Abstract
Background. Sofosbuvir-containing regimens have been approved for treatment of hepatitis C virus (HCV) infection in human immunodeficiency virus (HIV)-infected patients. We assessed the effect of treatment with sofosbuvir and ribavirin on patient-reported outcomes (PROs) in individuals with HIV/HCV coinfection.
Methods. HIV/HCV-coinfected patients were treated for 12 or 24 weeks with sofosbuvir and ribavirin. Matched HCV-monoinfected controls were also evaluated. All subjects completed standard PRO questionnaires before, during, and after treatment.
Results. Included were 497 participants from the PHOTON-1 and PHOTON-2 clinical trials. At baseline, more impairment in PRO scores was noted in HIV/HCV-coinfected patients, compared with HCV-monoinfected patients. During treatment, moderate decrements in PRO scores (change, up to −6.8% on a 0%–100% scale; P = .0053) were experienced regardless of treatment duration and were similar to those for HCV-monoinfected patients (all P > .05). In 413 HIV/HCV-coinfected patients with a virologic response sustained for 12 weeks after treatment cessation, most PRO scores improved (change, up to +7.6%; P < .0001), similar to findings for HCV-monoinfected patients. In multivariate analysis, in addition to clinico-demographic predictors, coinfection with HIV was associated with PRO impairment at baseline (beta, up to −7.6%; P < .002) but not with treatment-emergent changes in PRO scores (all P > .05).
Conclusions. Patients with HIV/HCV coinfection tolerate interferon-free sofosbuvir-based anti-HCV regimens well and, despite the presence of some baseline impairment, have treatment-emergent changes in PRO scores that are similar to those of patients with HCV monoinfection.
Clinical Trials Registration. NCT01667731 (PHOTON-1), NCT01783678 (PHOTON-2), NCT01604850 (FUSION), and NCT01682720 (VALENCE).
Keywords: Hepatitis C treatment, HCV/HIV coinfection, health-related quality of life, patient-reported outcomes, sofosbuvir, ribavirin, cirrhosis
(See the editorial commentary by Modi and Saab on pages 343–4.)
Chronic hepatitis C is a major cause of mortality and morbidity worldwide. In particular, hepatitis C virus (HCV) infection not only has a negatively influence on clinical and patient-reported outcomes (PROs) but is also associated with tremendous economic societal burden [1–4]. Given shared routes of transmission, the prevalence of HCV infection in individuals who are infected with human immunodeficiency virus (HIV) is high. In particular, among HIV-infected individuals with a history of injection drug use, the rate of coinfection with HCV is reported to be as high as 72%–95% [5–9]. Recently, an increase in the incidence of HCV infection has also been reported in HIV-infected men who have sex with men [10–12].
In addition to the high prevalence of HCV infection in HIV-infected patients, consequences of chronic hepatitis C in these patients can be more severe. Early studies of HCV-infected patients with hemophilia suggested an increase in HCV load after coinfection with HIV [13]. Other studies have reported higher rates of cirrhosis, decompensated liver disease, and hepatocellular carcinoma in HIV/HCV-coinfected patients, compared with HCV-monoinfected patients [14–17].
With the widespread use of antiretroviral therapy, HIV infection is now viewed by many as a chronic disease, with significant improvements in AIDS-related mortality [18, 19]. On the other hand, liver-related mortality is now the most common cause of death in HIV/HCV-coinfected individuals [20] and remains a major cause of death for all HIV-infected patients [21, 22]. Given the importance of the clinical burden of HCV infection in HIV-infected individuals, treatment of HCV infection in HIV/HCV-coinfected patients is a priority.
The initial standard treatment for HCV infection, pegylated interferon alfa and ribavirin, was plagued with low efficacy and high rates of side effects [23, 24]. Furthermore, a high prevalence of comorbidities in HIV/HCV-coinfected individuals further limited the number of patients treated with interferon-based regimens [25]. Although the development of direct-acting antiviral agents with the first-generation protease inhibitors improved sustained virologic response (SVR) rates [26–28], the complexity of the regimen, its substantial side effects, and its drug-interaction profile limited the usefulness of those direct-acting antiviral agents in clinical practice [29, 30]. In 2013, the approval of sofosbuvir (NS5B nucleotide polymerase inhibitor) brought a new treatment option with high efficacy and a significantly improved safety profile to coinfected patients. In fact, the oral regimen with sofosbuvir and ribavirin (sofosbuvir/RBV) for 24 weeks is now a preferred regimen for HIV/HCV-coinfected patients without cirrhosis [31, 32].
Currently available interferon-free regimens are more efficacious and safer than previous regimens for patients with chronic hepatitis C, and they have also improved PROs, such as health-related quality of life (HRQL), fatigue, and work productivity [33, 34]. At present, it is unclear whether these PRO improvments can also be seen in HIV/HCV-coinfected patients. Nevertheless, it is reasonable to expect that patients with HIV/HCV coinfection have more-impaired PROs at baseline, owing to both the aggressive course of HCV infection in HIV-infected patients [13–17] and the influence of HIV infection itself [35, 36].
Therefore, the aims of this study were, first, to assess PROs in patients with HIV/HCV coinfection before the initiaion of treatment and, second, to assess changes in the PROs during treatment with sofosbuvir/RBV and after acheiving SVR. We also aimed to assess independent predictors of each PRO before, during, and after treatment. We compared baseline PRO scores and changes in PRO scores between HIV/HCV-coinfected and HCV-monoinfected patients.
METHODS
Study Cohort
The HIV/HCV-coinfected study population was pooled from the PHOTON-1 and PHOTON-2 phase 3 clinical trials, which investigated the safety and efficacy of sofosbuvir 400 mg once daily plus weight-based ribavirin (1000 or 1200 mg/day) in HIV/HCV-coinfected patients. Patients were naive to anti-HCV treatment or treatment-experienced, and all HCV genotypes were represented [31, 37]. In those trials, PROs were collected as secondary end points. Patients were required to be receiving antiretroviral therapy and to have an HIV RNA load of ≤50 copies/mL and a CD4+ T-cell counts of >200 cells/µL or were required to have untreated HIV infection and a CD4+ T-cell count of >500 cells/µL.
HCV-monoinfected controls were identified from 2 registered trials, FUSION and VALENCE, which investigated the same sofosbuvir/RBV regimen administered for 12, 16, or 24 weeks [33, 34, 38]. Cases (HIV/HCV-coinfected patients) and controls (HCV-monoinfected patients, defined as all FUSION and VALENCE participants who completed PRO questionnaires) were matched by a propensity score that included treatment history, age, sex, body mass index, cirrhosis, baseline HCV RNA load, history of anxiety, depression, insomnia, clinically overt fatigue, and type 2 diabetes.
Study Definitions
Baseline history of depression, anxiety, clinically overt fatigue, sleep disorders, and type 2 diabetes or hyperglycemia were extracted from medical history collected at screening. The presence of hepatic cirrhosis was evaluated by liver biopsy, Fibroscan, or Fibrotest in combination with the aspartate transaminase to platelet ratio, as originally described by Sulkowski et al [31]. At day 1 of treatment (baseline), HCV RNA load, hemoglobin level, alanine transaminase level, and CD4+ T-cell count were measured for all study participants.
Adverse events evaluated in this study (identified by the investigators as being related to the study) were grouped into blood and lymphatic system disorders, gastrointestinal disorders, musculoskeletal and connective tissue disorders, nervous system disorders, psychiatric disorders, skin and subcutaneous tissue disorders, fatigue-related disorders, influenza-like symptom disorders, and all other disorders.
PROs and Health Utility
Data on PROs and health utility were collected and assessed using 4 PRO instruments: the Short-Form 36 (SF-36) questionnaire, the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire, the Chronic Liver Disease Questionnaire–Hepatitis C Virus (CLDQ-HCV) instrument, and the Work Productivity and Activity–Specific Health Problem (WPAI:SHP) instrument. These PRO instruments were administered to patients at baseline (day 1), during treatment (weeks 4, 12, and 24, where applicable), and at follow-up (weeks 4 and 12 after treatment cessation). The validity of the SF-36 and FACIT-F questionnaires has been previously assessed in patients with HIV infection [39–41].
The SF-36 questionnaire is a generic instrument that is widely used for assessment of HRQL [42]. It uses 8 individual scales: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health, as well as 2 summary scores, the physical component summary score and the mental component summary score. The FACIT-F questionnaire is a fatigue-specific PRO instrument [43]. It assesses 5 individual scales: physical well-being, emotional well-being, social well-being, functional well-being, and fatigue. The CLDQ-HCV instrument is a disease-specific PRO instrument that assesses the HRQL of patients with chronic hepatitis C [44]. It assesses 4 individual domains: activity and energy, emotional, worry, and systemic. The WPAI:SHP instrument assesses impairment in daily activities and work associated with a specific health problem. It includes the work productivity impairment domain and the activity impairment domain [45]. Health utility scores were assessed using the SF-6D metric derived from the SF-36 instrument by a nonparametric Bayesian model [46].
Statistical Analysis
Clinico-demographic parameters and PROs were described as frequencies (percentages) or mean values ± standard deviation in the study arms separately. The Wilcoxon nonparametric test and the χ2 test for heterogeneity were used for pairwise comparisons. We also calculated the changes in PROs and utility scores from baseline to all time points and tested those changes for statistical significance by using the Wilcoxon signed rank test for matched pairs.
For the case-control analysis of HIV/HCV coinfection versus HCV monoinfection, a bipartite matching algorithm with the reverse-squared Euclidean distance for propensity score was used. Only cases and controls with propensity scores differing by ≤0.05 were included in the case-control analysis.
Independent predictors of PRO and health utility scores at baseline, during treatment, and after treatment were assessed in a series of multiple linear regressions with stepwise selection of predictors (P = .2 for entering the model, and P = .05 for staying in the model). A complete list of potential PRO and health utility predictors to be evaluated is as follows: age; sex; ethnicity (white vs other races/ethnicities); location (United States vs other locations); baseline body mass index; baseline hemoglobin level; treatment-emergent adverse events; history of prior anti-HCV treatment (treatment naive vs treatment experienced); pretreatment history of anxiety, depression, insomnia, clinically overt fatigue, and type 2 diabetes (all derived from medical history collected at screening); baseline HCV load (<106 vs >106 copies/mL); baseline alanine transaminase level (<1.5 vs >1.5 times the upper limit of normal); duration of treatment in weeks; and achievement of SVR (at the last day of treatment and at follow-up visits only). In the case-control multivariate analysis, the presence of HIV infection was also included as one of the PRO/health utility predictors.
All analyses were performed in SAS 9.3 (SAS Institute, Cary, North Carolina). The study was separately approved by each site's institutional review board.
RESULTS
Baseline Clinical Presentation and PROs in PHOTON-1 and PHOTON-2
In PHOTON-1 and PHOTON-2, 497 HIV/HCV-coinfected patients were enrolled. Overall, 81% of patients were treatment naive, the mean age (±SD) was 48.2 ± 8.1 years, 82% were male, and 15.3% were cirrhotic. Ninety-six percent were receiving antiretroviral therapy, and 51% reported being employed as of the first day of treatment (Table 1).
Table 1.
Baseline Clinico-demographic Parameters of the PHOTON-1 and PHOTON-2 Cohorts
| Parameter | PHOTON-1 (n = 223) | PHOTON-2 (n = 274) | P Values | All (n = 497) |
|---|---|---|---|---|
| Treatment naive | 182 (81.6) | 219 (80.0) | .64 | 401 (80.7) |
| Age, y | 49.4 ± 8.7 | 47.3 ± 7.4 | .0001 | 48.2 ± 8.1 |
| Male sex | 185 (83.0) | 221 (80.7) | .51 | 406 (81.7) |
| White | 156 (70.0) | 259 (94.5) | <.0001 | 415 (83.5) |
| Black | 52 (23.3) | 3 (1.1) | <.0001 | 55 (11.1) |
| Employed | 97 (44.9) | 148 (55.6) | .019 | 245 (50.8) |
| BMIa | 27.3 ± 4.9 | 24.3 ± 3.7 | <.0001 | 25.7 ± 4.5 |
| Hemoglobin level, g/dL | 14.7 ± 1.5 | 14.9 ± 1.3 | .07 | 14.8 ± 1.4 |
| Receiving ART | 212 (95.1) | 265 (96.7) | .35 | 477 (96.0) |
| CD4+ T-cell count >500 cells/mm3 | 139 (62.3) | 162 (59.3) | .50 | 301 (60.7) |
| HCV RNA load >106 IU/mL | 173 (77.6) | 193 (70.4) | .07 | 366 (73.6) |
| ALT level >1.5 × ULN | 117 (52.5) | 153 (55.8) | .45 | 270 (54.3) |
| Cirrhosis | 22 (9.9) | 54 (19.7) | .0024 | 76 (15.3) |
| Pretreatment history | ||||
| Type 2 diabetes | 20 (9.0) | 6 (2.2) | .0007 | 26 (5.2) |
| Anxiety | 52 (23.3) | 23 (8.4) | <.0001 | 75 (15.1) |
| Depression | 122 (54.7) | 66 (24.1) | <.0001 | 188 (37.8) |
| Insomnia | 65 (29.2) | 40 (14.6) | <.0001 | 105 (21.1) |
| Fatigue | 25 (11.2) | 8 (2.9) | .0002 | 33 (6.6) |
| Treatment with sofosbuvir/RBV | ||||
| Treated for 12 wks | 68 (30.5) | 19 (6.9) | <.0001 | 87 (17.5) |
| Treated for 24 wks | 155 (69.5) | 255 (93.1) | <.0001 | 410 (82.5) |
| SVR12 | 176 (78.9) | 237 (86.5) | .0251 | 413 (83.1) |
| Adverse event | ||||
| Anemia | 21 (9.4) | 22 (8.0) | .58 | 43 (8.7) |
| Fatigue | 81 (36.3) | 74 (27.0) | .026 | 155 (31.2) |
| Influenza-like symptoms | 4 (1.8) | 5 (1.8) | .98 | 9 (1.8) |
| Gastrointestinal symptoms | 45 (20.2) | 61 (22.3) | .57 | 106 (21.3) |
| Musculoskeletal symptoms | 18 (8.1) | 20 (7.3) | .75 | 38 (7.6) |
| Nervous symptoms | 36 (16.1) | 45 (16.4) | .93 | 81 (16.3) |
| Psychiatric symptoms | 58 (26.0) | 69 (25.2) | .83 | 127 (25.6) |
| Skin symptoms | 26 (11.7) | 50 (18.3) | .042 | 76 (15.3) |
| Other | 48 (21.5) | 76 (27.7) | .11 | 124 (25.0) |
| None | 79 (35.4) | 95 (34.7) | .86 | 174 (35.0) |
Data are no. (%) of patients or mean value ± SD.
Abbreviations: ALT, alanine transaminase; ART, antiretroviral therapy; HCV, hepatitis C virus; IU, international units; RBV, ribavirin; SD, standard deviation; SVR12, sustained virologic response for 12 weeks after the end of treatment; ULN, upper limit of normal.
a Body mass index (BMI) is defined as the weight in kilograms divided by the height in meters squared.
Since PHOTON-1 was primarily conducted in the United States and PHOTON-2 was primarily conducted in Europe, some demographic and baseline clinical parameters were different between the cohorts (Table 1). In particular, participants in PHOTON-1 were older and less likely to be white and had a higher body mass index and a lower prevalence of cirrhosis. Furthermore, the rates of all studied comorbidities, such as depression, anxiety, insomnia, fatigue, and diabetes, were substantially higher in PHOTON-1 (Table 1). Owing to differences in study protocols, the proportion of patients treated for 24 weeks rather than 12 weeks was higher in PHOTON-2 (93.1% vs 69.5%).
Similar to previous reports [38], scores for the general health and mental health components of the SF-36 questionnaire, together with the social well-being domain of the FACIT-F questionnaire, were lower in PHOTON-2 patients, compared with PHOTON-1 patients, while the bodily pain scale on the SF-36 questionnaire was higher (P < .05). Scores for other baseline PROs were similar between the PHOTON-1 and PHOTON-2 cohorts (Table 2).
Table 2.
Baseline Patient-Reported Outcomes Among PHOTON-1 and PHOTON-2 Participants
| Instrument, Scale | PHOTON-1 | PHOTON-2 | P Values | Overall |
|---|---|---|---|---|
| SF-36 | ||||
| Physical functioning | 78.4 ± 25.1 | 81.9 ± 22.5 | .33 | 80.3 ± 23.7 |
| Role physical | 73.7 ± 26.5 | 72.8 ± 25.6 | .54 | 73.2 ± 26.0 |
| Bodily pain | 66.0 ± 26.7 | 73.7 ± 26.4 | .0011 | 70.2 ± 26.8 |
| General health | 64.0 ± 23.0 | 57.5 ± 20.8 | .0023 | 60.5 ± 22.1 |
| Vitality | 60.0 ± 23.2 | 57.6 ± 19.6 | .13 | 58.6 ± 21.3 |
| Social functioning | 74.9 ± 26.1 | 72.1 ± 26.2 | .20 | 73.4 ± 26.1 |
| Role emotional | 78.3 ± 24.4 | 73.1 ± 25.4 | .0233 | 75.5 ± 25.1 |
| Mental health | 71.2 ± 20.3 | 67.0 ± 18.2 | .0037 | 68.9 ± 19.3 |
| Physical component summary | 49.0 ± 9.2 | 50.7 ± 8.7 | .037 | 49.9 ± 8.9 |
| Mental component summary | 48.3 ± 10.8 | 45.3 ± 10.1 | .0008 | 46.7 ± 10.5 |
| FACIT-F | ||||
| Physical well-being | 22.6 ± 5.2 | 23.1 ± 4.6 | .60 | 22.9 ± 4.9 |
| Emotional well-being | 18.1 ± 4.6 | 18.0 ± 3.9 | .45 | 18.1 ± 4.3 |
| Social well-being | 19.6 ± 7.0 | 18.3 ± 7.0 | .0219 | 18.9 ± 7.0 |
| Functional well-being | 19.5 ± 6.6 | 18.7 ± 5.8 | .07 | 19.1 ± 6.2 |
| Fatigue | 38.5 ± 11.7 | 38.6 ± 10.7 | .53 | 38.6 ± 11.2 |
| Total FACIT-F | 118.4 ± 28.2 | 116.7 ± 25.5 | .32 | 117.5 ± 26.8 |
| CLDQ-HCV | ||||
| Activity/energy | 5.23 ± 1.34 | 5.17 ± 1.23 | .38 | 5.20 ± 1.28 |
| Emotional | 5.27 ± 1.28 | 5.26 ± 1.08 | .42 | 5.26 ± 1.17 |
| Worry | 5.44 ± 1.36 | 5.50 ± 1.14 | .83 | 5.47 ± 1.25 |
| Systemic | 4.80 ± 1.38 | 5.00 ± 1.18 | .15 | 4.91 ± 1.28 |
| Total CLDQ-HCV | 5.18 ± 1.21 | 5.24 ± 0.98 | .93 | 5.21 ± 1.09 |
| WPAI:SHP | ||||
| Work productivity impairment | 0.14 ± 0.25 | 0.11 ± 0.17 | .45 | 0.12 ± 0.21 |
| Absenteeism | 0.05 ± 0.16 | 0.01 ± 0.04 | .42 | 0.03 ± 0.11 |
| Presenteeism | 0.09 ± 0.18 | 0.10 ± 0.16 | .23 | 0.09 ± 0.17 |
| Activity impairment | 0.18 ± 0.25 | 0.19 ± 0.25 | .33 | 0.19 ± 0.25 |
| Health utility | ||||
| SF-6D | 0.68 ± 0.15 | 0.68 ± 0.12 | .46 | 0.68 ± 0.14 |
Data are mean score ± SD.
Abbreviations: CLDQ-HCV, Chronic Liver Disease Questionnaire–Hepatitis C Virus instrument; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue questionnaire; SD, standard deviation; SF-36, Short-Form 36 questionnaire; WPAI:SHP, Work Productivity and Activity–Specific Health Problem instrument.
PROs During Sofosbuvir/RBV Treatment
After initiation of treatment with sofosbuvir/RBV, moderate decrements in some PROs were observed as early as treatment week 4. In particular, significant decrements by this time were found in the fatigue scale and total score on the FACIT-F questionnaire and the work productivity and activity impairment domains of the WPAI:SHP instrument (P < .05; Supplementary Table 1).
By the end of treatment, decrements in these PROs became more substantial (all P < .05), and the decrement in the mental component summary of the SF-36 questionnaire and a number of individual scales (the physical functioning, role physical, vitality, social functioning, role emotional, and mental health scales of the SF-36 questionnaire; the physical well-being and functional well-being domains of the FACIT-F questionnaire; and the activity and energy domain, emotional domain, and systemic domain of the CLDQ-HCV instrument, including both presenteeism and absenteeism of work productivity) also became statistically significant (Supplementary Table 1 and Figure 1A). The only PRO scores that improved in patients by the end of treatment with sofosbuvir/RBV were the emotional well-being domain of the FACIT-F questionnaire and the worry domain of the CLDQ-HCV instrument (both P < .05; Figure 1A).
Figure 1.
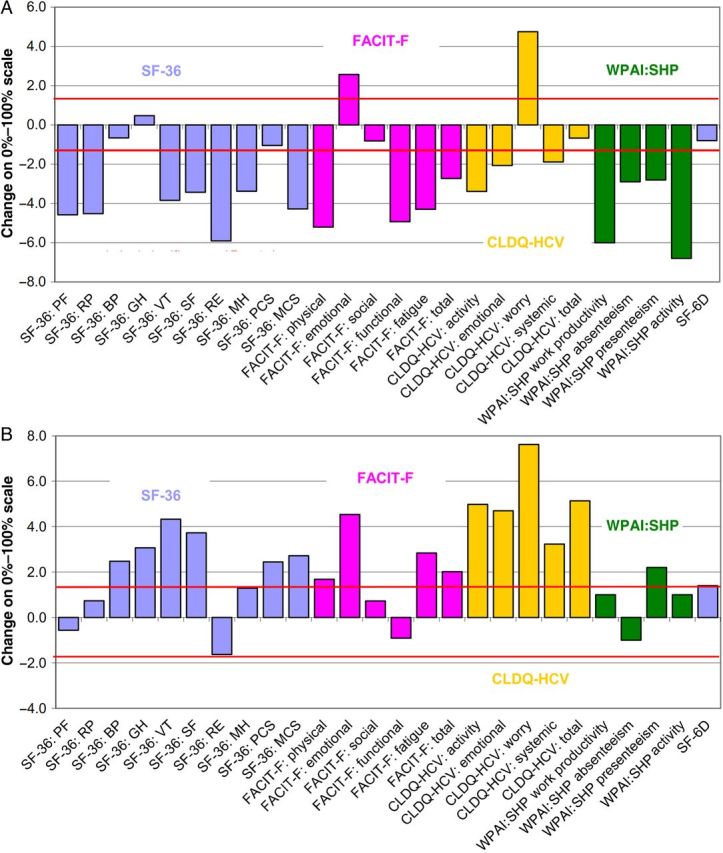
Changes in patient-reported outcomes at the end of treatment (A) and after sustaining a virologic response for 12 weeks after treatment cessation (B) in patients coinfected with human immunodeficiency virus and hepatitis C virus (HCV). Values above the upper and below the lower red lines denote statistically significant changes. Abbreviations: BP, bodily pain scale; CLDQ-HCV, Chronic Liver Disease Questionnaire–Hepatitis C Virus; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue questionnaire; MCS, mental component summary score; MH, mental health scale; PCS, physical component summary score; PF, physical functioning scale; RE, role emotional scale; RP, role physical scale; SF, social functioning scale; SF-36, Short-Form 36 questionnaire; VT, vitality scale; WPAI:SHP, Work Productivity and Activity–Specific Health Problem questionnaire.
Despite this, by week 4 after treatment, all PRO scores returned to their baseline levels, while all domains of the CLDQ-HC instrument significantly improved (change, up to +6.7% on a 0%–100% normalized PRO scale; all P < .05; Supplementary Table 1).
Between the 2 study cohorts, no difference in treatment-emergent PRO changes was observed throughout treatment (all P > .05). However, by week 4 after treatment, improvements in CLDQ-HCV and SF-6D scores, compared with baseline values, were statistically significant only in PHOTON-1 (Supplementary Table 1).
PROs After Achieving SVR for 12 Weeks After Treatment Cessation (SVR12) in Patients With HIV/HCV Coinfection
Of the entire cohort, 413 patients (83%) achieved SVR (Table 1). In patients with SVR12, most PRO scores improved from baseline levels (Supplementary Table 1 and Figure 1B). The greatest improvement was observed in the worry domain of the CLDQ-HCV instrument (change, +7.6% on a 0%–100% normalized PRO scale; P < .0001). Also, similar to findings from week 4 after the end of treatment, post-SVR12 improvements in the overall CLDQ-HCV score, the SF-6D score, and the bodily pain scale of the SF-36 questionnaire were greater in PHOTON-1 patients, compared with PHOTON-2 patients, while post-SVR changes in other PRO scores were similar between the 2 study cohorts (Supplementary Table 1).
Among HIV/HCV-coinfected patients who did not achieve SVR and completed PRO questionnaires at follow-up week 12 (n = 23), no improvement was observed in any PRO score. Furthermore, residual decrement from baseline in the general health scale of the SF-36 questionnaire (change, −5.7; P = .0259, compared with patients with SVR) and the physical well-being (−1.7; P = .0047) and fatigue (−2.5; P = .0199) scales of the FACIT-F questionnaire was observed in those patients.
PRO Scores in HIV/HCV-Coinfected Patients Versus Those in HCV-Monoinfected Patients
To compare HIV/HCV coinfection with HCV monoinfection, we matched HIV/HCV-coinfected patients to HCV-monoinfected controls from the FUSION and VALENCE trials [33, 34, 38]. Of PHOTON-1 and PHOTON-2 participants, 211 had matched monoinfected controls.
A number of baseline PRO scores were lower in the HIV/HCV-coinfected group, including the physical functioning, role physical, general health, social functioning, role emotional, and physical component summary scales of the SF-36 questionnaire; the physical well-being, social well-being, and functional well-being domains of the FACIT-F questionnaire; the systemic domain of the CLDQ-HCV instrument; and the SF-6D instrument (Table 3). In multivariate analysis, HIV infection was also independently associated with a lower baseline physical component summary scale (β = −2.65; P = .0013), after adjustment for sex, treatment history, pretreatment depression, fatigue, and insomnia.
Table 3.
Baseline Patient-Reported Outcomes Among PHOTON-1 and PHOTON-2 Participants With Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) Coinfection and Matched HCV-Monoinfected Controls
| Instrument, Scale | Coinfected (n = 211) | Monoinfected (n = 211) | P Values |
|---|---|---|---|
| SF-36 | |||
| Physical functioning | 79.4 ± 23.5 | 85.9 ± 19.3 | .0024 |
| Role physical | 70.9 ± 25.3 | 77.8 ± 26.0 | .0011 |
| Bodily pain | 69.7 ± 27.4 | 74.9 ± 25.4 | .06 |
| General health | 57.0 ± 21.9 | 61.2 ± 23.5 | .036 |
| Vitality | 57.9 ± 19.9 | 60.2 ± 23.4 | .10 |
| Social functioning | 71.6 ± 26.2 | 80.3 ± 24.7 | .0002 |
| Role emotional | 72.3 ± 24.7 | 77.5 ± 27.1 | .0078 |
| Mental health | 68.4 ± 18.7 | 67.7 ± 21.0 | .84 |
| Physical component summary | 49.2 ± 9.2 | 52.1 ± 7.9 | .0027 |
| Mental component summary | 46.1 ± 9.9 | 46.8 ± 11.5 | .19 |
| FACIT-F | |||
| Physical well-being | 22.8 ± 4.9 | 23.7 ± 4.8 | .025 |
| Emotional well-being | 17.9 ± 4.2 | 17.7 ± 4.5 | .65 |
| Social well-being | 19.1 ± 6.9 | 21.3 ± 5.8 | .0019 |
| Functional well-being | 18.8 ± 5.9 | 19.8 ± 5.9 | .046 |
| Fatigue scale | 38.7 ± 10.6 | 39.6 ± 11.4 | .13 |
| Total FACIT-F | 117.1 ± 25.6 | 122.1 ± 26.6 | .026 |
| CLDQ-HCV | |||
| Activity/energy | 5.10 ± 1.28 | 5.26 ± 1.41 | .09 |
| Emotional | 5.23 ± 1.11 | 5.29 ± 1.23 | .30 |
| Worry | 5.37 ± 1.18 | 5.40 ± 1.27 | .60 |
| Systemic | 4.85 ± 1.24 | 5.12 ± 1.30 | .029 |
| Total | 5.14 ± 1.02 | 5.27 ± 1.14 | .10 |
| WPAI:SHP | |||
| Work productivity impairment | 0.12 ± 0.21 | 0.15 ± 0.25 | .60 |
| Absenteeism | 0.02 ± 0.07 | 0.03 ± 0.11 | .50 |
| Presenteeism | 0.10 ± 0.18 | 0.12 ± 0.20 | .79 |
| Activity impairment | 0.20 ± 0.26 | 0.18 ± 0.26 | .31 |
| Health utility | |||
| SF-6D | 0.66 ± 0.12 | 0.70 ± 0.14 | .0042 |
Data are mean score ± SD.
Abbreviations: CLDQ-HCV, Chronic Liver Disease Questionnaire–Hepatitis C Virus; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue questionnaire; SF-36, Short-Form 36 questionnaire; WPAI:SHP, Work Productivity and Activity–Specific Health Problem questionnaire.
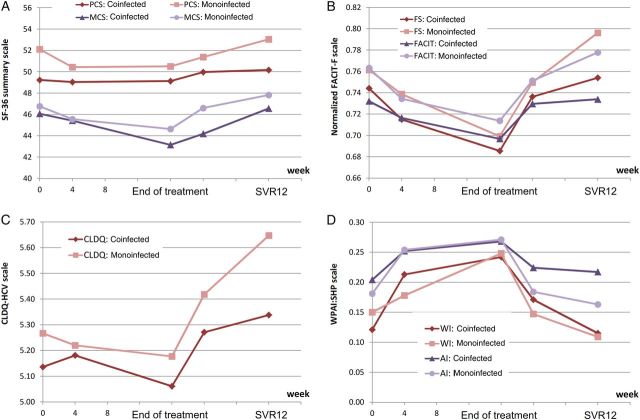
Throughout treatment, some of the PRO scores remained lower in patients with HIV infection (Figure 3). Furthermore, similar to baseline findings, the absolute values of most PRO scores remained lower in HIV/HCV-coinfected patients than in HCV-monoinfected patients, even after achieving SVR. Despite this, all treatment-emergent decrements and post-SVR12 improvements in PRO scores were similar between patients with HIV/HCV coinfection and those with HCV monoinfection (all P < .05; Table 4).
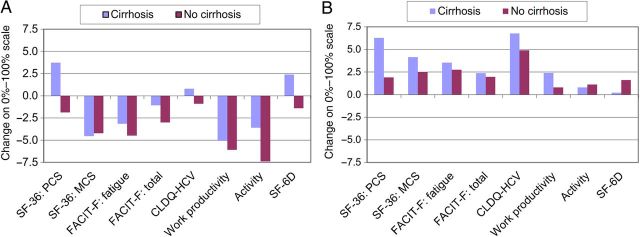
Figure 3.
Normalized changes in patient-reported outcomes at the end of treatment (A) and after sustaining a virologic response for 12 weeks after treatment cessation (B) in patients coinfected with human immunodeficiency virus and hepatitis C virus who did (n = 76) or did not (n = 421) have cirrhosis. All P values were > .05 for comparisons between cirrhosis and noncirrhosis cohorts. Abbreviations: CLDQ-HCV, Chronic Liver Disease Questionnaire–Hepatitis C Virus instrument; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue questionnaire; MCS, mental component summary score; PCS, physical component summary score; SF-36, Short-Form 36 questionnaire.
Table 4.
Treatment-Emergent Changes in Patient-Reported Outcome Scores Among Patients Coinfected With Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) and Matched Controls with HCV Monoinfection
| PRO, Time Point | Coinfected | Monoinfected | P Values |
|---|---|---|---|
| SF-36: physical component summary | |||
| Baseline | 49.23 ± 9.16 | 52.11 ± 7.90 | .0027 |
| Changea by: | |||
| Week 4 | −0.62 ± 6.05 | −1.36 ± 5.85c | .07 |
| End of treatment | −0.46 ± 7.53 | −1.64 ± 6.54c | .11 |
| 4 wks after treatment | 0.99 ± 6.51c | −0.31 ± 7.04 | .07 |
| SVR12 | 0.74 ± 6.95 | 0.73 ± 6.47 | .99 |
| SF-36: mental component summary | |||
| Baseline | 46.06 ± 9.90 | 46.77 ± 11.49 | .19 |
| Changea by: | |||
| Week 4 | −0.65 ± 8.20 | −0.96 ± 8.60 | .57 |
| End of treatment | −2.38 ± 9.16c | −1.79 ± 9.10c | .72 |
| 4 wks after treatment | −1.60 ± 9.17c | 0.12 ± 9.43 | .05 |
| SVR12 | 1.33 ± 8.86c | 1.62 ± 9.18 | .77 |
| Fatigue (FACIT-F) | |||
| Baseline | 38.70 ± 10.56 | 39.60 ± 11.38 | .13 |
| Changea by: | |||
| Week 4 | −1.35 ± 8.33 | −0.84 ± 8.36 | .81 |
| End of treatment | −2.48 ± 9.25c | −2.99 ± 9.56c | .54 |
| 4 wks after treatment | −0.34 ± 9.41 | 0.08 ± 9.53 | .67 |
| SVR12 | 1.19 ± 8.50c | 1.54 ± 8.20c | .80 |
| Total FACIT-F | |||
| Baseline | 117.13 ± 25.64 | 122.10 ± 26.58 | .026 |
| Changea by: | |||
| Week 4 | −2.41 ± 18.09 | −4.16 ± 15.64c | .14 |
| End of treatment | −5.05 ± 19.04c | −7.45 ± 20.61c | .74 |
| 4 wks after treatment | −0.49 ± 20.55 | −0.54 ± 20.08 | .76 |
| SVR12 | 2.15 ± 19.81 | 3.30 ± 17.37c | .21 |
| CLDQ-HCV | |||
| Baseline | 5.14 ± 1.02 | 5.27 ± 1.14 | .10 |
| Changea by: | |||
| Week 4 | 0.08 ± 0.74 | −0.04 ± 0.64 | .19 |
| End of treatment | −0.07 ± 0.86 | −0.10 ± 0.78 | .87 |
| 4 wks after treatment | 0.14 ± 0.80c | 0.20 ± 0.83c | .37 |
| SVR12 | 0.23 ± 0.91c | 0.34 ± 0.84c | .22 |
| Work productivity impairment (WPAI:SHP) | |||
| Baseline | 0.121 ± 0.208 | 0.150 ± 0.248 | .60 |
| Changeb by: | |||
| Week 4 | 0.090 ± 0.275c | 0.045 ± 0.153c | .64 |
| End of treatment | 0.110 ± 0.241c | 0.114 ± 0.237c | .47 |
| 4 wks after treatment | 0.050 ± 0.254 | −0.005 ± 0.262 | .30 |
| SVR12 | 0.014 ± 0.235 | 0.009 ± 0.170 | .18 |
| Activity impairment (WPAI:SHP) | |||
| Baseline | 0.204 ± 0.256 | 0.181 ± 0.259 | .31 |
| Changeb by: | |||
| Week 4 | 0.047 ± 0.271c | 0.070 ± 0.233c | .70 |
| End of treatment | 0.062 ± 0.264c | 0.083 ± 0.261c | .33 |
| 4 wks after treatment | 0.016 ± 0.255 | −0.022 ± 0.223 | .32 |
| SVR12 | −0.001 ± 0.269 | −0.009 ± 0.226 | .84 |
| SF-6D health utility | |||
| Baseline | 0.663 ± 0.116 | 0.702 ± 0.142 | .0042 |
| Changea by: | |||
| Week 4 | −0.010 ± 0.099 | −0.031 ± 0.118c | .19 |
| End of treatment | −0.008 ± 0.122 | −0.031 ± 0.130c | .13 |
| 4 wks after treatment | 0.002 ± 0.131 | −0.007 ± 0.132 | .87 |
| SVR12 | 0.001 ± 0.113 | 0.015 ± 0.123 | .90 |
Data are mean score ± SD.
a A positive change indicates improvement.
b A positive change indicates decrement.
c Significant difference from patients’ own baseline value (P < .05, by a paired nonparametric test).
During and after treatment, HIV infection was independently associated with lower physical component summary scale, fatigue scale, and total scores on the FACIT-F questionnaire and CLDQ-HCV instrument at different time points after adjustment for clinico-demographic confounders (β, up to −7.0%; P = .0088). However, no association between HIV infection and treatment-emergent and posttreatment changes in PRO scores was found (all P > .05).
PRO Scores and Cirrhosis in HIV/HCV-Coinfected Patients
There were 76 HIV/HCV-coinfected patients (15.3%) with cirrhosis enrolled in PHOTON-1 and PHOTON-2. At baseline, most of the PRO scores were lower in patients with cirrhosis, including the physical functioning, role physical, general health, social functioning, role emotional, and physical component summary scales of the SF-36 questionnaire; the physical well-being, emotional well-being, and fatigue scales and total score of the FACIT-F questionnaire score; the activity and energy, worry, and systemic domains and total score of the CLDQ-HCV instrument; and the work productivity, presenteeism, and activity domains of the WPAI:SHP instrument (change, up to 11.9%; all P < .05), compared with patients without cirrhosis (data not shown). Throughout treatment and after achieving SVR, some of the PRO scores remained lower in patients with cirrhosis. Despite this, all treatment-emergent and post-SVR12 changes in PRO scores (Figure 2) were similar between cirrhotic and noncirrhotic patients (all P > .05), except for the general health scale of the SF-36 questionnaire, which improved more in cirrhotic patients (change, +11.3%), compared with noncirrhotic patients (change, +1.9%; P = .0002).
Figure 2.
Patient-reported outcomes throughout treatment with sofosbuvir/ribavirin in patients coinfected with human immunodeficiency virus and hepatitis C virus (HCV) and matched controls with HCV monoinfection. The physical component summary (PCS) score is significantly lower in HIV/HCV-coinfected patients, compared with HCV-monoinfected patients (P < .05) at baseline, 12 weeks after treatment cessation in those who achieved sustained virologic response (SVR12); mental component summary at posttreatment week 4; fatigue scale at SVR12; total Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire baseline, SVR12; Chronic Liver Disease Questionnaire–Hepatitis C Virus (CLDQ-HCV) instrument at SVR12; work productivity and activity impairment of Work Productivity and Activity–Specific Health Problem (WPAI:SHP) questionnaire are similar between HIV/HCV-coinfected patients and HCV-monoinfected subjects at all time points (all P > .05). Abbreviations: AI, activity impairment; FS, fatigue scale; WI, work productivity impairment.
In multivariate analysis, cirrhosis during HIV/HCV coinfection was independently associated with lower PRO scores, including the physical component summary scale, fatigue scale, CLDQ-HCV instrument, and activity impairment domain (β, up to −12.9%; P < .0001; Supplementary Table 2). However, no association between cirrhosis and treatment-emergent or post-SVR changes in PROs was found (all P > .05).
PROs and Treatment Duration in HIV/HCV Coinfection
In PHOTON-1 and PHOTON-2, 87 patients were treated for 12 weeks, whereas 410 received sofosbuvir/RBV for 24 weeks. When end-of-treatment PRO scores were compared between patients who had received the 12-week regiment and those who had received the 24-week regimen, no difference was found for any summary or individual PRO scale (all P > .05).
Independent Predictors of PROs in HIV/HCV-Coinfected Patients
In multivariate analysis (Supplementary Table 2), consistent predictors of PRO scores at different time points were baseline depression (β, up to −14.5%), anxiety (up to −10.2%), fatigue (up to −15.4%), insomnia (up to −7.3%), cirrhosis (up to −12.9%), male sex (up to +15.1%), and being enrolled in the United States (up to +14.4%). Being treatment naive and younger were associated with a higher physical component summary (changes, up to +8.5% and −0.53% per year, respectively).
Achieving SVR12 was associated with lower work productivity impairment at posttreatment week 4 (β, −0.18) and with lower absenteeism (β, −0.13; both P < .05). In addition to these, experiencing adverse events was associated with lower PRO scores at different time points, including treatment-emergent fatigue (β, up to −12.5%), gastrointestinal disorders (up to −10.7%), musculoskeletal disorders (up to −11.3%), nervous disorders (up to −12.4%), psychiatric disorders (up to −7.7%), and skin disorders (up to −20.8%; all P < .05; Supplementary Table 2). Finally, treatment duration was not associated with any end-of-treatment PRO score (all P > .05).
DISCUSSION
This is the first in-depth evaluation of patient experience based on the assessment of PROs in HIV/HCV-coinfected individuals with 4 validated PRO instruments before, during, and after treatment with any interferon-free anti-HCV regimen.
Our data show that patients with HIV/HCV coinfection have greater impairment in PROs before the initiation of treatment than matched controls with HCV infection alone. These results contrast with those of a prior study that reported no difference in HRQL between HCV/HIV-coinfected patients and HCV-monoinfected patients [47]. Although the exact reasons are unknown, we suspect that differences in the patient populations that were included in these 2 studies (patients from tertiary care centers vs clinical trial subjects) can explain the difference.
Our data also show that, despite some baseline impairment, HIV/HCV-coinfected patients tolerate sofosbuvir/RBV quite well, with high SVR12 rates and substantial gains in PRO scores. The minimal PRO score decrements seen during the treatment regimen are similar to those reported for HCV-monoinfected patients [33, 34, 38]. In fact, these minimal decrements in PRO scores during treatment have previously been shown to be associated with RBV-related side effects [48].
Another very important finding of our study is the significant improvement of some PRO scores in patients HIV/HCV coinfection who achieved SVR12. These improvements were seen in a number of PROs and are also similar to improvements seen for patients with HCV monoinfection [33, 34, 38]. Furthermore, these benefits were seen in individuals with HIV/HCV infection who had severe liver disease, as documented by the presence of cirrhosis, despite the fact that, similar to previous reports [49], patients with cirrhosis had more impairment in PRO scores at baseline. In fact, improvement in the general health scale of the SF-36 questionnaire was more substantial in cirrhotic patients than in noncirrhotic patients who achieved SVR12. Given the increasing incidence of cirrhosis in HIV/HCV-coinfected patients, the improvement in PRO scores in cirrhotic patients with HIV/HCV could have important implications.
Finally, our multivariate analysis indicated that, similar to HCV-monoinfected patients [33, 34, 38, 48, 49], depression, fatigue, and RBV-associated side effects were independent predictors of PRO scores in patients with HIV/HCV coinfection.
In summary, our data indicate that HIV/HCV-coinfected patients tolerated treatment with sofosbuvir/RBV quite well and to an extent similar to that for monoinfected patients. In fact, PRO scores improved in HIV/HCV-coinfected patients receiving the interferon-free regimen who achieved SVR. Furthermore, these improvements in PRO scores were similar to those for patients with HCV monoinfection, despite the lower baseline scores for these patients. Our data support the fact that these patients benefit from not only achieving a high SVR rate, along with its beneficial clinical implication, but also from experiencing significant improvement in PROs, along with its beneficial patient experience implication. The combination of both clinical and PRO benefits should lead to the prioritization of treatment for HIV/HCV-coinfected patients, given their more aggressive liver disease progression, even in the highly active antiretroviral therapy era. We believe that treatment of HIV/HCV-coinfected patients with these highly effective and safe treatment regimens is certainly good for patients and cost-effective from a societal perspective.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by Gilead Sciences.
Potential conflicts of interest. Z. M. Y. is a consultant to Intercept, AbbVie, Gilead Sciences, BMS, and Salix. M. S., S. N., M. P., C. O. received research support from Gilead Sciences. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 2011; 140:1182–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 2014; 39:518–31. [DOI] [PubMed] [Google Scholar]
- 3.Kallman J, O'Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci 2007; 52:2531–9. [DOI] [PubMed] [Google Scholar]
- 4.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med 2014; 161:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–9. [DOI] [PubMed] [Google Scholar]
- 6.Denis F, Adjide CC, Rogez S, Delpeyroux C, Rogez JP, Weinbreck P. Seroprevalence of HBV, HCV and HDV hepatitis markers in 500 patients infected with the human immunodeficiency virus. Pathol Biol 1997; 45:701–8. [PubMed] [Google Scholar]
- 7.Roca B, Suarez I, Gonzalez J, et al. Hepatitis C virus and human immunodeficiency virus coinfection in Spain. J Infect 2003; 47:117–24. [DOI] [PubMed] [Google Scholar]
- 8.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US Adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–7. [DOI] [PubMed] [Google Scholar]
- 9.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med 2003; 138:197–207. [DOI] [PubMed] [Google Scholar]
- 10.Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS 2009; 23:F1–7. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men--New York City, 2005–2010. MMWR Morb Mortal Wkly Rep 2011; 60:945–50. [PubMed] [Google Scholar]
- 12.van der Helm JJ, Prins M, del Amo J, et al. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS 2011; 25:1083–91. [DOI] [PubMed] [Google Scholar]
- 13.Eyster ME, Fried MW, Di Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood 1994; 84:1020–3. [PubMed] [Google Scholar]
- 14.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008; 22:1979–91. [DOI] [PubMed] [Google Scholar]
- 15.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418–31. [DOI] [PubMed] [Google Scholar]
- 16.Lo Re V, Tate J, Kallan M, et al. Increased risk of hepatic decompensation and hepatocellular carcinoma in HIV/HCV-co-infected patients compared to HCV-mono-infected patients despite combination antiretroviral therapy [abstract WEAB0102] Presented at: 19th International AIDS Conference, Washington, D. C., 2012. http://pag.aids2012.org/Abstracts.aspx?SID=213&AID=17867 Accessed 25 September 2014. [Google Scholar]
- 17.Merchante N, Merino E, López-Aldeguer J, et al. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis 2013; 56:143–50. [DOI] [PubMed] [Google Scholar]
- 18.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoll B, Lassmann B, Temesgen Z. Current status of HIV infection: a review for non-HIV-treating physicians. Int J Dermatol 2007; 46:1219–28. [DOI] [PubMed] [Google Scholar]
- 20.Morlat P, Roussillon C, Henard S, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS 2014; 28:1181–91. [DOI] [PubMed] [Google Scholar]
- 21.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013; 14:195–207. [DOI] [PubMed] [Google Scholar]
- 22.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 23.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140:346–55. [DOI] [PubMed] [Google Scholar]
- 24.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004; 351:438–50. [DOI] [PubMed] [Google Scholar]
- 25.Butt AA, Justice AC, Skanderson M, Good C, Kwoh CK. Rates and predictors of hepatitis C virus treatment in HIV/HCV-coinfected subjects. Aliment Pharmacol Ther 2006; 24:585–91. [DOI] [PubMed] [Google Scholar]
- 26.Sulkowski MS, Sherman KE, Soriano V, et al. Telaprevir in combination with peginterferon alfa-2a/Ribavirin in HCV/HIV co-infected patients: SVR24 final study results. Hepatology 0000; 56(suppl 1):219A.22334397 [Google Scholar]
- 27.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–16. [DOI] [PubMed] [Google Scholar]
- 28.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hézode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol 2013; 59:434–41. [DOI] [PubMed] [Google Scholar]
- 30.Gordon SC, Muir AJ, Lim JK, et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real-world experience from HCV-TARGET. J Hepatol 2015; 62:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014; 312:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieterich D, Rockstroh JK, Orkin C, et al. Simeprevir (TMC435) with peginterferon/ribavirin in patients coinfected with HCV genotype-1 and HIV-1: a phase III study. Clin Infect Dis 2014; 59:1579–87. [DOI] [PubMed] [Google Scholar]
- 33.Younossi ZM, Stepanova M, Henry L, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in Chronic Hepatitis C (CH-C). J Hepatol 2014; 60:741–7. [DOI] [PubMed] [Google Scholar]
- 34.Younossi ZM, Stepanova M, Henry L, et al. Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2014; 12:1349–59. [DOI] [PubMed] [Google Scholar]
- 35.Miners AH, Sabin CA, Mocroft A, Youle M, Fisher M, Johnson M. Health-related quality of life in individuals infected with HIV in the era of HAART. HIV Clin Trials 2001; 2:484–92. [DOI] [PubMed] [Google Scholar]
- 36.Hays RD, Cunningham WE, Sherbourne CD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med 2000; 108:714–22. [DOI] [PubMed] [Google Scholar]
- 37.Molina JM, Orkin C, Iser DM, et al. All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotypes 1, 2, 3 and 4 infection in patients co-infected with HIV (PHOTON-2) [abstract MOAB0105LB] Presented at: 20th International AIDS Conference, Melbourne, Australia, 2014. [Google Scholar]
- 38.Younossi ZM, Stepanova M, Zeuzem S, et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J Hepatol 2014; 61:228–34. [DOI] [PubMed] [Google Scholar]
- 39.Riley ED, Bangsberg DR, Perry S, Clark RA, Moss AR, Wu AW. Reliability and validity of the SF-36 in HIV-infected homeless and marginally housed individuals. Qual Life Res 2003; 12:1051–8. [DOI] [PubMed] [Google Scholar]
- 40.Hsiung PC, Fang CT, Chang YY, Chen MY, Wang JD. Comparison of WHOQOL-bREF and SF-36 in patients with HIV infection. Qual Life Res 2005; 14:141–50. [DOI] [PubMed] [Google Scholar]
- 41.Butt Z, Lai JS, Rao D, Heinemann AW, Bill A, Cella D. Measurement of fatigue in cancer, stroke, and HIV using the Functional Assessment of Chronic Illness Therapy - Fatigue (FACIT-F) scale. J Psychosom Res 2013; 74:64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med 2001; 33:350–7. [DOI] [PubMed] [Google Scholar]
- 43.Webster K, Odom L, Peterman A, Lent L, Cella D. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Validation of version 4 of the core questionnaire. Quality Life Res 1999; 8:604. [Google Scholar]
- 44.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999; 45:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993; 4:353–65. [DOI] [PubMed] [Google Scholar]
- 46.Brazier JE, Rowen D, Hanmer J. Revised SF-6D scoring programmes: a summary of improvements. PRO Newsletter 2008; 40(Fall):14–5.
- 47.Fleming CA, Christiansen D, Nunes D, et al. Health-related quality of life of patients with HIV disease: impact of hepatitis C coinfection. Clin Infect Dis 2004; 38:572–8. [DOI] [PubMed] [Google Scholar]
- 48.Younossi Z, Stepanova M, Marcellin P, Afdhal N, Hunt S. Ledipasvir (LDV) and sofosbuvir (SOF) combination improves patient-reported outcomes (PRO) during treatment of chronic hepatitis C (CH-C) patients: results from the ION-1 clinical trial [abstract P1324] Presented at: 49th Annual Meeting of European Association for the Study of the Liver, London, United Kingdom, 2014. [Google Scholar]
- 49.Younossi ZM, Stepanova M, Nader F, et al. Patient-reported outcomes in chronic hepatitis C patients with cirrhosis treated with sofosbuvir-containing regimens. Hepatology 2014; 59:2161–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.