Abstract
Objective
The aim of this study was to investigate the effect of neutrophil-to-lymphocyte ratio on the prognosis of patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy.
Methods
One hundred and twenty-five patients with locoregionally advanced laryngeal carcinoma (cT3–4 N0–3M0) treated with chemoradiotherapy were reviewed retrospectively. Chemoradiotherapy comprised external beam radiotherapy to the larynx (70 Gy) with three cycles of cisplatin at 3 week intervals. The survival rate was calculated using the Kaplan–Meier method, and a multivariate analysis was used to identify significant factors associated with prognosis, using a Cox proportional hazards model.
Results
During the median (range) follow-up of 45 months, the median neutrophil-to-lymphocyte ratio was 3.02. The high neutrophil-to-lymphocyte ratio group (neutrophil-to-lymphocyte ratio > 3.0) contained 69 patients and the low neutrophil-to-lymphocyte ratio group (neutrophil-to-lymphocyte ratio < 3.0) contained 46 patients. The low neutrophil-to-lymphocyte ratio group patients had a significantly higher chemoradiotherapeutic disease control rate (86.96 vs. 69.57%, P = 0.031). Forty-six patients had a low neutrophil-to-lymphocyte ratio (<3.0) before chemoradiotherapy and their progression-free survival and 75% overall survival were significantly better than that of the high neutrophil-to-lymphocyte ratio patients (P = 0.015, P = 0.045). Multivariate analysis showed that neutrophil-to-lymphocyte ratio and N stage were independent prognostic indicators for progression-free survival (with a hazard ratio of 1.79, P = 0.003 and a hazard ratio of 1.28, P = 0.034) and overall survival (with a hazard ratio of 1.51, P = 0.029 and a hazard ratio of 1.21, P = 0.043), respectively.
Conclusion
Pre-treatment neutrophil-to-lymphocyte ratio is a useful prognostic marker in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy.
Keywords: laryngeal carcinoma, neutrophil-to-lymphocyte ratio, chemoradiotherapy, progression-free survival
Introduction
Conventional management approaches to locoregionally advanced laryngeal cancer have included radical surgery and adjuvant radiotherapy for resectable disease and radical radiotherapy for unresectable disease. Treatment outcomes with these approaches have been generally poor. Recent developments in improving disease control, survival and functional outcome in these patients have focused upon combining radiotherapy with chemotherapy (1).
Radiotherapy with concurrent chemotherapy should be considered standard care for patients desiring laryngeal preservation whose cancer is within the categories of disease, and laryngectomy should be performed only as salvage therapy. So chemoradiotherapy is the standard regimen in the treatment of locoregionally advanced laryngeal carcinoma (2).
However, in some patients the disease progressed after chemoradiotherapy within a few years. Therefore, identifying a prognostic factor for this would allow a better therapeutic approach to patients with locoregionally advanced laryngeal carcinoma.
Increasing evidence suggests that inflammatory cells are an essential component of the tumor microenvironment and have a role in tumor progression (3,4). Tumor cells often constitutively produce several inflammatory chemokines, including neutrophil-attracting cysteine-X-cysteine chemokines (3). Markers of inflammation, such as the neutrophil-to-lymphocyte ratio (NLR), and their clinical significance in renal cell carcinoma patients are still under evaluation. NLR is an easily measurable parameter of systemic inflammation. Increased pre-treatment NLR has been demonstrated to be associated with poor outcome for various types of cancers, including advanced pancreatic cancer (5), colorectal liver metastases (6) non-small cell lung cancer (7), gastric cancer (8) and soft-tissue sarcoma (9).
The aim of the present study was to examine the effect of NLR on the prognosis of patients with locoregionally advanced laryngeal carcinoma. To our knowledge, this is the first study to show the prognostic effect of NLR in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy.
Patients and methods
Study population
Medical records from patients diagnosed with locoregionally advanced laryngeal carcinoma (UICC stage T3–4,N0–3,M0) treated with chemoradiotherapy in our institution between January 2007 and December 2013 were retrospectively reviewed. All patients were histologically confirmed (squamous cell carcinoma) and underwent chemoradiotherapy. The inclusion criteria were as follows: (i) age 18–70 years; (ii) UICC stage T3–4,N0–3,M0; (iii) at least one measurable or evaluable lesion; (iv) Eastern Cooperative Oncology Group (ECOG) performance status ≤2; (v) no prior antitumor treatment, such as chemotherapy, surgery or radiotherapy; (vi) life expectancy ≥12 weeks; (vii) adequate bone marrow function (absolute neutrophil count ≥1.5 × 109/l and platelet count ≥100 × 109/l); (viii) adequate renal function (serum creatinine <1.5 mg/dl or calculated creatinine clearance ≥60 ml/min); and (ix) adequate hepatic function [total bilirubin level ≤2 times institutional upper limit of normal (ULN) and serum transaminase level ≤3 times the institutional ULN]. Those who did not fit the inclusion criteria were excluded. Finally, 125 patients were enrolled in this study.
Peripheral blood parameters
Venous blood was sampled one day before the first chemoradiotherapy treatment and collected in ethylenediaminetetraacetic acid-containing tubes. Blood cell counts were analyzed using The XE-2100 Automated Hematology System (Model, XE2100, Sysmex Co., Kobe, Japan). The normal range of white blood cell count was from 4000 to 10 000 cells/mm3 (4–10 × 109/l). Baseline NLR was calculated as neutrophil count divided by lymphocyte count. The patients were dichotomized at the median value of NLR. The cut-off value for abnormal NLR was set at the median value of NLR (8,10). The choice of the median value of NLR as the cut-off value is acceptable in some similar studies (8,10). The cut-off value for abnormal hemoglobin was set at the median value of hemoglobin, which was just as NLR.
Treatments and response evaluation
The NLR (a single value for each patient) was defined as that estimated at dawn before treatment; this value was unavailable in 10 patients. Consequently, 115 patients comprised the study group; at diagnosis, none of these patients had any inflammatory disease. Chemoradiotherapy comprised external beam radiotherapy (70 Gy) and cisplatin-based chemotherapy. All patients underwent three cycles of chemotherapy (two cycles of concurrent chemotherapy and one cycle of chemotherapy after radiotherapy) after diagnosis. Sixty-two patients received fluorouracil and cisplatin (5-fluorouracil at a dose of 750 mg/m2 by 24 h continuous infusion for 5 days, cisplatin 75 mg/m2 on Day 1 for three cycles with a 21-day interval), and 24 were treated with cisplatin or carboplatin and docetaxel (cisplatin 75 mg/m2 on Day 1 or carboplatin (AUC = 4), docetaxel 75 mg/m2 Day 1 for three cycles with a 21-day interval). The other 29 patients had been treated with a combination of carboplatin and paclitaxel regimens [carboplatin (AUC = 4) on Day 1, paclitaxel 175 mg/m2 Day 1 for three cycles with a 21-day interval]. All patients received external beam radiation therapy (RT) on a linear accelerator (Siemens ONCOR Medical Linear Accelerator) at 100 cm source–axis distance with the appropriate technique of bilateral parallel opposed portals, to cover the primary site and the upper neck nodes. In principle, radiation to the larynx was given to a dose of 70 Gy with the daily fractional dose of 1.8 or 2 Gy in a 5-day week. The duration was 7–9 weeks. The lower neck and supraclavicular nodes were treated with a matched anterior field (60–70 Gy). The radiation dose was reduced in four patients (3.5%) due to acute larynx toxicity (median total dose 50 Gy; range 45.0–65.0).
The tumor response was assessed 1 month after treatment. We adopted the response evaluation criteria in solid tumors as follows: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) (14). Objective response was defined as CR or PR, while disease control was defined as CR, PR or SD. Response was analyzed by intention-to-treat analysis.
Progression-free survival (PFS) was defined as the duration of time from the date of the initial chemoradiotherapy to the date of the first sign of tumor progression or death. Overall survival (OS) was defined as the period from the date of initial chemoradiotherapy to the date of death. Patients who were lost to follow-up were censored. PFS and OS were calculated and analyzed using the Kaplan–Meier method and log-rank test. Univariate and multivariate analysis used the Cox proportional hazards model, with factors before chemoradiotherapy including age, sex, tumor stage, nodal status, tumor site, regimen of chemotherapy, performance status, treatment after chemoradiotherapy, treatment response and NLR. In all statistical tests the significance level was determined at P < 0.05.
This study is approved by the independent institutional review board of our center and the study protocol is consistent with the ethical guidelines of the 1975 Declaration of Helsinki.
Results
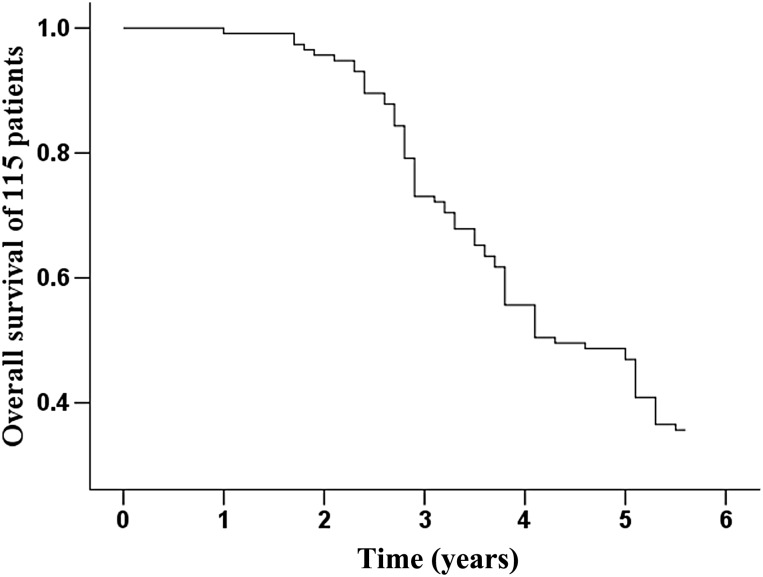
Baseline characteristics of the 115 patients are shown in Table 1. During the median (range) follow-up of 45 (3–66) months, 74 patients (64.35%) died from laryngeal cancer. The 5-year survival rate was 35.65%, as shown in Fig. 1. The median NLR was 3.02 (range 0.16–16.37). We determined the cut-off value of NLR for prognostic stratification. Based on the median value of NLR, the patients were divided into two groups (Table. 1): the low NLR group (NLR ≤ 3.0) and the high NLR group (NLR > 3.0). The high NLR group had a higher N stage than the low NLR group (P < 0.001). The median hemoglobin was 125 g/l (range 109–157). We determined the cut-off value of hemoglobin for prognostic analysis. Other clinicopathological characteristics were not significantly different between the two groups.
Table 1.
The relationship between NLR and clinical characteristics of patients treated with chemoradiotherapy
| Variable | NLR (≤3.0 or >3.0) |
P | |
|---|---|---|---|
| Group | Low (n = 46) | High (n = 69) | |
| Mean age in years | 57 (50–75) | 58 (45–73) | |
| Male | 26 (56.52) | 38 (55.07) | |
| Female | 20 (43.48) | 31 (44.93) | 0.878 |
| T stage | |||
| T3 | 32 (69.57) | 44 (63.77) | |
| T4 | 14 (30.43) | 25 (36.23) | 0.520 |
| N stage | |||
| N0 | 10 (21.74) | 17 (24.64) | |
| N1 | 18 (39.13) | 31 (44.92) | |
| N2 | 15 (32.61) | 15 (21.74) | |
| N3 | 3 (6.52) | 6 (8.70) | <0.01 |
| Tumor site | |||
| Glottic | 26 (56.52) | 45 (65.22) | |
| Supraglottic | 15 (32.61) | 17 (24.64) | |
| Subglottic | 5 (10.87) | 7 (10.14) | 0.612 |
| Performance status | |||
| 0 | 7 (15.22) | 10 (14.49) | |
| 1 | 35 (76.09) | 50 (72.47) | |
| 2 | 4 (8.70) | 9 (13.04) | 0.771 |
| Chemotherapy regimens | |||
| Fluorouracil and cisplatin | 29 (63.04) | 33 (47.83) | |
| Cisplatin or carboplatin and docetaxel | 9 (19.57) | 15 (21.74) | |
| Carboplatin and paclitaxel | 8 (17.39) | 21 (30.43) | 0.211 |
| Treatment after chemoradiotherapy | |||
| Neck dissection | 5 (10.87) | 12 (17.39) | |
| Salvage laryngectomy | 2 (4.35) | 6 (8.70) | 0.990 |
| Treatment response | |||
| RR (response rate) | 31 (67.39) | 39 (56.52) | |
| No RR | 15 (32.61) | 30 (43.48) | 0.242 |
NLR, neutrophil-to-lymphocyte ratio.
Figure 1.
Overall survival of 115 patients.
Chemoradiotherapeutic response
The distribution of the chemoradiotherapeutic response after the treatment with reference to NLR subgroup is shown in Table 2. Overall, 9 (7.76%) and 61 (53.04%) patients had CR and PR, while 18 (15.65%) and 27 (23.48%) patients had SD and PD, respectively. The objective response rates (RR, CR + PR) were not different according to the NLR (67.39 vs. 56.52%, P = 0.242). However, the low NLR group had a significantly higher disease control rate (CR + PR + SD) than the high NLR group (86.96 vs.69.57%, P = 0.031). Local or locoregional residual represented the major patterns of treatment failure. For those patients who did not obtain CR, 8 patients had salvage laryngectomies and 17 patients had neck dissections after chemoradiotherapy.
Table 2.
Treatment response to chemoradiotherapy according to NLRa
| Response | Total patients (n = 115) | Low NLR group (n = 46) | High NLR group (n = 69) |
|---|---|---|---|
| Complete response | 9 (7.83) | 4 (8.70) | 5 (7.25) |
| Partial response | 61 (53.04) | 27 (58.70) | 34 (49.28) |
| Stable disease | 18 (15.65) | 9 (19.57) | 9 (13.04) |
| Progressive disease | 27 (23.48) | 6 (13.03) | 21 (30.43) |
aP = 0.031 for disease control rate between the low NLR group and the high NLR group.
Treatment compliance
The completion rate of RT was 100%, the median duration of RT was 8.2(7.0–9.0) weeks, the radiation dose was reduced in four patients (3.5%) and the delay of chemotherapy was present in 11 patients (9.6%, mostly in the third cycle of chemotherapy).
Progression-free and overall survival
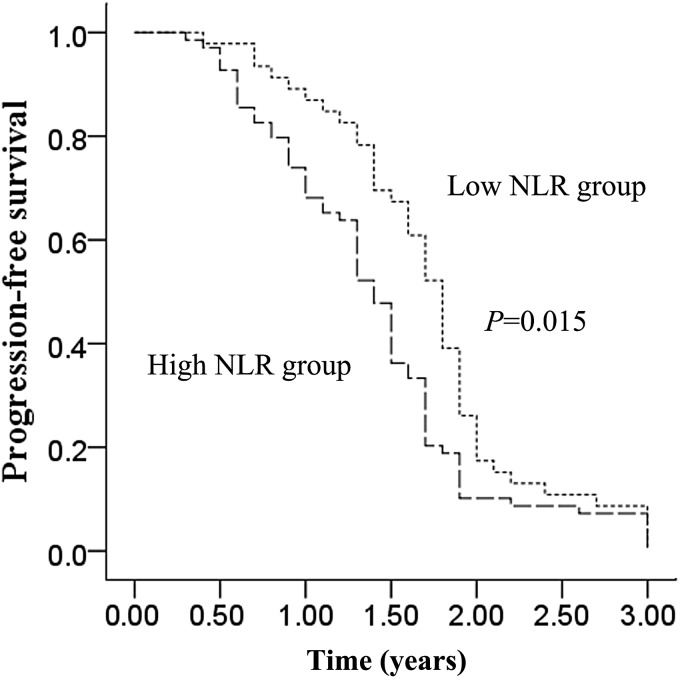
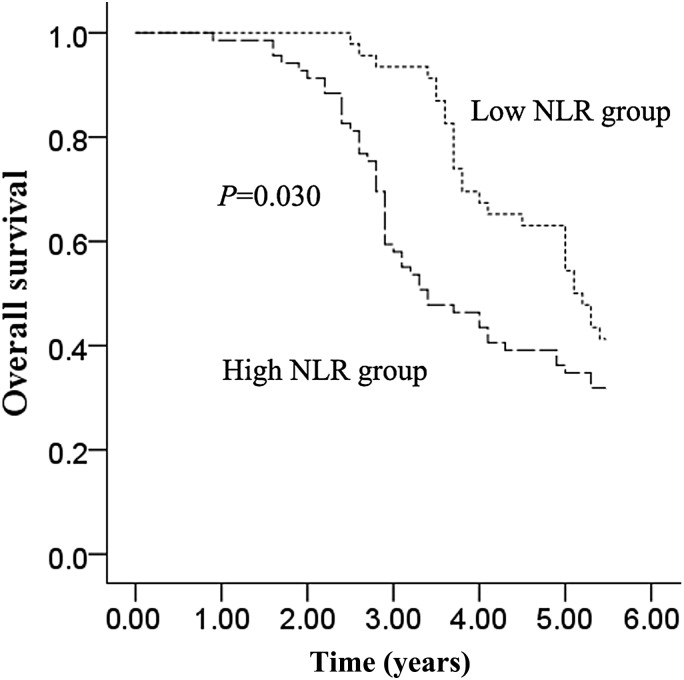
The median PFS (all patients progressed and none were censored) and OS were1.5 years [95% confidence interval (CI), 1.35–1.65] and 4.3 years (95% CI, 3.46–5.14), respectively. Median PFS was longer in the low NLR group than in the high NLR group [1.8 years (95% CI, 1.67–1.93) vs. 1.4 years (95% CI, 1.27–1.53), P = 0.015; Fig. 2.]. Moreover, the median OS was also longer in the low NLR group than in the high NLR group (5.1 years (95% CI, 4.51–5.69) vs. 3.4 years (95% CI, 2.49–4.32), P = 0.030; Fig. 3.). For OS analysis, there were no censoring data in both arms. We took 5.5 years as the follow-up endpoint for both arms. Clinicopathological variables for prediction of prognosis were tested in univariate and multivariate analyses (Tables 3 and 4). Univariate predictors of PFS were N stage (P = 0.013), tumor site (P = 0.017) and NLR (P = 0.026). In multivariate analysis, NLR [hazard ratio (HR) 1.79, 95% CI, 1.21–2.64; P = 0.003] and N stage (HR 1.28, 95% CI, 1.02–1.60; P = 0.034) were also independent predictors of PFS. Univariate predictors of OS were N stage (P = 0.011), tumor site (P = 0.045) and NLR (P = 0.020). In multivariate analysis, NLR (HR 1.51, 95% CI, 1.04–2.20; P = 0.029) and N stage (HR 1.21, 95% CI, 1.01–1.45; P = 0.043) were independent predictors of OS.
Figure 2.
Progression-free survival of the low neutrophil-to-lymphocyte ratio (NLR) group and the high NLR group.
Figure 3.
Overall survival of the low NLR group and the high NLR group.
Table 3.
Univariate and multivariate analyses of PFS of patients treated with chemoradiotherapy
| Variable | Patient number (n) | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years ≥60 vs.<60) | 55/60 | 0.74 | 0.51–1.08 | 0.122 | |||
| Male vs. female | 64/51 | 0.79 | 0.54–1.14 | 0.208 | |||
| T stage (T3 vs. T4) | 76/39 | 1.22 | 0.81–1.84 | 0.345 | |||
| N stage (N0 vs. N1 vs. N2 vs. N3) | 27/49/30/9 | 1.28 | 1.05–1.55 | 0.013 | 1.28 | 1.02–1.60 | 0.034 |
| Tumor site (glottic vs. supraglottic vs. subglottic) | 71/32/12 | 1.54 | 1.08–2.18 | 0.017 | 1.36 | 0.92–2.02 | 0.127 |
| Performance status (0 vs. 1 vs. 2) | 17/85/13 | 1.25 | 0.96–1.64 | 0.099 | |||
| Chemotherapy regimen (carboplatin and paclitaxel vs. cisplatin or carboplatin and docetaxel vs. fluorouracil and platinum) | 62/24/29 | 1.16 | 0.92–1.47 | 0.218 | |||
| Treatment after chemoradiotherapy (neck dissection vs. salvage laryngectomy vs. none) | 17/8/90 | 0.83 | 0.62–1.12 | 0.219 | |||
| Hemoglobin (≤125 g/L vs >125 g/L) | 21/94 | 1.24 | 0.89–1.77 | 0.458 | |||
| NLR(≤3.0 vs >3.0) | 46/69 | 1.52 | 1.05–2.21 | 0.026 | 1.79 | 1.21–2.64 | 0.003 |
HR, hazard ratio.
Table 4.
Univariate and multivariate analysis of OS of patients treated with chemoradiotherapy
| Variable | Patient number (n) | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | |||||
| Age (years ≥60 vs. <60) | 55/60 | 0.83 | 0.56–1.22 | 0.330 | |||
| Male vs. female | 64/51 | 0.88 | 0.61–1.27 | 0.502 | |||
| T stage (T3 vs. T4) | 76/39 | 1.08 | 0.72–1.62 | 0.716 | |||
| N stage (N0 vs. N1 vs. N2 vs. N3) | 27/49/30/9 | 1.26 | 1.05–1.50 | 0.011 | 1.21 | 1.01–1.45 | 0.043 |
| Tumor site (glottic vs. supraglottic vs. subglottic) | 71/32/12 | 1.33 | 1.01–1.74 | 0.045 | 1.21 | 0.92–1.61 | 0.171 |
| Performance status (0 vs. 1 vs. 2) | 17/85/13 | 1.29 | 0.97–1.71 | 0.084 | |||
| Chemotherapy regimen (carboplatin and paclitaxel vs. cisplatin or carboplatin and docetaxel vs. fluorouracil and platinum) | 62/24/29 | 1.03 | 0.81–1.30 | 0.813 | |||
| Treatment after chemoradiotherapy (neck dissection vs. salvage laryngectomy vs. none) | 17/8/90 | 0.84 | 0.62–1.14 | 0.268 | |||
| NLR (≤3 vs. >3) | 46/69 | 1.55 | 1.07–2.25 | 0.020 | 1.51 | 1.04–2.20 | 0.029 |
Discussion
To our knowledge this is the first study to show the prognostic effect of NLR in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. This study shows that pre-treatment NLR in the peripheral blood of patients with locoregionally advanced laryngeal carcinoma can be used as a prognostic marker to predict chemoradiotherapeutic response and survival time. In the present study, NLR and N stage before chemoradiotherapy predicted a poor prognosis in these patients.
In recent decades, the understanding of the inflammatory microenvironment of cancer has increased rapidly, and there is growing interest in the relationship between inflammation and cancer. Recently reported predictive inflammatory markers include interleukin-6 (11), NLR (10) and CRP levels (12). NLR reflects systemic inflammation and previous results support the use of NLR as predictive inflammatory markers and as an independent predictor of clinical benefit in various cancer patients (5–8).
Our study indicates that a longer PFS (P = 0.015; Fig. 2) and OS (P = 0.030; Fig. 3) in the low NLR group than in the high NLR group. In addition, NLR showed a significant relationship with both PFS and OS in multivariate analysis. Similar to our findings, previous studies also showed that high baseline NLR predicts poor survival in patients with nasopharyngeal carcinoma (10). These studies indicated that NLR could be a simple and reliable prognostic factor for risk stratification and might provide better treatment allocation in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. There are several possible explanations for the association between elevated NLR and poor cancer prognosis. One explanation is decreased lymphocyte count and function. Lymphocytes are a crucial component of the host's cellular adaptive immunity against cancer cells and can attack cancer cells and eliminate nascent tumor cells (13). Patients with lymphocyte infiltration around the tumor may have a better prognosis than those without infiltration (14), and a clinical study has demonstrated that a low total lymphocyte count can be used as an index of an adverse outcome in non-small cell lung cancer (15). Another possible explanation is increased neutrophil number and function. There is some evidence to suggest that neutrophils may actually promote cancer cell growth and metastasis and/or suppress lymphocyte activity (16). Circulating neutrophils have been shown to contain and secrete pro-angiogenic factors including vascular endothelial growth factor (VEGF). Therefore, an elevated neutrophil count stimulates tumor angiogenesis and aids the progression of neoplasm (17,18).
Interestingly, in the present study, the low NLR group had a significantly higher disease control rate than the high NLR group (86.96 vs. 69.57%, P = 0.031). Although prediction of chemoradiotherapeutic response in advance cancer patients is important, there are insufficient data on novel markers predicting chemoradiotherapeutic response. In this study, low NLR was associated with improved clinical benefit and the NLR was confirmed to independently predict PFS and OS in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy.
Therefore, NLR could be a practical adjunct in stratification before and after treatment of patients with locoregionally advanced laryngeal carcinoma. Moreover, in assessing NLR elevation, the presence of other inflammatory disease should be considered because NLR is a nonspecific inflammatory marker (19). However, measuring the NLR level is simple and of low cost. Therefore, despite this weak sensitivity, NLR could be routinely measured as a practical clinical marker in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Higher pre-treatment serum NLR may also identify candidates for more aggressive surveillance and treatment.
However, this study has a relatively small sample size and retrospective design. Therefore, the results of our study should be confirmed through a large-scale, prospective study. The complete response of this study is low. The following reasons might affect the disease response. There was a high proportion of cases with more advanced T stage (T4, 39/115) and N stage (N2–3, 39/115) in this study. The conventional radiotherapy having more side effects might lengthen the duration of treatment, which might affect the disease response. The time from confirmed diagnosis to the start of treatment is important for response evaluation. But in this study, these data were not available, which might be a limitation of this study. In conclusion, pre-treatment NLR is a useful prognostic indicator in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81201803).
Conflicts of interest statement
None declared.
References
- 1.Mantz CA, Vokes EE, Kies MS et al. Sequential induction chemotherapy and concomitant chemoradiotherapy in the management of locoregionally advanced laryngeal cancer. Ann Oncol 2001;12:343–7. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091–8. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 5.An X, Ding PR, Li YH et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516–22. [DOI] [PubMed] [Google Scholar]
- 6.Kishi Y, Kopetz S, Chun YS et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol 2009;16:614–22. [DOI] [PubMed] [Google Scholar]
- 7.Sarraf KM, Belcher E, Raevsky E et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425–8. [DOI] [PubMed] [Google Scholar]
- 8.Cho IR, Park JC, Park CH et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014;17:703–10. [DOI] [PubMed] [Google Scholar]
- 9.Szkandera J, Pichler M, Liegl-Atzwanger B et al. The elevated pre-operative plasma fibrinogen level is an independent negative prognostic factor for cancer-specific, disease-free and overall survival in soft-tissue sarcoma patients. J Surg Oncol 2014;109:1395–144. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Ye X, He C et al. Pretreatment neutrophil-to-lymphocyte ratio as predictor of survival for patients with metastatic nasopharyngeal carcinoma. Head Neck 2015;37:69–75. [DOI] [PubMed] [Google Scholar]
- 11.Duffy SA, Taylor JM, Terrell JE et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008;113:750–7. [DOI] [PubMed] [Google Scholar]
- 12.Zeng YC, Xue M, Chi F et al. C-reactive protein level predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Tumour Biol 2012;33:891–5. [DOI] [PubMed] [Google Scholar]
- 13.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 2006;90:1–50. [DOI] [PubMed] [Google Scholar]
- 14.Martinet L, Garrido I, Filleron T et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011;71:5678–87. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi N, Usui S, Kikuchi S et al. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer 2012;75:223–7. [DOI] [PubMed] [Google Scholar]
- 16.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Carlo E, Forni G, Musiani P. Neutrophils in the antitumoral immune response. Chem Immunol Allergy 2003;83:182–203. [DOI] [PubMed] [Google Scholar]
- 18.Shamamian P, Schwartz JD, Pocock BJ et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol 2001;189:197–206. [DOI] [PubMed] [Google Scholar]
- 19.Bakirci EM, Topcu S, Kalkan K et al. The role of the nonspecific inflammatory markers in determining the anatomic extent of venous thromboembolism. Clin Appl Thromb Hemost 2015;21:181–5. [DOI] [PubMed] [Google Scholar]