value compared with LASS2 or TGF-β1 alone.
Table 2. Univariate analyses of factors associated with recurrence and survival in hepatocelluar carcinoma patients from test and validation cohorts.
| Variables | Test |
Validation |
||||||
|---|---|---|---|---|---|---|---|---|
| OS |
TTR |
OS |
TTR |
|||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Sex (male vs female) | 1.558(0.911–2.664) | 0.105 | 1.298(0.786–2.142) | 0.308 | 0.659(0.204–2.132) | 0.486 | 0.612(0.221–1.690) | 0.343 |
| Age (>50 vs ≤50) | 1.004(0.674–1.495) | 0.984 | 1.067(0.748–1.523) | 0.719 | 1.418(0.746–2.695) | 0.286 | 1.365(0.792–2.354) | 0.262 |
| HBsAg (positive vs negative) | 1.400(0.765–2.565) | 0.275 | 1.256(0.752–2.098) | 0.384 | 1.504(0.591–3.826) | 0.392 | 1.840(0.789–4.291) | 0.158 |
| Serum AFP (>20 vs ≤20) | 2.928(1.787–4.797) | <0.001* | 1.890(1.278–2.794) | 0.001* | 1.252(0.651–2.408) | 0.501 | 1.635(0.906–2.951) | 0.103 |
| Liver cirrhosis (yes vs no) | 1.144(0.726–1.804) | 0.562 | 1.192(0.796–1.786) | 0.394 | 2.107(1.059–4.195) | 0.034* | 3.127(1.651–5.921) | <0.001* |
| TNM stage (I vs II vs III–IV) | 1.569(1.144–2.152) | 0.005* | 1.813(1.352–2.432) | <0.001* | 2.877(1.766–4.687) | <0.001* | 2.405(1.587–3.644) | <0.001* |
| Child-pugh (A vs B) | 1.881(1.004–3.527) | 0.049* | 2.080(1.167–3.708) | 0.013* | 1.233(0.485–3.138) | 0.660 | 0.997(0.428–2.324) | 0.994 |
| Tumor size (>3 cm vs ≤3 cm) | 2.682(1.464–4.912) | 0.001* | 1.691(1.073–2.665) | 0.024* | 1.876(0.868–4.055) | 0.110 | 2.504(1.227–5.108) | 0.012* |
| Tumor number (multiple vs single) | 1.721(1.103–2.686) | 0.017* | 2.279(1.533–3.388) | <0.001* | 4.550(2.464–8.405) | <0.001* | 3.038(1.748–5.283) | <0.001* |
| Tumor differentiation (well vs moderate vs poor) | 1.856(0.939–3.670) | 0.075 | 1.841(1.031–3.288) | 0.039* | 1.982(0.479–8.202) | 0.345 | 2.826(0.689–11.595) | 0.149 |
| Microvascular invasion (yes vs no) | 1.495(0.971–2.301) | 0.068 | 1.515(1.034–2.219) | 0.033* | 1.574(0.837–2.960) | 0.159 | 1.500(0.875–2.570) | 0.140 |
| LASS2 (low vs high) | 0.514(0.334–0.791) | 0.002* | 0.517(0.354–0.756) | 0.001* | 0.347(0.154–0.781) | 0.011* | 0.367(0.190–0.710) | 0.003* |
| TGF-β1 (low vs high) | 0.507(0.340–0.757) | 0.001* | 0.577(0.403–0.828) | 0.003* | 0.354(0.178–0.705) | 0.003* | 0.581(0.339–0.996) | 0.049* |
| LASS2 and TGF-β1†GI vs GII vs GIII vs GIV | 0.716(0.601–0.852) | <0.001* | 0.733(0.629–0.855) | <0.001* | 0.558(0.397–0.786) | 0.001* | 0.640(0.492–0.833) | 0.001* |
Univariate analysis: Cox proportional hazards regression model.
Validation cohort: GI, LASS2−/TGF-β1−(n = 49); GII, LASS2−/TGF-β1 + (n = 29); GIII, LASS2+/TGF-β1-(n = 16); GIV, LASS2+/TGF-β1 + (n = 24).
HbsAg: Hepatitis B surface antigen; HR: Hazard ratio; OS: Overall survival; TTR: Time to recurrence.
*p-value smaller than 0.05.
†Test cohort: GI, LASS2−/TGF-β1−(n = 53); GII, LASS2−/TGF-β1 + (n = 56); GIII, LASS2+/TGF-β1-(n = 16); GIV, LASS2+/TGF-β1 + (n = 59).
Confirmation of prognostic ability in an independent validation cohort
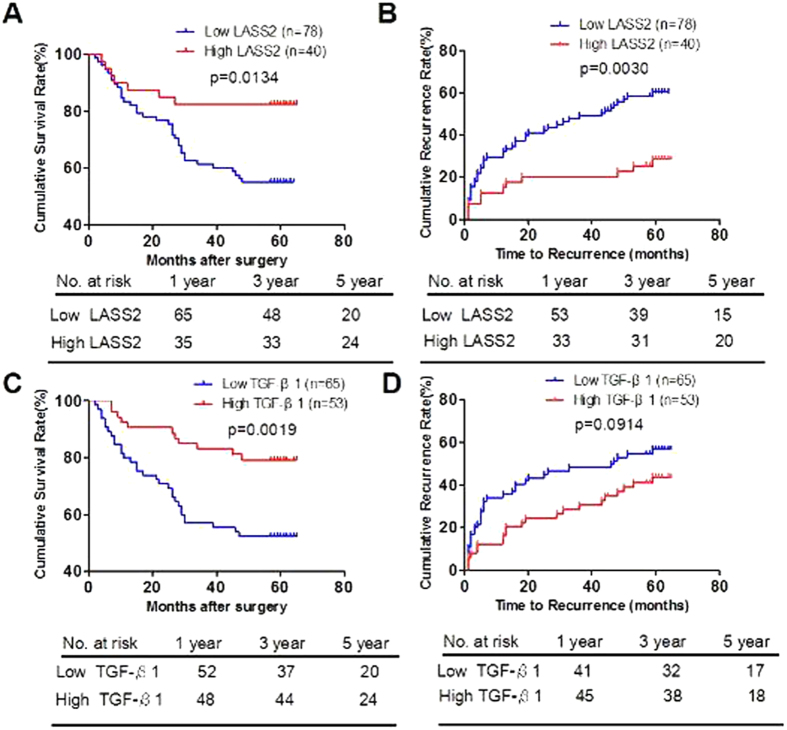
We observed similar prognostic results of LASS2 and TGF-β1 in another independent cohort consisting of 114 HCC patients after surgery. Among the 114 HCC patients of validation cohort, 40 (35.1%) and 53 (46.5%) cases had high expression of LASS2 and TGF-β1, respectively. Kaplan–Meier analysis showed that low LASS2 group had the shorter OS (median months: 44.7, 95% CI: 39.6–49.9) and TTR (median months: 36.1, 95% CI: 30.2–42.0), whereas high LASS2 group had the longrer OS (median months: 55.8, 95% CI: 49.4–62.1) and TTR (median months: 52.4, 95% CI: 45.1–59.6) (Fig. 4A,B).
Figure 4. Low expression of LASS2 and TGF-β1 significantly correlates with poor prognosis of hepatocelluar carcinoma in the validation cohort.
(A,B). Kaplan-Meier analysis of the correlation between LASS2 expression and OS (A) or TTR (B) in the validation cohort. (C,D). Kaplan-Meier analysis of the correlation between TGF-β1 expression and OS (C) or TTR (D) in the validation cohort. Log-rank tests were used to determine statistical significance.
Furthermore, we found that patients with low TGF-β1 expression also had shorter OS (42.7 versus 56.2 months, p = 0.0019, Fig. 4C) and TTR (36.4 versus 48.5 months, p = 0.0914, Fig. 4D). Importantly, low LASS2 low TGF-β1 group had the shortest OS (median months: 39.5, 95% CI: 32.8–46.3) and TTR (median months: 31.2, 95% CI: 23.5–38.8), whereas high LASS2 high TGF-β1 group had the longest OS (median months: 58.8, 95% CI: 52.1–65.5) and TTR (median months: 52.5, 95% CI: 43.4–61.6) (Fig. 5). LASS2/TGF-β1 combination was associated with OS (p = 0.001) and TTR (p = 0.001) by univariate analysis (Table 2) and was an independent predictor for both OS (p = 0.009) and TTR (p = 0.025) by multivariate analysis (Table 3).
Figure 5. The combination of LASS2 and TGF-β1 exhibited the improved prognostic value for HCC patients in the validation cohort.
(A,B). Kaplan-Meier analysis of the correlation between LASS2/TGF-β1 and OS (A) or TTR (B) in the validation cohort. GI, LASS2−/TGF-β1−(n = 49); GII, LASS2−/TGF-β1 + (n = 29); GIII, LASS2+/TGF-β1-(n = 16); GIV, LASS2+/TGF-β1 + (n = 24).
Table 3. Multivariate analyses of factors associated with survival and recurrence in hepatocelluar carcinoma patients from test and validation cohorts.
| Variables | OS |
TTR |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Test cohort | ||||
| Serum AFP (>20 vs ≤20) | 2.860(1.742–4.693) | <0.001 | 1.879(1.265–2.790) | 0.002 |
| TNM stage (I vsI I vs III–IV) | NA | NA | NA | NA |
| Child-pugh (A vs B) | NA | NA | 2.438(1.353–4.392) | 0.003 |
| Tumor size (>3 cm vs ≤3 cm) | 2.191(1.183–4.059) | 0.013 | NA | NA |
| Tumor number (multiple vs single) | 1.763(1.127–2.759) | 0.013 | 2.493(1.666–3.731) | <0.001 |
| Tumor differentiation (well vs moderate vs poor) | NA | NA | NA | NA |
| Microvascular invasion (yes vs no) | NA | NA | NA | NA |
| LASS2 (low vs high) | NA | NA | NA | NA |
| TGF-β1 (low vs high) | NA | NA | NA | NA |
| LASS2 and TGF-β1† | ||||
| GI vs GII vs GIII vs GIV | 0.753(0.631–0.898) | 0.002 | 0.705(0.603–0.824) | <0.001 |
| Validation cohort | ||||
| Liver cirrhosis (yes vs no) | 2.179(1.087–4.369) | 0.028 | 3.755(1.958–7.200) | <0.001 |
| TNM stage (I vsI I vs III–IV) | NA | NA | 1.896(1.234–2.914) | 0.004 |
| Tumor size (>3 cm vs ≤3 cm) | NA | NA | 2.719(1.304–5.671) | 0.008 |
| Tumor number (multiple vs single) | 4.036(2.149–7.580) | <0.001 | NA | NA |
| LASS2 (low vs high) | NA | NA | NA | NA |
| TGF-β1 (low vs high) | NA | NA | NA | NA |
| LASS2 and TGF-β1† | ||||
| GI vs GII vs GIII vs GIV | 0.629(0.444–0.891) | 0.009 | 0.732(0.557–0.962) | 0.025 |
Multivariate analysis: Cox proportional hazards regression model. Variables were adopted for their prognostic significance by univariate analysis.
HbeAg: Hepatitis B e-antigen; HR: Hazard ratio; NA: Not applicable; OS: Overall survival; TTR: Time to recurrence.
Discussion
Hepatocellular carcinoma is one of the most aggressive cancers featured with high rate of metastasis and mortality. Although several molecular biomarkers have been reported to have clinical significance for predicting HCC prognosis29,30,31, clinical and molecular features to be used as prognostic parameters in clinical practice are still substantially needed. Critical oncogenes or tumor suppressors involved in different stages of growth, cell cycle progression, disease initiation and responses to environmental stimuli provide important clues to identify prognostic biomarkers of cancer. LASS2 is the gene identified from a human liver cDNA library by our laboratory and interacts with proteolipid subunit of vacuolar H+ ATPase (VPL)7. A growing amount of research has reported that LASS2 mainly acts as a tumor suppressor and is closely associated with a variety of tumor progression, including prostate9, breast10, bladder11 and liver cancer12. LASS2 can induce apoptosis of prostate cancer, inhibit growth and invasion of breast cancer cell in vitro through interacting with vacuolar ATPase32,33. Our previous studies have shown that low expression of LASS2 is associated with poor prognosis in patients with breast cancer10, and deletion of LASS2 is associated with a high risk of spontaneous or DEN-induced HCC in hepatocyte-specific LASS2-KO mice models13,14. Recent research also revealed that LASS2 was the target of miR-9, and involved in the chemoresistance of bladder cancer11. In addition, it has been reported that decreased expression of LASS2 is associated with worse prognosis in meningiomas34. However, the prognostic significance of LASS2 expression in HCC is still unclear. In current study, we found that the expression of LASS2 level was negatively correlated with the tumor size, tumor differentiation, and TNM stage. Moreover, the Kaplan-Meier survival analysis showed that the OS and TTR of HCC patients with low LASS2 expression were shorter than those with high LASS2 expression. These results suggested that LASS2 may act as a critical tumor suppressor in HCC progression.
Our previous study showed that knockout of LASS2 significantly upregulated the expression of TGF-β1 in DEN-induced liver tumourigenesis of mice13. This clue strongly prompted us to further study the correlation between LASS2 and TGF-β1 in HCC patients. Interestingly, we were surprised to find that a significant positive correlation between expression of LASS2 and TGF-β1 was observed in HCC patients. This discrepancy may be mainly explained by the following points: 1) In the process of cancer development, TGF-β1 plays biphasic functions in tumor tumorigenesis, having a growth inhibitory effect at early stages, but at later stages enhancing the malignant conversion. This contradictory results may be due to the function switch of TGF-β1 in different stages of carcinogenesis, which is still largely unclear since now. 2) Although the animal models of DEN-induced HCC were used as a tool to test preventive treatments for HCC, the mice model of DEN-induced hepatocarcinogenesis and the actual process of human liver cancer are still different. For example, our study was performed mainly on hepatitis B virus (HBV)-related HCC, which is a dominant etiology of Asian HCC patients. According to the statistics, 157 of 184 (85.3%) HCC patients had positve hepatitis B surface antigen (HBsAg) in the test cohorts of this study, and 99 of 114 (86.8%) HCC patients had positve HBsAg in our validation cohort. 3) The correlation between LASS2 and TGF-β1 was firstly observed in a hepatocyte-specific LASS2-KO transgenic mouse model, which was generated using the Cre/LoxP system for site-specific excisional DNA recombination. The particular mouse model also might lead to its inconsistent with clinical results. However, the underlying mechanism remains to be further investigated.
TGF-β1 particularly achieves its tumor suppressive effect through the inhibition of proliferation and the induction of apoptosis or senescense in the early stage-tumors18,19. Consistent with a tumor-suppressor role for TGF-β1, analysis of clinical samples and tumor-derived cell lines suggested that it is a common occurrence that reduction in epithelial responsiveness to TGF-β1 during carcinogenic progression in many tissues35. It was reported that TGF-β1 can induce p53-independent and p16-independent, and reactive oxygen species (ROS)-dependent senescence arrest in well-differentiated HCC cells18. In our study, most HCC patients of our study were well and moderate differentiation. According to the statistics, 182 of 184 (98.9%) and 114 of 114 (100%) HCC patients were well and moderate differentiation in the test cohort and validation cohort, respectively. The Kaplan-Meier survival analysis also showed that the OS and TTR of HCC patients with low TGF-β1 expression were shorter than those with high TGF-β1 expression.
In the present study, we provided the evidence that high level of LASS2/TGF-β1 expression predicted a favorable OS and TTR rate for HCC patients. The 1-, 3- and 5-year OS rates of HCC patients with high level of LASS2 or TGF-β1 expression were remarkably higher than those of HCC patients with low levels of LASS2 or TGF-β1 expression, respectively. Accordingly, the 1-, 3- and 5-year TTR rates of HCC patients with high level of LASS2 or TGF-β1 expression were significantly lower than those of HCC patients with low levels of LASS2 or TGF-β1 expression, respectively. As combination of multiple markers might yield more information for predicting clinical outcome of HCC patients, combination of LASS2 and TGF-β1 expression were therefore used as a predictor of clinical outcome. In both test cohort and validation cohort, HCC patients with both high expression of LASS2 and TGF-β1 had the most favorable OS and TTR rates, whereas those patients with both low expression of LASS2 and TGF-β1 had the poorest OS and TTR rates. Besides, we used univariate and multivariate analyses to further study the prognostic value of LASS2 and TGF-β1, the results showed that combination of LASS2 and TGF-β1 was significant and independent prognostic factor for HCC patients, but either LASS2 or TGF-β1 was not.
In conclusion, our study demonstrated that high levels of LASS2 expression and TGF-β1 expression have a positive relationship with prognosis of HCC patients. The combination of LASS2 and TGF-β1 was more sensitive than either of them alone with respect to OS and TTR, and could be used as a new independent prognostic marker of HCC patients.
Materials and Methods
Patients and tissue samples
This study involved two independent cohorts of patients with HCC: test cohort (184 HCC patients) and validation cohort (118 HCC patients), obtained from Eastern Hepatobiliary Surgery Hospital (Shanghai, PR China) between January 2003 and April 2006. For suspected cases with elevated serum AFP level (>20 ng/ml as positive), computed tomography (CT) and/or magnetic resonance imaging (MRI) were used to verify tumor recurrence. No patient received either radiotherapy or chemotherapy before the surgery, and no other cancers co-occurrence. The overall survival (OS) was defined as the length of time between the surgery and death or the last follow-up examination. The time to recurrence (TTR) was calculated from the date of tumor resection until the detection of tumor recurrence, death or the last observation. Tumor stage was defined according to the American Joint Committee on Cancer (AJCC 2010, 7th edition) TNM staging system36. The grade of tumor differentiation was assigned by the Edmondson-Steiner grading system. Micrometastases were defined as tumors adjacent to the border of the main tumor that was only observed under the microscope37.
For the use of clinical materials for research purposes, prior patients’ consents and approval were obtained from the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine and EHBH of the Second Military Medical University. Written informed consent was obtained from all subjects for publication of this study. All experiments were performed in accordance with approved guidelines of Shanghai Jiao Tong University School of Medicine.
Immunohistochemistry and Scoring
HCC tumor tissues were resected from the patients, and then were fixed with 10% formalin and embedded in paraffin before prepared for tissue microarray. Briefly, 1-mm cores were taken from intratumoral tissue and serial sections (4-μm thick) were placed on slides coated with 3-aminopropyltriethoxysilane. The immunohistochemistry analysis was conducted as described previously30. Immunostaining scores were independently evaluated by two pathologists who were blinded to the clinical outcome. The sections were incubated with primary goat anti-human LASS2 polyclonal antibody (clone sc-65102, 1:80, Santa Cruz Biotechnology)38 and rabbit anti-human TGF-β1 polyclonal antibody (18978-1-AP, 1:100, Proteintech)39 at 4 °C overnight, and then secondary antibody within 30 minutes. The average sum of integrated optical density (IOD) of each sample was calculated using ImageJ software.
Statistical Analysis
Differences among variables were assessed by χ2 analysis or two-tailed Student t test. Kaplan-Meier analysis was used to assess survival. Log-rank tests were used to compare survival of patients between subgroups. Multivariate analyses were performed by multivariate Cox proportional hazard regression model. Data were presented as mean ± SEM. Differences were considered to be statistically significant for p < 0.05.
Additional Information
How to cite this article: Ruan, H. et al. Co-expression of LASS2 and TGF-β1 predicts poor prognosis in hepatocellular carcinoma. Sci. Rep. 6, 32421; doi: 10.1038/srep32421 (2016).
Acknowledgments
This work was supported by grants from National Key Basic Research Program of China (973 Program: 2015CB553905), National Natural Science Foundation of China (81371883, 81572311), National Key Sci-Tech Special Project of China (2012ZX10002011-004), projects of Special Research Fund for Healthy (201402003), the Shanghai Natural Science Foundation of China (16ZR1434700),Shanghai Jiao Tong University School of Medicine (YG2015QN34,YG2014MS44), State Key Laboratory of Oncogenes and Related Genes (SB16-04) and Key Discipline and Specialty Foundation of Shanghai Municipal Commission of Health and Family Planning.
Footnotes
Author Contributions H.J. and W.Q. designed the study; H.R., T.W., C.Y. and D.G. collected the tissue samples; G.J., X.D. and C.W. performed and evaluated the IHC analysis; H.J., H.R. and G.J. collected clinical data and participated in the evaluation of the IHC data; H.R. and H.J. drafted the manuscript; H.J. and W.Q. supervised the study. All authors read and approved the final manuscript.
References
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- El-Serag H. B. Hepatocellular carcinoma. N Engl J Med 365, 1118–1127 (2011). [DOI] [PubMed] [Google Scholar]
- Ganapathy-Kanniappan S., Kunjithapatham R. & Geschwind J. F. Glyceraldehyde-3-phosphate dehydrogenase: a promising target for molecular therapy in hepatocellular carcinoma. Oncotarget 3, 940–953 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. B. & Zhu A. X. Hepatocellular carcinoma: the need for progress. J Clin Oncol 23, 2892–2899 (2005). [DOI] [PubMed] [Google Scholar]
- Ueno M. et al. Adjuvant chemolipiodolization reduces early recurrence derived from intrahepatic metastasis of hepatocellular carcinoma after hepatectomy. Ann Surg Oncol 18, 3624–3631 (2011). [DOI] [PubMed] [Google Scholar]
- Kamiyama T. et al. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol 16, 1560–1571 (2009). [DOI] [PubMed] [Google Scholar]
- Pan H. et al. Cloning, mapping, and characterization of a human homologue of the yeast longevity assurance gene LAG1. Genomics 77, 58–64 (2001). [DOI] [PubMed] [Google Scholar]
- Laviad E. L. et al. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem 283, 5677–5684 (2008). [DOI] [PubMed] [Google Scholar]
- Xu X., You J. & Pei F. Silencing of a novel tumor metastasis suppressor gene Lass2/TMSG1 promotes invasion of prostate cancer cell in vitro through increase of vacuolar ATPase activity. J Cell Biochem 113, 2356–2363 (2012). [DOI] [PubMed] [Google Scholar]
- Fan S. et al. LASS2 enhances chemosensitivity of breast cancer by counteracting acidic tumor microenvironment through inhibiting activity of V-ATPase proton pump. Oncogene 32, 1682–1690 (2013). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour Biol 36, 9631–9640 (2015). [DOI] [PubMed] [Google Scholar]
- Imgrund S. et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem 284, 33549–33560 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. et al. Enhancement of DEN-induced liver tumourigenesis in hepatocyte-specific Lass2-knockout mice coincident with upregulation of the TGF-β1-Smad4-PAI-1 axis. Oncol Rep 31, 885–893 (2014). [DOI] [PubMed] [Google Scholar]
- Lu X. et al. Knockout of the HCC suppressor gene Lass2 downregulates the expression level of miR-694. Oncol Rep 32, 2696–2702 (2014). [DOI] [PubMed] [Google Scholar]
- Derynck R. & Zhang Y. E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584 (2003). [DOI] [PubMed] [Google Scholar]
- Pickup M., Novitskiy S. & Moses H. L. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 13, 788–799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi S. K. et al. Role of TGF-β signaling in uterine carcinosarcoma. Oncotarget 6, 14646–14655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senturk S. et al. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 52, 966–974 (2010). [DOI] [PubMed] [Google Scholar]
- Lin H. K., Bergmann S. & Pandolfi P. P. Cytoplasmic PML function in TGF-beta signalling. Nature 431, 205–211 (2004). [DOI] [PubMed] [Google Scholar]
- Forrester E. et al. Effect of conditional knockout of the type II TGF-β receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res 65, 2296–2302 (2005). [DOI] [PubMed] [Google Scholar]
- Tian F. et al. Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res 64, 4523–4530 (2004). [DOI] [PubMed] [Google Scholar]
- Wakefield L. M. & Roberts A. B. TGF-β signaling: Positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev 12, 22–29 (2002). [DOI] [PubMed] [Google Scholar]
- Massagué J. TGFbeta in Cancer. Cell 134, 215–230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulmeester E. & Ten Dijke P. The dynamic roles of TGF-β in cancer. J Pathol 223, 205–218 (2011). [DOI] [PubMed] [Google Scholar]
- Siegel P. M. & Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 3, 807–821 (2003). [DOI] [PubMed] [Google Scholar]
- Boulanger C. A., Wagner K. U. & Smith G. H. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene 24, 552–560 (2005). [DOI] [PubMed] [Google Scholar]
- Acosta J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15, 978–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E. et al. Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous stem cell proliferation. Nat Med 20, 350–359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. D. et al. Overexpression Of Hepatocyte Nuclear Factor-1beta Predicting Poor Prognosis Is Associated With Biliary Phenotype In Patients With Hepatocellular Carcinoma. Sci Rep 5, 13319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H. et al. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci Rep 5, 10466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. H. et al. TMPRSS4 facilitates epithelial-mesenchymal transition of hepatocellular carcinoma and is a predictive marker for poor prognosis of patients after curative resection. Sci Rep 5, 12366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F. et al. LASS2/TMSG1 inhibits growth and invasion of breast cancer cell in vitro through regulation of vacuolar ATPase activity. Tumour Biol 36, 2831–2844 (2015). [DOI] [PubMed] [Google Scholar]
- Yu W. et al. A novel tumor metastasis suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through its homeodomain. J Cell Biochem 114, 570–583 (2013). [DOI] [PubMed] [Google Scholar]
- Ke R. H. et al. Decreased expression of LASS2 is associated with worse prognosis in meningiomas. J Neurooncol 118, 369–376 (2014). [DOI] [PubMed] [Google Scholar]
- Fynan T. M. et al. Resistance to inhibition of cell growth by transforming growth factor-beta and its role in oncogenesis. Crit Rev Oncog 4, 493–540 (1993). [PubMed] [Google Scholar]
- Edge S. B. & Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17, 1471–1474 (2010). [DOI] [PubMed] [Google Scholar]
- Bruix J. et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35, 421–430 (2001). [DOI] [PubMed] [Google Scholar]
- Xu X. et al. Silencing of LASS2/TMSG1 enhances invasion and metastasis capacity of prostate cancer cell. J Cell Biochem. 115, 731–743 (2014). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Mucin1 mediates autocrine transforming growth factor beta signaling through activating the c-Jun N-terminal kinase/activator protein 1 pathway in human hepatocellular carcinoma cells. Int J Biochem Cell Biol. 59, 116–125 (2015). [DOI] [PubMed] [Google Scholar]