Abstract
Temperature–food interactions in the larval environment can affect life history and population growth of container mosquitoes Aedes aegypti (L.) and Aedes albopictus Skuse, the primary vectors of chikungunya and dengue viruses. We used Ae. aegypti, Ae. albopictus, and dengue-1 virus (DENV-1) from Florida to investigate whether larval rearing temperature can alter the effects of larval food levels on Ae. aegypti and Ae. albopictus life history and DENV-1 infection and vertical transmission. Although we found no effect of larval treatments on survivorship to adulthood, DENV-1 titer, or DENV-1 vertical transmission, rates of vertical transmission up to 16–24% were observed in Ae. albopictus and Ae. aegypti, which may contribute to maintenance of this virus in nature. Larval treatments had no effect on number of progeny and DENV-1 infection in Ae. aegypti, but the interaction between temperature and food affected number of progeny and DENV-1 infection of the female Ae. albopictus parent. The cooler temperature (24°C) yielded the most progeny and this effect was accentuated by high food relative to the other conditions. Low and high food led to the highest (∼90%) and lowest (∼65%) parental infection at the cooler temperature, respectively, whereas intermediate infection rates (∼75–80%) were observed for all food conditions at the elevated temperature. These results suggest that temperature and food availability have minimal influence on rate of vertical transmission and a stronger influence on adults of Ae. albopictus than of Ae. aegypti, which could have consequences for dengue virus epidemiology.
Keywords: larval ecology, infection, transmission, dengue
Dengue viruses are responsible for up to 390 million infections per year (Kroeger et al. 2004, WHO 2012). Transmission to humans occurs by widespread invasive mosquitoes Aedes aegypti and Aedes albopictus (WHO 2012). Horizontal (human–mosquito–human) transmission is the best known mechanism of DENV transmission. However, vertical transmission, the transfer of virions from an infected female mosquito to her progeny has also been documented in natural populations of Ae. aegypti and Ae. albopictus (Angel and Joshi 2008, Martins et al. 2012, Espinosa et al. 2014). Additionally, DENV vertical transmission has been demonstrated in both mosquito species in the laboratory (Rosen et al. 1983, Shroyer 1990, Bosio et al. 1992, Castro et al. 2004, Buckner et al. 2013). Though the occurrence of DENV vertical transmission is clear, its role in the DENV transmission cycle is not well-understood. A frequently cited hypothesis is that vertical transmission serves as a mechanism that allows DENV to persist in nature during inter-epidemic periods (Rosen et al. 1983, Hull et al. 1984, Joshi et al. 2002, Guo et al. 2007, Angel and Joshi 2008).
Mosquitoes possess a complex life cycle in which the immature and adult stages occupy distinctly different environments. An expansive literature demonstrates that the environmental factors experienced by an adult female mosquito can affect her vector competence, the innate ability of the vector to support replication and transmission of a pathogen (Turell 1993, Richards et al. 2007). Additionally, growing evidence suggests that effects of the larval environment can continue through the adult stage to also influence vector competence, because innate immunity, infection barriers, and escape barriers develop during larval stages (Grimstad and Walker 1991; Reiskind and Lounibos 2009; Muturi et al. 2011a, 2012; Alto and Lounibos 2013). In particular, the interaction between larval temperature and nutrient availability may affect mosquito biology and vector competence, especially for container mosquitoes (Padmanabha et al. 2011). In urban and suburban areas in DENV endemic climates (≈20–30°C), Ae. aegypti and Ae. albopictus larvae primarily inhabit water-filled artificial containers like water storage containers, plant vases, and tires. Container habitats often are associated with episodic and irregular inputs of basal nutrients and crowded larval conditions causing larvae to experience food limitation (Southwood et al. 1972, Subra and Mouchet 1984, Arrivillaga and Barrera 2004, Juliano 2007, Padmanabha et al. 2010), which may affect adult nutrient reserves (Briegel 1990) and so may influence adult morphology (size) and physiology (immune functions; Ahmed et al. 2002, Schwartz and Koella 2004, Telang et al. 2012, Kim and Muturi et al. 2013). Temperature in these container habitats may also affect mosquito larval development and survival within food-limited conditions by modifying feeding rate, efficiency of food conversion into biomass, or ability to survive starvation (Rashed and Mulla 1989, Padmanabha et al. 2011). Therefore, by affecting adult morphology, physiology, and survival, temperature–food interactions in the larval environment could have consequences for Ae. aegypti and Ae. albopictus biology and DENV vector competence. If observed larval environment-induced changes in infection and horizontal transmission also affect changes in vertical infection (Turell 1993, Alto and Bettinardi 2013), then the parental environment may contribute to infection and transmission by the progeny. Further, we may expect that the relative role of vertical transmission in dengue epidemiology is dynamic and changes with environmental conditions. Based on findings investigating single treatment effects, we predict that nutrient stress and elevated temperature may reduce barriers to dengue virus vertical transmission (Zhang et al. 1993, Alto and Bettinardi 2013, Alto and Lounibos 2013).
Previously, for dengue-1 virus (DENV-1) isolated during the 2010 Key West, Florida outbreak, we demonstrated infection and dissemination rates of 93.3 and 80.2% for Ae. aegypti and 93.0 and 84.9% for Ae. albopictus, respectively, which had fed on infected blood with viremia levels observed in humans (Buckner et al. 2013). In this same study, we documented DENV-1 vertical transmission rates of 8.3% for Ae. aegypti and 11.1% for Ae. albopictus (Buckner et al. 2013). In the current work, we test the hypothesis that larval temperature alters the effects of larval food levels that continue through the adult stage and influence DENV-1 infection and transmission to their progeny (vertical transmission). We interpret this information in the context of measurements on mosquito life history: survivorship to adulthood, development time, and number of progeny of Ae. aegypti and Ae. albopictus.
Materials and Methods
Rearing of Ae. aegypti and Ae. albopictus
We manipulated temperature and food level during the immature stages of the parental (P1) generation of Ae. aegypti and Ae. albopictus mosquitoes using a two-level full factorial experimental design with six replicates per treatment for 24 experimental units. Experiments were conducted independently for each species to increase the number of replicates per treatment. The P1 generation used in the Ae. aegypti experiment was the F6 progeny of larvae collected from Key West, FL. The P1 generation used in the Ae. albopictus experiment was the F2 progeny of larvae collected from the campus of the Florida Medical Entomology Laboratory in Vero Beach, FL. Experimental units consisted of 7.5-liter containers (height by diameter: 24 by 23.5 cm) with 5 liters of tap water and 500 first-instar larvae. We controlled larval temperature by maintaining experimental units in incubators at 24 ± 0.5°C and 30 ± 0.5°C. These temperatures were chosen because they are within the range in which Ae. aegypti and Ae. albopictus develop and dengue virus may circulate. Larval food level was controlled by providing containers with either 250 mg or 500 mg of an equal mixture of brewer’s yeast and lactalbumin on the day larvae were added. Supplemental food resources (250 mg or 500 mg depending on treatment) were added to containers on days 4 and 11 after addition of larvae.
The time until pupation and the number of mosquitoes surviving to pupation for each experimental unit were recorded, and pupae were transferred to water-filled cups and placed in 0.3-m3 adult cages labeled according to experimental unit and date. Only pupae collected for five consecutive days from the same experimental unit were placed in the same adult cage, which narrowed the ages of adult mosquitoes in each cage to a 5-d period to ensure that infectious bloodmeals would later be provided to similarly aged adult females to minimize the possibility for age-related variation in measurements (Buckner et al. 2013). After emergence, male and female mosquitoes were held together for approximately 7–12 d at 30 ± 0.5°C to allow mating and provided with 20% sucrose solution, except for 48 hours before infectious bloodmeals, when the sucrose solution was replaced by tap water. Adults were then aspirated from each cage, cold-anesthetized, and sorted on a chill table (BioQuip Products, Rancho Dominguez, CA). Males were separated from females and discarded. Females were transferred to 1-liter cylindrical cartons covered by screen, with approximately 75 adults per carton for the feeding trials with DENV-1-infected blood.
Preparation of Infectious Bloodmeal
Propagation of DENV-1 virus for bloodmeals followed previously established methods (Buckner et al. 2013). Briefly, confluent monolayers of African green monkey kidney (Vero) cells were inoculated with 250 μl of DENV-1 (GenBank accession no. JQ675358, originally isolated from a human infected in Key West, FL, in 2010 and passaged three times in Vero cells) at a multiplicity of infection of 0.3. After one hour of incubation at 35°C in a 5% CO2 atmosphere, 25 ml of media (199 media, 10% fetal bovine serum, 0.2% antimycotic, and 2% penicillin-streptomycin) were added to each flask. After 7 d, media and virus were collected from flasks and combined with defibrinated bovine blood (Hemostat, Dixon, CA) in a 1:1 ratio of blood: media + virus. Aliquots of DENV-1-infected blood were taken just before feeding trials and stored at −80°C. Viral titers of infected blood were determined using quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR; Buckner et al. 2013).
Oral Infection of Mosquitoes
Females were given the opportunity to feed on DENV-1-infected blood using an artificial membrane feeding system (Hemotek, Discovery Workshops, Accrington, Lancashire, UK) for approximately 45 min. After offering the infectious bloodmeal, females were cold-anesthetized and blood-fed were separated from unfed females based on abdominal color and distention under a 10 × stereo microscope. Fully engorged mosquitoes were housed together in cages with 10% sucrose solution, and females that did not initially feed were given subsequent opportunities to feed on infectious blood for each of the next 3 d. The six replicates per treatment were retained throughout the infection study.
During and after feeding on infectious blood, females were held within incubators at 30 ± 0.5°C on a daily photoperiod of 14:10 (L:D) h. After imbibing infectious blood, females were provided with 10% sucrose solution and held for 12 d, which approximates the extrinsic incubation period of dengue viruses at 30°C (Watts et al. 1987, Chan and Johansson 2012). During this time, females were allowed to lay their first gonotrophic cycle eggs. However, these eggs were not assayed for DENV-1 RNA because vertical transmission of DENV during the first gonotrophic cycle is rare (Mourya et al. 2001).
Provision of Noninfectious Bloodmeal
Twelve days after imbibing infectious blood, to stimulate a second gonotrophic cycle, females were given the opportunity to feed for approximately 45 min on defibrinated bovine blood. After the noninfectious bloodmeal, mosquitoes were cold-anesthetized, and blood-fed and unfed females were separated using criteria described earlier. Fully engorged mosquitoes were placed individually in 37-ml plastic tubes (h by d: 8 by 3 cm), which were covered with screen and lined with moist germination paper for oviposition. Mosquitoes were given 5 d to lay their eggs.
Determination of Infection Status of P1 and F1 Generations
After the P1 generation females laid eggs from their second gonotrophic cycle, they were anesthetized using CO2 and stored in centrifuge tubes at −80°C. Hatching of F1 eggs was stimulated by immersing germination papers with eggs in individual 0.47-liter water-filled containers containing 100 mg of larval food. Eggs from each female were hatched separately. The F1 generation was reared to late larval instars, counted, and frozen in centrifuge tubes at −80°C. All progeny from each P1 generation female were pooled into one tube to allow for determining vertical transmission rates for each larval treatment (the proportion of P1 generation-infected females that infected at least one of their progeny). Whole-body P1 generation females and their progeny samples were then homogenized using a TissueLyser (Qiagen®, Valencia, CA) in centrifuge tubes containing 0.9 ml BA-1 diluent and two zinc-plated steel BBs.
The homogenized samples were clarified by centrifugation and total nucleic acids were extracted from 250 μl samples using a MagNA Pure Total Nucleic Acid kit (Roche Diagnostics, Chicago, IL) and a MagNA Pure LC robot (Roche Diagnostics, Chicago, IL). Following extraction of total nucleic acids, qRT-PCR was conducted on the samples using SuperScript III Platinum one step qRT-PCR (Invitrogen Company, Carlsbad, CA) and fluorogenic probe hydrolysis (TaqMan, Applied Biosystems, Foster City, CA) technology. Quantitative RT-PCR reactions were performed in 96-well reaction plates, with each containing: 0.4 µl SuperScript III RT/Platinum Taq mix, 10 µl 2x reaction mix, 1 µl DENV-1 specific forward primer (10 µmol/L), 1 µl DENV-1 specific reverse primer (10 µmol/L), 0.4 µl fluorogenic probe (10 µmol/L), 2.2 µl H2O, and 5 µl test sample. Amplification and quantification of DENV-1 RNA in positive samples were performed by running the 96-well reaction plate in a LC480 thermocycler machine (Roche Diagnostics, Chicago, IL) programmed for the following: 60°C for 30 minutes, 2 minutes at 95°C, followed by 40 cycles of PCR (15 seconds at 95°C and 1 minute at 60°C), and 1 second at 45°C (Callahan et al. 2001). A negative control (water) and positive control standard (DENV-1 RNA 10−2 dilution, GenBank accession no. JQ675358) were included on each 96-well reaction plate. The titer of each mosquito sample and blood from feeding trials was expressed in PFUeq/ml by a standard curve method that compares the known titer of the positive standard control to the titer of unknown samples (Buckner et al. 2013).
Calculations and Statistical Analysis
As a result of the separate experiments performed for Ae. aegypti and Ae. albopictus, data for the two species were analyzed separately. This allowed for qualitative but not quantitative comparisons of results between species. The following mosquito life history information was measured for each experimental unit for each species: the mean number of days to pupation, proportion of P1 generation mosquitoes surviving to pupation, and mean number of F1 progeny that hatched from second gonotrophic cycle eggs. Additionally, the mean DENV-1 titer of individual females was calculated for each experimental unit. P1 generation infection rates of females were calculated by dividing the number of females infected with DENV-1 by the total number of females that imbibed an infectious bloodmeal, expressed as a percent. Vertical transmission was calculated by dividing the number of P1 generation females that had DENV-1-infected progeny by the total number of DENV-1-infected P1 generation females, expressed as a percent. Here we calculate infection, viral titer, and vertical transmission only for mosquitoes that fed on both an infectious and a noninfectious bloodmeal.
All statistical analyses were performed using the SAS statistical package version 9.3 (SAS Institute, Cary, NC). Some of the measurements did not meet the assumptions of normality and homoscedasticity. Therefore, the effects of larval treatments on number surviving to pupation, time to pupation in days, number of F1 progeny that hatched from second gonotrophic cycle eggs, and DENV-1 titer of infected females were analyzed using separate nonparametric Kruskal–Wallis tests. Post hoc comparisons used the least significant difference in mean ranks. The Bonferroni correction was used to adjust P-values for all post hoc comparisons. We tested for the interaction of temperature and food factors by reorganizing the data so that each temperature–food combination represented a single treatment, which yielded four individual treatments. Significant effects of treatment were further analyzed by determining the least significant difference in mean ranks. We tested for differences in the number of progeny from infected and uninfected parental mosquitoes using the Wilcoxon–Mann–Whitney test. The number of mosquitoes available for testing for DENV-1 infection was much lower than the number of mosquitoes surviving to adulthood, attributable to low blood feeding rates and mortality during the adult stage. The effects of larval treatments and their interaction on the number of Ae. aegypti and Ae. albopictus females that developed DENV-1 infections and vertically transmitted DENV-1 to their progeny were analyzed by maximum likelihood categorical analyses of contingency tables (PROC CATMOD, SAS Institute 2010, Agresti 1990). When significant interactions were detected, we reorganized the data in the manner described above and used pairwise contrasts to determine if effects of treatments were statistically different.
Results
Mosquito Life History
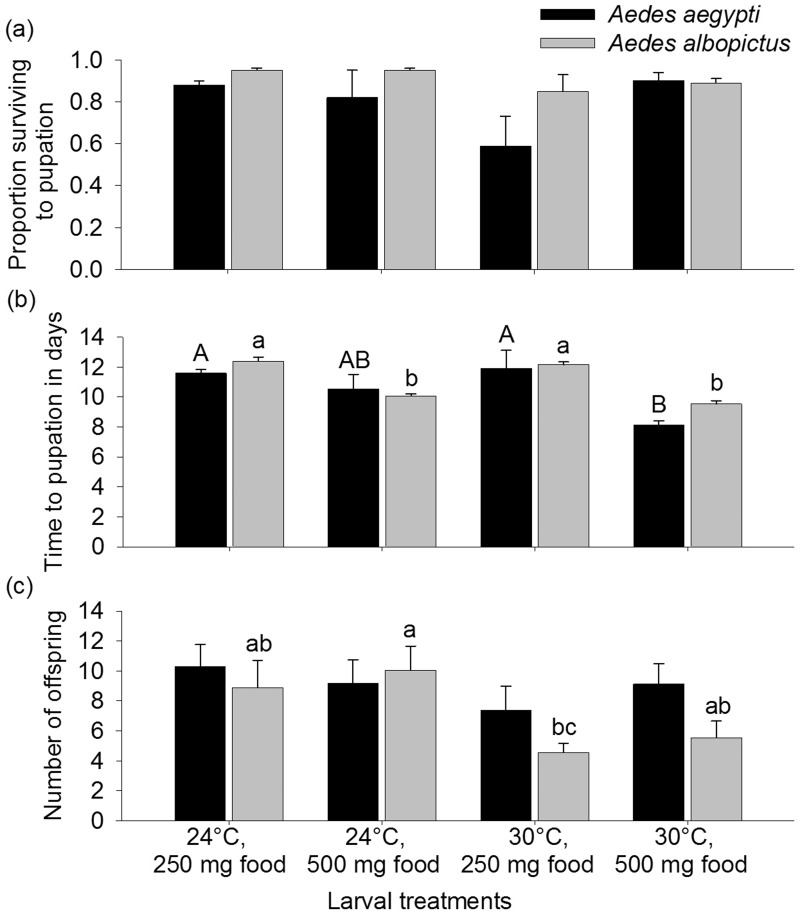
Temperature and food treatments did not affect Ae. aegypti or Ae. albopictus survival (Ae. aegypti, H = 4.80, df = 3, P = 0.19, Ae. albopictus, H = 6.59, df = 3, P = 0.09) but did affect days to pupation for both species (Ae. aegypti: H = 12.99, df = 3, P = 0.005; Ae. albopictus: H = 18.33, df = 3, P = 0.0004). Food, but not temperature, altered development time for Ae. albopictus. Specifically, more food resulted in significantly shorter times to pupation (Fig. 1). For Ae. aegypti, more food resulted in fewer days to pupation, but only at 30°C (Fig. 1). Larval factors did affect the number of Ae. albopictus progeny (H = 8.73, df = 3, P = < 0.033) but not Ae. aegypti progeny (H = 1.62, df = 3, P = 0.65). Ae. albopictus females from the 24°C, 500 mg food larval treatment produced more progeny than cohorts from the 30°C, 250 mg larval food treatment (Fig. 1).
Fig. 1.
Means (±SE) of proportion of Aedes aegypti and Aedes albopictus mosquitoes surviving to pupation (a), time to pupation in days (b), and number of 2nd gonotrophic cycle progeny (c) for larval treatments, which varied temperature (24°C or 30°C) and food levels (250 mg or 500 mg as initial amounts). Means followed by different letters (upper case for Ae. aegypti and lower case for Ae. albopictus) show significant differences in ranks. Post hoc comparisons used the least significant difference in mean ranks.
Dengue Virus Infection, Body Titer, and Vertical Transmission
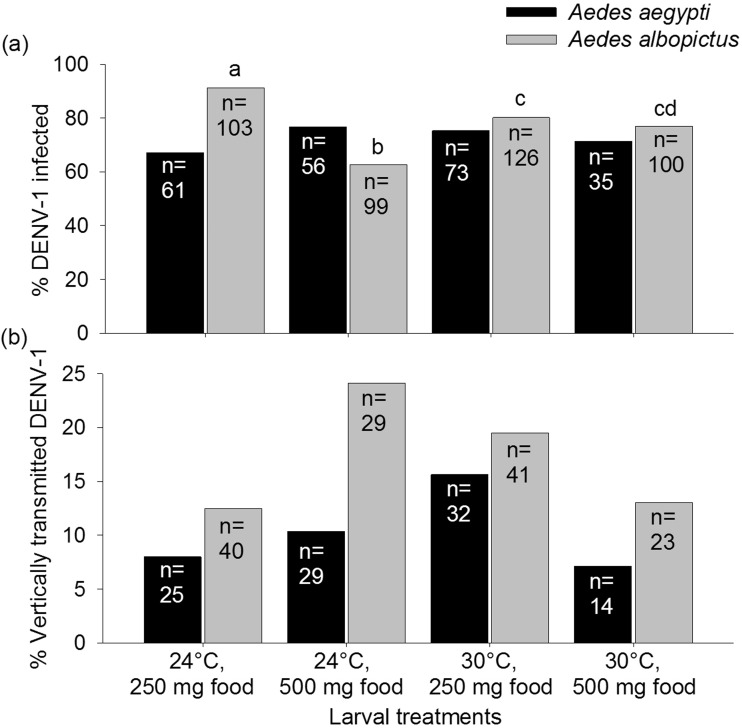
The mean ± standard error viral titers of the infectious bloodmeals provided to Ae. aegypti and Ae. albopictus mosquitoes were determined to be 7.03 ± 0.06 and 6.12 ± 0.16 log10 PFUeq DENV/mL, respectively, which is within the range of viremia levels observed in humans (Gubler et al. 1981, Vaughn et al. 2000, Stramer et al. 2012). Out of the 225 Ae. aegypti and 428 Ae. albopictus females that imbibed infected blood, 164 (72.9%) and 334 (78.0%) developed DENV-1 infections, respectively. Vertical transmission rates for Ae. albopictus ranged between 13–24% among treatments and 7–16% for Ae. aegypti (Fig. 2).
Fig. 2.
Percentages of Aedes aegypti and Aedes albopictus mosquitoes infected with DENV-1 (a) and vertically transmitted DENV-1 to progeny (b) for larval treatments, which varied temperature and food levels. Numbers (n) associated with treatment groups indicate the total number of females provided with a DENV-1 bloodmeal (a) and total number of DENV-1-infected mothers (b) for each treatment. Percentages followed by different lowercase letters show significant differences determined by maximum likelihood follow-up contrasts (temperature – Ae. albopictus: χ2 = 0.04, df = 1, P = 0.83, Ae. aegypti: χ2 = 0.06, df = 1, P = 0.81; food – Ae. albopictus: χ2 = 0.10, df = 1, P = 0.76, Ae. aegypti: χ2 = 0.16, df = 1, P = 0.69; food × temperature interaction – Ae. albopictus: χ2 = 2.01, df = 1, P = 0.16, Ae. aegypti: χ2 = 0.61, df = 1, P = 0.44). Post hoc comparisons used the least significant difference in mean ranks.
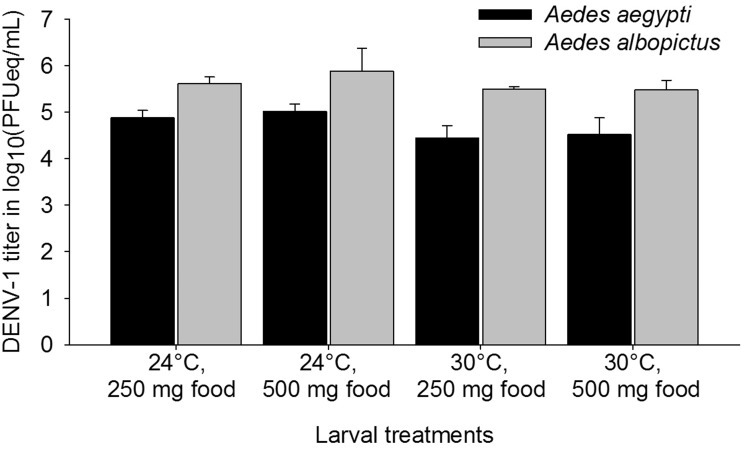
Food quantity and temperature, as well as their interaction, had no influence on the proportion of DENV-1-infected Ae. aegypti, the titer of Ae. aegypti and Ae. albopictus infected mosquitoes, and the proportion of Ae. aegypti and Ae. albopictus that vertically transmitted DENV-1 to their progeny (P > 0.08 in all instances; Figs. 2 and 3). However, food quantity and the interaction between food quantity and temperature did influence the proportion of DENV-1-infected Ae. albopictus (food: χ2 = 15.01, df = 1, P = 0.0001; food × temperature: χ2 = 9.94, df = 1, P = 0.002), while temperature alone did not (χ2 = 0.24, df = 1, P = 0.62; Fig. 2). Infection rates were highest at low-temperature–low-nutrient treatment and lowest in low-temperature–high-nutrient treatment (Table 1; Fig. 2). Infection status of the female parent (uninfected vs infected) did not influence the number of progeny during the second gonotrophic cycle (Wilcoxon–Mann–Whitney test, Ae. aegypti, z =−0.36, P = 0.72; Ae. albopictus, z = 0.08, P = 0.93).
Fig. 3.
Means (±SE) of DENV-1 titer in log10(PFUeq/mL) of infected Aedes aegypti and Aedes albopictus mosquitoes for larval treatments, which varied temperature (24°C or 30°C) and food levels (250 mg or 500 mg as initial amounts). Post hoc comparisons used the least significant difference in mean ranks.
Table 1.
Maximum likelihood follow-up contrasts for proportions of Aedes albopictus females infected with DENV-1 from each treatment
| Contrast | χ2 value | df | P value |
|---|---|---|---|
| 24°C, 250 mg vs 24°C, 500 mg | 20.31 | 1 | <0.0001* |
| 24°C, 250 mg vs 30°C, 250 mg | 5.26 | 1 | 0.02* |
| 24°C, 250 mg vs 30°C, 500 mg | 7.26 | 1 | 0.01* |
| 24°C, 500 mg vs 30°C, 250 mg | 8.32 | 1 | 0.004* |
| 24°C, 500 mg vs 30°C, 500 mg | 4.81 | 1 | 0.03* |
| 30°C, 250 mg vs 30°C, 500 mg | 0.33 | 1 | 0.62 |
An asterisk (*) denotes statistically significant differences between treatment groups. All P-values are adjusted using the Bonferroni approach.
Discussion
We tested the hypothesis that larval temperature alters the effects of larval food level on Ae. aegypti and Ae. albopictus life history and DENV-1 infection and vertical transmission. Larval treatments were not limiting factors that influenced survivorship to adulthood, which supports results from experiments using similar larval rearing conditions (Lounibos et al. 2002; Muturi et al. 2011b, 2012; Yoshioka et al. 2012; Alto and Bettinardi 2013). However, we did observe species-specific patterns of treatments on larval development time. Ae. albopictus accelerated development with increased food quantity but was unaffected by larval rearing temperature. Larval diet has previously been shown to be a significant predictor of time to pupation for Ae. albopictus, but our finding of the lack of effect of larval temperature on Ae. albopictus development time contrasts with other studies that have documented higher larval temperature decreasing Ae. albopictus development time (Muturi et al. 2011c, Yoshioka et al. 2012, Alto and Bettinardi 2013). As predicted, more food led to a significantly shorter development time only at the elevated larval temperature in Ae. aegypti, demonstrating an interactive effect of larval temperature and food on Ae. aegypti development. Likewise, Padmanabha et al. (2011) documented an interactive effect of larval temperature and food on wing cell size in Ae. aegypti mosquitoes, consistent with our findings on temperature modulation of the effects of larval food availability in that mosquito species.
Larval rearing conditions dictate nutrient reserves of teneral adults and so are likely to influence the biological processes of female adults such as immune responses (Ahmed et al. 2002, Schwartz and Koella 2004) and reproduction (Briegel 1990). Specifically, infection with pathogens may decrease reproductive fitness (Sheldon and Verhulst 1996). We observed no relationship between the number of eggs laid by female mosquitoes and their infection rates, providing indirect support that reproductive output was not compromised by different rates of DENV-1 infection. A caveat to this interpretation is that our experimental design did not include groups of mosquitoes that were unexposed to DENV-1, and so we cannot distinguish between individuals that mount an immune response associated with DENV-1 exposure and those that don’t. The effects of larval treatment on number of progeny were only significant for Ae. albopictus. Females reared at the cooler temperature and provided with more food experienced a 44% increase in the number of progeny compared with females reared at the elevated temperature and provided with less food. These results suggest that elevated temperature and less nutrition may induce reproductive stress relative to females from other rearing conditions (Briegel 1990, Telang and Wells 2004). While body sizes of mosquitoes were not determined in our study, larger Ae. albopictus females have previously been found to be more fecund than their smaller counterparts (Blackmore and Lord 2000). Body size, temperature, and food levels are known to have complex, population-dependent relationships in this species (Reiskind and Zarrabi 2012). Larger adult size has been shown to be associated with cooler larval conditions (e.g., <25°C) in mosquitoes, which may explain why females reared at the cooler larval temperature and provided with more food produced more progeny than those reared at the elevated larval temperature and provided with less food (Padmanabha et al. 2011).
Previous studies have demonstrated either larval nutrient or temperature-mediated changes in vector competence for arboviruses, but, to our knowledge, our study is one of the first to investigate the potential interaction between these factors and arbovirus infection and transmission. Cooler larval temperature and less food enhanced Ae. albopictus DENV-1 infection by approximately 33% relative to high food. The elevated larval temperature (30°C) resulted in similar rates of DENV-1 infection of adults from both low- and high-nutrient provisioned larvae. However, infection rates at the elevated temperature were intermediate and distinct from mosquitoes between low and high food at the cooler temperature, suggesting a complex interaction between these factors. These results provide partial support for our hypothesis that temperature alters the effects of larval food level on Ae. albopictus DENV-1 infection, although these effects do not appear to alter rates of vertical transmission, which indicate a unique reproductive barrier(s) to transmission. These results are consistent with Westbrook et al. (2010), who showed that Ae. albopictus reared at a cooler larval temperature (18°C) had higher chikungunya virus infection and dissemination rates compared with those reared at a warmer larval temperature (32°C). Zhang et al. (1993) observed small adult Ae. albopictus derived from a poor larval diet experienced enhanced DENV-2 dissemination relative to adults from nutrient-rich larval conditions. Additionally, these authors showed that field collections of Ae. albopictus had sizes that more closely reflected those from poor larval diet, suggesting that the nutrient conditions used in the study were within the range encountered in nature. Along the same lines, nutrient-deprivation during the larval stages enhanced infection and transmission (horizontal and vertical) of LaCrosse encephalitits virus by Aedes triseriatus (Say; Grimstad and Haramis 1984, Patrican and DeFoliart 1985, Grimstad and Walker 1991). Together these observations are consistent with observations that temperature and nutrition may alter the expression of immunity-related genes and viral competence (Telang et al. 2012, Muturi et al. 2011c). Specifically, cooler rearing conditions may compromise the immune system in Ae. albopictus and lead to higher flavivirus and alphavirus infection, perhaps accentuated by nutrient deprivation (Adelman et al. 2013). Ae. albopictus adult mosquitoes that develop in areas of the world with cooler ambient temperatures (e.g., <25°C) and nutrient-poor conditions as larvae may be more likely infected with DENV compared with those exposed to nutrient-rich conditions as larvae. By contrast, other investigations have found little evidence relating larval nutrition to measurements of vector competence (e.g., Culex annulirostris Skuse and Murray Valley encephalitis virus, Kay et al. 1989; Aedes vigilax Skuse and Ross River virus, Jennings and Kay 1999; Culex tarsalis Coquillett and West Nile virus, Dodson et al. 2011). However, these studies used a single-temperature experimental design (range, 27–28°C), limiting the ability to identify possible nutrient-mediated changes in adult competence for arboviruses that may vary across a temperature gradient.
While treatment factors altered infection in Ae. albopictus, we did not observe effects on Ae. aegypti DENV-1 infection, suggesting that the stress of larval treatments used in our study may have been enough to influence Ae. aegypti development time to pupation but not infection barriers such as immune system function. Alto et al. (2008) also documented species-specific differences in effects of larval competition on DENV-2 infection rates in Ae. aegypti and Ae. albopictus. Specifically, they found that larval competition enhanced components of vector competence in Ae. albopictus, but not Ae. aegypti. While the reasons for differences between the two Aedes species in response to larval treatments are unknown, proposed explanations are species-specific differences in immune function and arbovirus infection barriers that may be differentially affected by the larval environment (Alto al. 2008). Together the results indicate that larval environmental conditions, such as the interaction between temperature and food availability or competition, may have a stronger impact on Ae. albopictus compared with Ae. aegypti for dengue viruses (Alto et al. 2008).
To our knowledge, our study is the first to examine the effects of the larval environment on DENV vertical transmission in mosquitoes. Though there was no effect of larval treatments on DENV-1 titer and vertical transmission, we demonstrated that vertical transmission rates of up to 15.6 and 24.1% are possible in Ae. aegypti and Ae. albopictus mosquitoes, respectively. Using a mathematical modeling approach, Adams and Boots (2010) suggest that a DENV vertical infection rate of 5% can lead to local survival of the virus in between outbreaks for a matter of months, and they define vertical infection rate as vertical transmission rate (the proportion of infected females that infect at least one of their progeny) × filial infection rate (the proportion of the progeny of an infected female that are infected given that vertical transmission occurred; Adams and Boots 2010). Additionally, these authors suggest that a DENV vertical infection rate of 10% may allow DENV to survive locally in the environment for up to two years in between outbreaks. In context of a vertical infection rate of 5% suggested by Adams and Boots (2010), if the filial infection rates exceed 25% in Ae. albopictus, then the vertical transmission rate of 24.1% in Ae. albopictus documented in our study could lead to the persistence of DENV for months between outbreaks (Adams and Boots 2010). Also, if filial infection rates exceed 35% in Ae. aegypti, then the vertical transmission rate of 15.6% in Ae. aegypti documented in our study could lead to the persistence of DENV for months between outbreaks. However, because we pooled the progeny from each P1 female and did not test each offspring separately for DENV, we cannot determine the actual filial infection rates for the progeny of each female. Filial infection rates have been found to be lower than rates suggested for the persistence of dengue viruses between outbreaks based on models by Adams and Boots (2010) (Ae. albopictus OAHU strain and DENV-1 Fiji strain, 0.4–5%, Shroyer 1990; DENV-1 Jamaican strain, 1.4–17.4%, Bosio et al. 1992; Ae aegypti and DENV-2 16681 from Thailand, 1.4-3.7%, Wasinpiyamongkol et al. 2003), bringing into question the importance that vertical transmission plays in the persistence of DENV-1.
Though the separate larval manipulation experiments conducted for Ae. aegypti and Ae. albopictus in this study prevented a quantitative comparison between the DENV-1 vertical transmission rates of the Aedes species, it is worth noting that qualitatively higher vertical transmission rates were documented in Ae. albopictus (∼13–24%) compared with Ae. aegypti (∼7–16%), despite having fed on a lower dose of DENV-1-infected blood. Previously, we also documented a higher DENV-1 vertical transmission rate in Ae. albopictus (11%) compared with Ae. aegypti (8%; Buckner et al. 2013). Our observations are consistent with other comparative studies demonstrating higher rates of vertical transmission of dengue viruses by Ae. albopictus than Ae. aegypti (reviewed by Lambrechts et al. 2010). The higher DENV-1 vertical transmission rates documented in Ae. albopictus compared with Ae. aegypti potentially suggest that while Ae. aegypti may the more efficient horizontal vector of DENV, Ae. albopictus could be the more efficient vertical vector of DENV. A meta-analysis of experimental laboratory studies revealed that overall Ae. albopictus is overall more susceptible to DENV midgut infection than Ae. aegypti, suggesting greater DENV adaptation to Ae. albopictus (Lambrechts et al. 2010). Therefore, the potential role of Ae. albopictus in the transmission cycle of DENV should not be overlooked.
Acknowledgments
We thank D. Bettinardi for assisting with blood-feeding trials and processing samples, N. Karr for experiment maintenance, and N. Nishimura for performing some of the statistical analyses. DENV-1 (strain BOL-KW010) was kindly provided by the Florida Department of Health Bureau of Laboratories. The research was funded by Florida Department of Agricultural and Consumer Services contract 016603 (University of Florida Project No. 00090369) and NIH grant R01 AI-044793.
References Cited
- Adams B., Boots M. 2010. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics 2: 1–10. [DOI] [PubMed] [Google Scholar]
- Adelman Z. N., Anderson M. A. E., Wiley M. R., Murreddu M. G., Samuel G. H., Morazzani E. M., Myles K. M. 2013. Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl. Trop. Dis. 7: e2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti A. 1990. An introduction to categorical data analysis. John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- Ahmed A. M., Baggott S. L., Hurd H. 2002. The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos 97: 371–377. [Google Scholar]
- Alto B. W., Bettinardi D. 2013. Temperature and dengue virus infection in mosquitoes: Independent effects on the immature and adult stages. Am. J. Trop. Med. Hyg. 88: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto B. W., Lounibos L. P. 2013. Vector competence for arboviruses in relation to the larval environment of mosquitoes, pp. 81–101. In Takken W., Koenraadt C. J. M. (eds.), Ecology of parasite-vector interactions, vol. 3 Wageningen Academic Publishers, Wageningen, NL. [Google Scholar]
- Alto B. W., Lounibos L. P., Mores C. N., Reiskind M. H. 2008. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc. R. Soc. B 275: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel B., Joshi V. 2008. Distribution and seasonality of vertically transmitted dengue viruses in Aedes mosquitoes in arid and semi-arid areas of Rajasthan, India. Vector Borne Zoonotic Dis. 45: 56–59. [PubMed] [Google Scholar]
- Arrivillaga J., Barrera R. 2004. Food as a limiting factor for Aedes aegypti in water-storage containers. J. Vector Ecol. 29: 11–20. [PubMed] [Google Scholar]
- Blackmore M., Lord C. 2000. The relationship between size and fecundity in Aedes albopictus. J. Vector Ecol. 25: 212–217. [PubMed] [Google Scholar]
- Bosio C., Thomas R., Grimstad P., Rai K. 1992. Variation in the efficiency of vertical transmission of dengue-1 virus by strains of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 29: 985–989. [DOI] [PubMed] [Google Scholar]
- Briegel H. 1990. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 36: 165–172. [Google Scholar]
- Buckner E. A., Alto B. W., Lounibos L. P. 2013. Vertical transmission of Key West dengue-1 virus by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes from Florida. J. Med. Entomol. 50: 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan J. D., Shuenn-Jue L., Dion-Schultz A., Mangold B. E., Peruski L. F., Watts D. M., Porter K. R., Murphy G. R., Suharyono W., King C. C., et al. 2001. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J. Clin. Microbiol. 39: 4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M. G., Nogueira R. M. R., Schatzmayr H. G., Miagostovich M. P., Lourenço-de-Oliveira R. 2004. Dengue virus detection by using reverse transcription -polymerase chain reaction in saliva and progeny of experimentally infected Aedes albopictus from Brazil. Mem. Inst. Oswaldo Cruz 99: 809–814. [DOI] [PubMed] [Google Scholar]
- Chan M., Johansson M. A. 2012. The incubation periods of dengue viruses. PLoS ONE 7: e50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson B. L., Kramer L. D., Rasgon J. L. 2011. Larval nutritional stress does not affect vector competence for West Nile virus (WNV) in Culex tarsalis. Vector-borne and Zoonotic Dis. 11: 1493–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa M., Giamperetti S., Abril M., Seijo A. 2014. Vertical transmission of dengue virus in Aedes aegypti collected in Puerto Iguazú, Misiones, Argentina. Rev. Inst. Med. Trop. Sao Paulo 56: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstad P. R., Walker E. D. 1991. Aedes triseriatus (Dipera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. J. Med. Entomol. 28: 378–386. [DOI] [PubMed] [Google Scholar]
- Grimstad P. R., Haramis L. D. 1984. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. III. Enhanced oral transmission by nutrition-deprived mosquitoes. J. Med. Entomol. 21: 249–256. [DOI] [PubMed] [Google Scholar]
- Gubler D. J., Suharyono W., Tan R., Abidin M., Sie A. 1981. Viremia in patients with naturally acquired dengue infection. Bull. World Health Org. 59: 623–630. [PMC free article] [PubMed] [Google Scholar]
- Guo X. X., Zhao T. Y., Dong Y. D., Lu B. L. 2007. Survival and replication of dengue-2 virus in diapausing eggs of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 44: 492–497. [DOI] [PubMed] [Google Scholar]
- Hull B., Tikasingh E., de Souza M., Martinez R. 1984. Natural transovarial transmission of dengue-4 virus in Aedes aegypti in Trinidad. Am. J. Trop. Med. Hyg. 33: 1248–1250. [DOI] [PubMed] [Google Scholar]
- Jennings C. D., Kay B. H. 1999. Dissemination barriers to Ross River virus in Aedes vigilax and the effects of larval nutrition on their expression. Med. Vet. Entomol. 13: 431–438. [DOI] [PubMed] [Google Scholar]
- Joshi V., Mourya D. T., Sharma R. C. 2002. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 67: 158–161. [DOI] [PubMed] [Google Scholar]
- Juliano S. A. 2007. Population dynamics, pp. 265–275. In Floore T. G. (ed.), Biorational control of mosquitoes. Supplement to the Journal of the Mosquito Control Association, vol. 23 American Mosquito Control Assn., Bulletin No. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B. H., Edman J. D., Fanning I. D., Mottram P. 1989. Larval diet and the vector competence of Culex annulirostris (Diptera: Culicidae) for Murray Valley encephalitis virus. J. Med. Entomol. 26: 487–488. [DOI] [PubMed] [Google Scholar]
- Kim C. -H., Muturi E. J. 2013. Effect of larval density and Sindbis virus infection on immune responses in Aedes aegypti. J. Insect Physiol. 59: 604–610. [DOI] [PubMed] [Google Scholar]
- Kroeger A., Nathan N., Hombach J. 2004. Focus: Dengue. Nat. Rev. Microbiol. 2: 360–361. [DOI] [PubMed] [Google Scholar]
- Lambrechts L., Scott T. W., Gubler D. J. 2010. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 4: e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L. P., Suárez S., Menéndez Z., Nishimura N., Escher R. L., O'Connell S. M., Rey J. R. 2002. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J. Vector Ecol. 27: 86–95. [PubMed] [Google Scholar]
- Martins V. E. P., Alencar C. H., Kamimura M. T., Araujo F. M. D., De-Simone S. G., Dutra R. F., Guedes M. I. F. 2012. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceara, Brazil. PLoS ONE 7: e41386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya D. T., Gokhale M., Basu A., Barde P., Sapkal G., Padbidri V., Gore M. 2001. Horizontal and vertical transmission of dengue virus type 2 in highly and lowly susceptible strains of Aedes aegypti mosquitoes. Acta Virol. 45: 67–71. [PubMed] [Google Scholar]
- Muturi E. J., Costanzo K., Kesavaraju B., Alto B. W. 2011a. Can pesticides and larval competition alter susceptibility of Aedes mosquitoes (Diptera: Culicidae) to arbovirus infection? J. Med. Entomol. 48: 429–436. [DOI] [PubMed] [Google Scholar]
- Muturi E. J., Lampman R., Costanzo K., Alto B. W. 2011b. Effect of temperature and insecticide stress on life-history traits of Culex restuans and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 48: 243–250. [DOI] [PubMed] [Google Scholar]
- Muturi E. J., Kim C.-H., Alto B. W., Schuler M. A., Berenbaum M. R. 2011c. Larval environmental stress alters adult mosquito fitness and competence for arboviruses. Trop. Med. Intl. Health 16: 955–964. [DOI] [PubMed] [Google Scholar]
- Muturi E. J., Blackshear M., Montgomery A. 2012. Temperature and density-dependent effects of larval environment on Aedes aegypti competence for an alphavirus. J. Vector Ecol. 37: 154–161. [DOI] [PubMed] [Google Scholar]
- Padmanabha H., Soto E., Mosquera M., Lord C. C., Lounibos L. P. 2010. Ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. Ecohealth 7: 78–90. [DOI] [PubMed] [Google Scholar]
- Padmanabha H., Bolker B., Lord C. C., Rubio C., Lounibos L. P. 2011. Food availability alters the effects of larval temperature on Aedes aegypti growth. J. Med. Entomol. 48: 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrican L. A., DeFoliart G. R. 1985. Lack of adverse effect of transovarially acquired La Crosse virus infection on the reproductive capacity of Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol. 22: 604–611. [DOI] [PubMed] [Google Scholar]
- Rashed S. S., Mulla M. S. 1989. Factors influencing ingestion of particulate materials by mosquito larvae (Diptera: Culicidae). J. Med. Entomol. 26: 210–216. [DOI] [PubMed] [Google Scholar]
- Reiskind M. H., Lounibos L. P. 2009. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med. Vet. Entomol. 23: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind M. H., Zarrabi A. A. 2012. Is bigger really better? Complex responses to temperature in measures of body size of the mosquito, Aedes albopictus. J. Insect Physiol. 58: 911–917. [DOI] [PubMed] [Google Scholar]
- Richards S. L., Mores C. N., Lord C. C., Tabachnick W. J. 2007. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 7: 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L., Shroyer D., Tesh R., Freier J., Lien J. 1983. Transovarial transmission of dengue viruses by mosquitoes Aedes albopictus and Aedes aegypti. Am. J. Trop. Med. Hyg. 32: 1108–1119. [DOI] [PubMed] [Google Scholar]
- (SAS) SAS Institute Inc. 2010. SAS/STAT® 9.22 User’s Guide. The CATMOD procedure. SAS Institute Inc., Cary, NC. [Google Scholar]
- Sheldon B. C., Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. T.R.E.E. 11: 317–321. [DOI] [PubMed] [Google Scholar]
- Shroyer D. 1990. Vertical maintenance of dengue-1 virus in sequential generations of Aedes albopictus. J. Am. Mosq. Control Assoc. 6: 312–314. [PubMed] [Google Scholar]
- Schwartz A., Koella J. C. 2004. The cost of immunity in the yellow fever mosquito, Aedes aegypti depends on immune activation. J. Evol. Biol. 17: 834–840. [DOI] [PubMed] [Google Scholar]
- Stramer S. L., Linnen J. M., Carrick J. M., Foster G. A., Krysztof D. E., Zou S. M., Dodd R. Y., Tirado-Marrero L. M., Hunsperger E., Santiago G. A., et al. 2012. Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion 52: 1657–1666. [DOI] [PubMed] [Google Scholar]
- Southwood T. R. E., Murdie B., Yasuno N., Tonn R. J., Reader P. M. 1972. Studies on the life budget of Aedes aegypti in Wat Samphaya, Bangkok, Thailand. Bull. World Health Org. 46: 211–226. [PMC free article] [PubMed] [Google Scholar]
- Subra R., Mouchet J. 1984. The regulation of preimaginal populations of Aedes aegypti (L) (Diptera: Culicidae) on the Kenya Coast 2: Food as a main regulatory factor. Ann. Trop. Med. Parasitol. 78: 63–70. [DOI] [PubMed] [Google Scholar]
- Telang A., Wells M. A. 2004. The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. J. Insect Physiol. 50: 677–685. [DOI] [PubMed] [Google Scholar]
- Telang A., Qayum A. A., Parker A., Sacchetta B. R., Byrnes G. R. 2012. Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti). Med. Vet. Entomol. 26: 271–281. [DOI] [PubMed] [Google Scholar]
- Turell M. J. 1993. Effect of environmental temperature on the vector competence of Aedes taeniorhynchus for Rift Valley fever and Venezuelan equine encephalitis viruses. Am. J. Trop. Med. Hyg. 49: 672–676. [DOI] [PubMed] [Google Scholar]
- Vaughn D. W., Green S., Kalayanarooj S., Innis B. L., Nimmannitya S., Suntayakorn S., Endy T. P., Raengsakulrach B., Rothman A. L., Ennis F. A., et al. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181: 2–9. [DOI] [PubMed] [Google Scholar]
- Wasinpiyamongkol L., Thongrungkiat S., Jirakanjanakit N., Apiwathnasorn C. 2003. Susceptibility and transovarial transmission of dengue virus in Aedes aegypti: A preliminary study of morphological variations. Southeast Asian J. Trop. Med. Public Health 34: 131–135. [PubMed] [Google Scholar]
- Watts D. M., Burke D. S., Harrison B. A., Whitmire R. E., Nisalak A. 1987. Effect of temperature on the vector efficiency of Aedes aegypti for dengue-2 virus. Am. J. Trop. Med. Hyg. 36: 143–152. [DOI] [PubMed] [Google Scholar]
- Westbrook C. J., Reiskind M. H., Pesko K. N., Green K. E., Lounibos L. P. 2010. Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to chikungunya virus. Vector Borne Zoonotic Dis. 10: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (WHO) World Health Organization. 2012Global strategy for dengue prevention and control, 2012–2020. WHO reference number: WHO/HTM/NTD/VEM/2012.5, (ISBN:9789241504034). [Google Scholar]
- Yoshioka M., Couret J., Kim F., McMillan J., Burkot T. R., Dotson E. M., Kitron U., Vazquez-Prokopec G. M. 2012. Diet and density dependent competition affect larval performance and oviposition site selection in the mosquito species Aedes albopictus (Diptera: Culicidae). Parasit. Vectors 5: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., He G., Xu L., Lin Q., Zhang S. 1993. Effects of larval nutrition on susceptibility of Aedes albopictus to dengue 2 virus. Arbovirus Res. Aust. 6: 44–48. [Google Scholar]