Abstract
The extent to which animal migrations shape parasite transmission networks is critically dependent on a migrant’s ability to tolerate infection and migrate successfully. Yet, sub-lethal effects of parasites can be intensified through periods of increased physiological stress. Long-distance migrants may, therefore, be especially susceptible to negative effects of parasitic infection. Although a handful of studies have investigated the short-term, transmission-relevant behaviors of wild birds infected with low-pathogenic avian influenza viruses (LPAIV), the ecological consequences of LPAIV for the hosts themselves remain largely unknown. Here, we assessed the potential effects of naturally-acquired LPAIV infections in Bewick’s swans, a long-distance migratory species that experiences relatively low incidence of LPAIV infection during early winter. We monitored both foraging and movement behavior in the winter of infection, as well as subsequent breeding behavior and inter-annual resighting probability over 3 years. Incorporating data on infection history we hypothesized that any effects would be most apparent in naïve individuals experiencing their first LPAIV infection. Indeed, significant effects of infection were only seen in birds that were infected but lacked antibodies indicative of prior infection. Swans that were infected but had survived a previous infection were indistinguishable from uninfected birds in each of the ecological performance metrics. Despite showing reduced foraging rates, individuals in the naïve-infected category had similar accumulated body stores to re-infected and uninfected individuals prior to departure on spring migration, possibly as a result of having higher scaled mass at the time of infection. And yet individuals in the naïve-infected category were unlikely to be resighted 1 year after infection, with 6 out of 7 individuals that never resighted again compared to 20 out of 63 uninfected individuals and 5 out of 12 individuals in the re-infected category. Collectively, our findings indicate that acute and superficially harmless infection with LPAIV may have indirect effects on individual performance and recruitment in migratory Bewick’s swans. Our results also highlight the potential for infection history to play an important role in shaping ecological constraints throughout the annual cycle.
Introduction
Migratory species are renowned for their ability to track seasonal fluctuations in environmental conditions and, in so doing, influence ecological networks worldwide (Bauer and Hoye 2014). In particular, there is growing interest in the role that these highly predictable, seasonally-pulsed movements play in the transmission and evolution of parasites (Altizer et al. 2011; Fritzsche McKay and Hoye, this volume). Because migrations form unique links between disparate locations, involve large numbers of individuals, and may increase parasite exposure through the use of multiple different habitats and increased interspecies interactions, animal migrations are widely assumed to enhance the cross-species transmission and global spread of parasites (Altizer et al. 2011; Fritzsche McKay and Hoye, this volume). However, the relative importance of migrants in parasite transmission networks is critically dependent on the migrant’s ability to tolerate infection and migrate successfully while infected (Galsworthy et al. 2011; Bauer et al. 2016).
In addition to parasites that cause rapid host death (so-called “killers”), or directly attack host reproductive organs (“castrators”), ecologists increasingly recognize that apparently benign parasites may in fact precipitate reductions in overall host fitness (so-called “debilitators”) (Lafferty and Kuris 2002). Demonstrated sub-lethal effects of endemic parasites include: the potential to alter reproduction timing (Telfer et al. 2005; Vandegrift et al. 2008; Tersago et al. 2012); reduce fecundity (Hudson et al. 1998; Telfer et al. 2005; Schwanz 2008), offspring growth (Vandegrift et al. 2008) and fledging success (Reed et al. 2008; O'Brien and Brown 2012); decrease movement (Lindstrom et al. 2003; Jansen et al. 2007; Fellous et al. 2011), time spent foraging (Jansen et al. 2007), and body mass (Vandegrift et al. 2008); increase metabolic rate (Booth et al. 1993) and the cost of thermoregulation (Schwanz 2006; Hawley et al. 2012); and reduce survival (Kallio et al. 2007; Burthe et al. 2008; Vandegrift et al. 2008; Martinez-de la Puente et al. 2010; Lachish et al. 2011; Knowles et al. 2012; Tersago et al. 2012). Ultimately, when these effects coalesce to reduce host survival or fecundity in a density-dependent manner, parasites can regulate (Yorinks and Atkinson 2000) host populations, both in theory (Anderson and May 1978) and in the wild (Hudson et al. 1998; Pedersen and Greives 2008).
Critically, experimental demonstrations of the regulatory effect of parasites have revealed that the weak effects of nematode infection in Peromyscus mice (Pedersen and Greives 2008) and Red grouse (Lagopus lagopus scotica) (Hudson et al. 1998) interact synergistically with at least one other main factor, food availability or territorial aggression. Because these interactions are mediated by physiological stress and reduced immunocompetence (Ostfeld 2008; Pedersen and Greives 2008), the negative effects of parasites may manifest from periods of increased physiological stress. As long-distance migration is one of the most physiologically demanding activities in the animal world, migrants may, therefore, be particularly susceptible to negative effects of parasitic infection (Ricklefs et al. 2005; Fritzsche McKay and Hoye, this volume).
An increasing body of evidence suggests that even when a migratory journey separates the location of infection from the location at which infection status and performance are assessed (such that infected individuals that do not complete migration are excluded, and any negative effect of infection on survival is unavoidably underestimated), infection has been shown to impose significant costs in migrants. For instance, blood parasite infections acquired on the wintering grounds have been associated with delayed arrival in spring (Ratti et al. 1993; Møller et al. 2004; DeGroote and Rodewald 2010; Asghar et al. 2011; Santiago-Alarcon et al. 2013), reduced body mass (Marzal et al. 2008), reduced fecundity (Marzal et al. 2005; Asghar et al. 2011), and reduced survival or longevity (Marzal et al. 2008; Asghar et al. 2015) of several species of European passerine birds, although these effects are often small and far from universal. Effects of infection appear to be more pronounced in studies where infection status (and ensuing performance) have been assessed at the time and location of infection. For instance, sockeye salmon (Oncorhynchus nerka) smolts that did not survive the first ∼1150 km of their migration to the North Pacific Ocean had gene expression profiles consistent with an immune response to one or more viral pathogens (Jeffries et al. 2014). In addition, monarch butterflies (Danaus plexippus) experimentally infected with a protozoan parasite (Ophryocystis elektroscirrha) exhibited shorter flight distances, slower flight speeds, and loss of proportionately more body mass for the distance flown in the laboratory (Bradley and Altizer 2005). Several lines of evidence also suggest that naturally infected monarchs experience substantially higher mortality than uninfected individuals during their migratory journeys (Altizer et al. 2000, 2015; Bartel et al. 2011; Satterfield et al. 2015).
Studies of avian influenza viruses (AIV) in wild birds have placed particular emphasis on migrant-mediated dispersal (Hill and Runstadler, this volume). Low-pathogenic avian influenza viruses (LPAIV) typically induce an acute, non-persistent infection that proceeds in the absence of clinical signs, with only a slight, transient increase in body temperature (Jourdain et al. 2010; Kuiken 2013). Collectively, these findings have led to the conclusion that AIV is avirulent in its natural hosts (Kuiken 2013). However, wild animals must satisfy a number of ecological demands—including procuring food, avoiding predation, thermoregulation, reproduction, and moving from one site to another—that may be impaired without clinical signs of disease (Kuiken 2013). Moreover, theoretical models suggest that infection-induced delays can alter the timing, location, and total incidence of infection (Galsworthy et al. 2011). Although transmission (and hence detectable geneflow) along flyways may persist (Hill and Runstadler, this volume), such delays have important ramifications for interspecies exchange, rates of co-infection and viral recombination, and ultimately viral evolution (Galsworthy et al. 2011).
Over the past decade, a handful of studies have investigated the behavior of wild birds infected with LPAIV, focusing on short-term, transmission-relevant behaviors. Overwintering greater white-fronted geese (Anser albifrons albifrons) dispersed similar distances to uninfected geese in the first 12 days post capture (Kleijn et al. 2010), and infected mallards (Anas platyrhynchos) on post-breeding migration exhibited no differences in movements during stopover (Bengtsson et al. 2016), duration of stopover, or net displacement of those recovered (dead) throughout the following winter and spring (Latorre-Margalef et al. 2009) compared to uninfected birds. Infected mallards on post-breeding migration were, on average, 20 g lighter than uninfected birds (Latorre-Margalef et al. 2009); however, no differences in body mass were detected during the breeding season in a resident population (van Dijk et al. 2015a). These resident mallards were, however, seen to fly ∼10% less when infected, as well as have ∼20% smaller home ranges and ∼30% shorter flight distances over a 3-day period (van Dijk et al. 2015b).
Only two studies have assessed the ecological consequences of LPAIV for the hosts themselves. During overwintering, two infected Bewick’s swans (Cygnus columbianus bewickii) foraged and accumulated body stores at lower rates, and departed later and displaced shorter distances from the capture site than four uninfected birds (van Gils et al. 2007); although body mass at capture and total body stores prior to migratory departure did not differ. In contrast, the resighting rate of ruddy turnstones (Arenaria interpres) 1 year after infection did not differ from that of uninfected birds on a key stopover site on spring migration (Maxted et al. 2012b). Critically, at the sites where the aforementioned mallard and turnstone studies were conducted, LPAIV prevalence can peak as high as 50% (Krauss et al. 2010; van Dijk et al. 2014) such that the majority of each of these populations becomes infected during the period of study (Latorre-Margalef et al. 2009b; Maxted et al. 2012a). Although investigation of transmission-relevant behaviors is not hindered, any comparison of seasonal or long-term effects of infection are inevitably underestimated given that individuals classified as uninfected are likely to have experienced infection, either before or after being sampled (Kuiken 2013).
This study aims to gain insight into the potential for short-term, endemic infections such as LPAIV to hamper the performance of migratory birds, both within and between seasons. We investigate the effect of infection in Bewick’s swans, a long-distance migratory species breeding in the Pechora Delta (NW Russia) from June to August, and wintering 3000–4000 km to the southwest in the Netherlands and the British Isles, from October to February (Rees 2006). During December and early January, this species experiences relatively low incidence of LPAIV infection (maximum prevalence < 8%; Supplementary Fig. S1). In light of the potential for events occurring during one period of the migratory cycle to have carry-over effects on individual performance in subsequent periods (Harrison et al. 2011), we monitored both the foraging and movement of swans in the winter that their infection status was assessed, as well as subsequent breeding behavior and inter-annual resighting probability. We hypothesized that if LPAIV were to hamper the performance of these migrants, this would be most apparent between seasons, given the potential for cascading effects on refueling, body stores, and timing throughout migration (Nolet and Drent 1998). We also incorporate data on infection history (in the form of detectable antibodies to AIV) to assess the hypothesis that any effects of infection are most apparent in naïve individuals experiencing their first AIV infection.
Materials and methods
Swan capture, sampling, and biometrics
Bewick’s swans were captured 6–8 weeks after arrival on their Dutch wintering grounds in one of the three successive winters (2006–2008 inclusive). Using cannon nets, each catch captured a small proportion of a single, cohesive flock of 200–400 swans foraging on sugar beet remains in the provinces of Noord Holland or Flevoland (Supplementary Fig. S2). Sterile cotton swabs were used to collect cloacal and oropharyngeal samples before being stored in transport medium (Hanks balanced salt solution containing 0.5% lactalbumin, 10% glycerol, 200 U/ml penicillin, 200 μg/ml streptomycin, 100 U/ml polymyxin B sulfate, 250 μg/ml gentamicin, and 50 U/ml nystatin (ICN, The Netherlands)), maintained at −70ºC until analysis. Whole blood (∼1 ml) was sampled from the brachial or tarsal vein, allowed to clot for ∼6 h, centrifuged, and serum maintained at −20 °C until analysis. All swans were weighed to the nearest 50 g and skull and wing length measured to the nearest mm. A scaled mass index (SMI) of body condition (shown to be the best indicator of the relative size of energy reserves and other body components; Peig and Green 2009) was calculated for each individual (see Supplementary Material). Birds were aged as either hatch-year or adult on the basis of plumage and sexed using molecular methods (Hoye et al. 2012b). Birds were held in new, individual jute sacks for the duration of sampling, after which they were released at a nearby water body. Swans were captured, ringed, and sampled under approvals CL06.06 and CL08.05 from the Animal Experimentation Committee (DEC) of the Royal Netherlands Academy of Arts and Sciences (KNAW), in accordance with Dutch regulations for animal experiments (Wet op de Dierproeven 1977), and permissions FF/75A/2008/060 and FF/75A/2008/060a from the Ministry of Agriculture, Nature and Food Quality (Ministerie van Landbouw, Natuurbeheer en Voedselkwaliteit; LNV). All efforts were made to minimize any suffering throughout the study.
Virus and antibody detection
Current infection with AIV was tested using a generic real-time reverse transcriptase PCR assay targeting the matrix gene on RNA isolated from the swab samples following the standard diagnostic procedure for the Dutch wild bird surveillance program (Munster et al. 2009). As this method has detected virus 24–48 h after experimental inoculation in waterfowl, including adult Bewick’s swans, and only for 3–14 days (Jourdain et al. 2010; Hoye 2011), this diagnostic method can be assumed to assess active infection with AIV. This quantitative PCR method allowed assessment of cycle threshold value (Ct)—the first real-time amplification cycle in which target gene amplification is detectable, such that a small Ct value indicates a high number of virus genome copies and thus virus particles in the sample, whereas a large Ct value indicates a small amount of virus (Munster et al. 2009). The highest Ct considered positive was 40, and 3 cycles equate to approximately a log10 difference in genome copies (Munster et al. 2009).
The presence of antibodies to the nucleoprotein gene in serum samples was tested using a commercially-available blocking enzyme-linked immunosorbent assay (MultiS-Screen AIV Antibody Test Kit, IDEXX Laboratories) with absorbance measured at 620 nm using a Tecan infinite 200 plate reader. Samples were considered negative for the presence of antibodies if the mean of the two signal-to-noise ratios (sample absorbance divided by negative control mean absorbance) for the sample were greater than 0.5.
Tracking individual movements and return
Each swan received a yellow-neck collar inscribed with an individual four-digit code in black (readable from up to 600 m; van Gils et al. 2007). Twelve neck collars also carried a custom made GPS-logging device (Madebytheo, Nijmegen, The Netherlands). Each GPS collar weighed 75–80 g, automatically recorded geographical positions (accuracy 25 m) at two prescheduled times per day, and was downloaded via Bluetooth from a distance of 300–400 m. Daily positions for the remaining birds were reconstructed from extensive volunteer resightings over the 4 weeks following each catch (n = 586 resightings from a total of 76 birds; average of 9.4 observations per bird). These resightings were reported by over 100 independent citizen scientists across the wintering range of the species (the Netherlands, southern England, and northern Belgium). Because we cannot know the movement of birds between resightings, three different assumptions were used to interpolate each bird’s location between successive resightings. The “minimum” movement assumption stipulates that a bird remained at the position it was last resighted until it was resighted elsewhere. The “maximum” movement assumption stipulates that a bird arrived at its next position the day after it was seen at its last known position, only to be seen at this new position some time later. The “average” movement assumption stipulates that a bird followed a linear trajectory between each pair of resighting locations. Each bird’s daily displacement from its catch site was then calculated on the basis of the three interpolation methods. Our database also recorded resightings of the birds in the years following capture, allowing us to quantify whether or not each bird was seen to return to the overwintering area. Because observers report the GPS co-ordinates at which each bird was sighted, in real time, we could visit these locations to make follow-up observations of foraging and migratory fueling behaviors, as well as breeding status (with or without offspring) of adults in the year of and the year after capture.
Foraging and migratory fueling
Throughout the winters of capture, we regularly observed the foraging and fueling behavior of individual swans prior to their departure on spring migration. On encountering a flock, we first estimated the abdominal profile index (API—a visual estimate of abdominal fat storage; Bowler 1994), on a scale of 1–6 for each collared bird (53 birds, mean ± SE 2.4 ± 0.2 observations per bird). We then performed focal scans, in which one collared swan was observed continuously for a 30-minute period using a 20–60 × spotting scope, recording the number of bites taken (43 birds, 2.4 ± 0.4 observations per bird) and, when the bird’s cloaca was visible, the number of droppings produced (26 birds, 2.4 ± 0.4 observations per bird). All observers were unaware of the infection status of the birds.
Data analysis
For each response variable we developed a set of candidate models and compared them using an information theoretic approach, based on Akaike’s information criteria (AIC) such that: where AIC differs (ΔAIC) by less than 2; both models are considered to be equally well-supported explanations of variation in the response variable; where ΔAIC is between 4 and 7, the model has considerably less support; and models having ΔAIC >10 have no support from the data (Burnham and Anderson 2002). All candidate model sets included a global model with all parameters followed by several increasingly simplified models, as well as a null model that contained all effects in the most parsimonious model with the exception of infection status (Supplementary Tables S2, S4, S6, S8, S10, S12, and S14). The number of variables fit to any global model was always less than 10% of the sample size. Model assumptions were validated by visual examination of diagnostic plots. Displacement distances were loge transformed to achieve normality. All analyses were performed in R 3.2.2 (R Core Team 2015). Factors predicting infection in an individual and proportion of birds seen the following winter were tested using generalized linear models (GLM). Factors predicting individual’s scaled mass index were tested using linear models (LM). Factors predicting bite rate, dropping rate, API, and displacement (based on each of the three movement interpolation methods) were tested using mixed models in the “lme” package (Bates et al. 2015). For each of these analyses, the global model was first constructed using three different structures for random effects: intercept; intercept and slope (with respect to date of observation); and fixed effect, and these structures were compared on the basis of AIC (Zuur et al. 2009). The most parsimonious random effects (ΔAIC to next lowest AIC >>10) were then used in all candidate models, being intercept only for all foraging responses, and intercept and slope for movement responses.
Results
Infection and immunity
Infection was detected in 25 of the 107 swans (23.4%; 95% CI: 15.9–31.8%; Table 1), with 4 birds showing positive cloacal and oropharyngeal swabs, 6 showing positive oropharyngeal swabs only, and 15 showing positive cloacal swabs only. As no response variables differed between these swab sample locations, we considered a bird to be infected if either site was positive for AIV. Infection was not uniform across the population with hatch-year birds 3.36 times more likely to be infected than adults (95% CI: 1.03–11.78; P = 0.048; 11/27 (41%) hatch-year birds infected), after accounting for significant effects of year and date of capture (day in December; Supplementary Table S1). Eighty-seven birds (76.6%; 95% CI: 68.2–84.1%) had antibodies to the nucleoprotein gene segment, indicative of prior AIV infection. Information on infection and serostatus were used to further categorize infected individuals as either “re-infected” or “naïve-infected”. Given that (1) viral shedding lasts 3–8 days in free-living birds (Latorre-Margalef et al. 2009; Hénaux et al. 2010), (2) seroconversion in naïve individuals occurs 6–14 days after infection (Jourdain et al. 2010; Tolf et al. 2013), and (3) all swans were at least 4 months old (and hence are extremely unlikely to possess maternally-derived antibodies; Garnier et al. 2011), we assumed that individuals that were positive for AIV infection and showed detectable antibodies were potentially experiencing a re-infection, referring to them as “re-infected” individuals in the remaining analyses. Although this diagnostic combination could also be achieved by individuals several days into their first infection, cycle threshold, a proxy for viral load, was not lower in birds in this category (Supplementary Table S16), as would be expected later in infection. Conversely, individuals that were positive for AIV infection but lacked detectable antibody responses were likely to have been experiencing their first infection, or to have seroreverted (as has been reported after 8–15 months in mallards (Fereidouni et al. 2010), and ∼12 months in geese (Hoye et al. 2011)). While it is unlikely that individuals that have seroreverted are immunologically similar to naïve individuals, hatch-year birds were 7.64 times more likely to be infected and lack detectable antibodies (95% CI: 1.00–101.01; P = 0.038; Table 1) and therefore likely to have been experiencing their first AIV infection. These birds are referred to as “naïve infected” individuals in the remaining analyses.
Table 1.
Age distribution of Bewick’s swans across LPAIV infection categories at capture
| Uninfected | Re-infected | Naïve-infected | |
|---|---|---|---|
| Hatch-year | 16 | 5 | 6 |
| Adult | 66 [47] | 7 [12] | 2 [1] |
Note: The number of individuals from whom post-capture metrics could be included are indicated in brackets.
Condition at capture
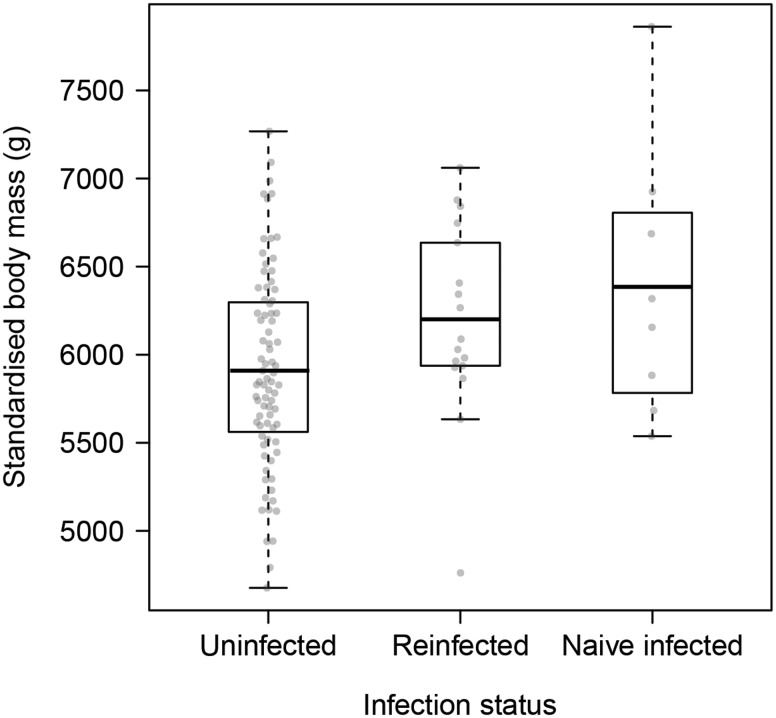
Individuals in the naïve-infected category had, on average, a scaled mass index 469 g higher (± SE 212 g; P = 0.03; Fig. 1; Supplementary Table S3) than uninfected birds, with no significant effect of age, sex, date of capture, or interaction between age and infection in any of the competing models (Supplementary Table S2). Individuals in the re-infected category had a scaled mass index that was not significantly different from their uninfected counterparts (P = 0.06; Fig. 1, Supplementary Table S3).
Fig. 1.
Scaled mass index (after Pieg and Green 2009) of Bewick’s swans at capture on the basis of infection status. Thick horizontal bars represent means, and shaded points indicate individual observations.
Foraging and migratory fueling
Twenty-five individuals were used in an experimental study following capture (Hoye et al. unpublished), and as a result all post-capture metrics (foraging and migratory fueling, movement, breeding success, and return) are limited to 82 individuals, of which 19 were infected: 12 re-infected and 7 naïve infected.
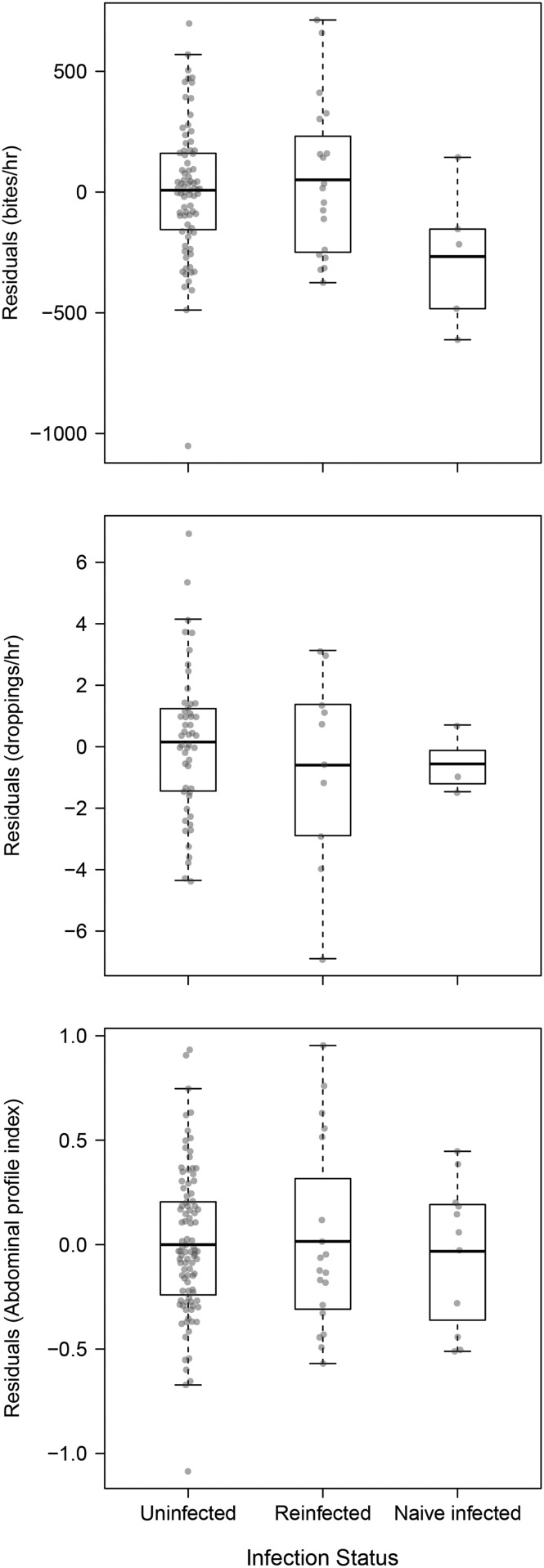
During the overwintering period, birds in the naïve-infected category took, on average, 617 (± SE 238) fewer bites per hour than uninfected birds (t = −2.59; P = 0.014; Fig. 2A; Supplementary Table S5), with the most parsimonious model including effects of sex, age, date of observation, year, field type, and a random effect of individual bird to account for repeated observations (Supplementary Table S4). Birds in the re-infected category took a similar number of bites to uninfected birds (t = 0.49; P = 0.63; Supplementary Table S5). Birds in the naïve-infected (t = −0.93; P = 0.37; Fig. 2B; Supplementary Table S7) and re-infected categories (t = −1.14; P = 0.27) produced a similar number of droppings per hour throughout the winter compared to uninfected birds, after accounting for effects of sex, age, date of observation, year, field type, and a random effect of individual to account for repeated observations (Supplementary Table S6). There was also no difference in accumulated body stores, as given by the abdominal profile index of birds in the naïve-infected (t = −0.39; P = 0.70; Fig. 2C; Supplementary Table S9) or re-infected categories (t = 0.17; P = 0.86) compared to uninfected birds, after accounting for the effects of date of observation, year, and a random effect of individual to account for repeated observations in the highest ranked model containing an effect of infection. However, the most parsimonious model (ΔAIC = 1.95) did not include an effect of infection (Supplementary Table S8).
Fig. 2.
Foraging and fueling behavior of Bewick’s swans on the basis of infection status. (A) Bites per hour, (B) droppings per hour, and (C) accumulated fat stores (API); observed during their overwintering period, presented as means (thick horizontal bars) of residual values after accounting for effects of sex, age, year, date of observation, and field type (Supplementary Tables S5, S7, and S9).
Movement
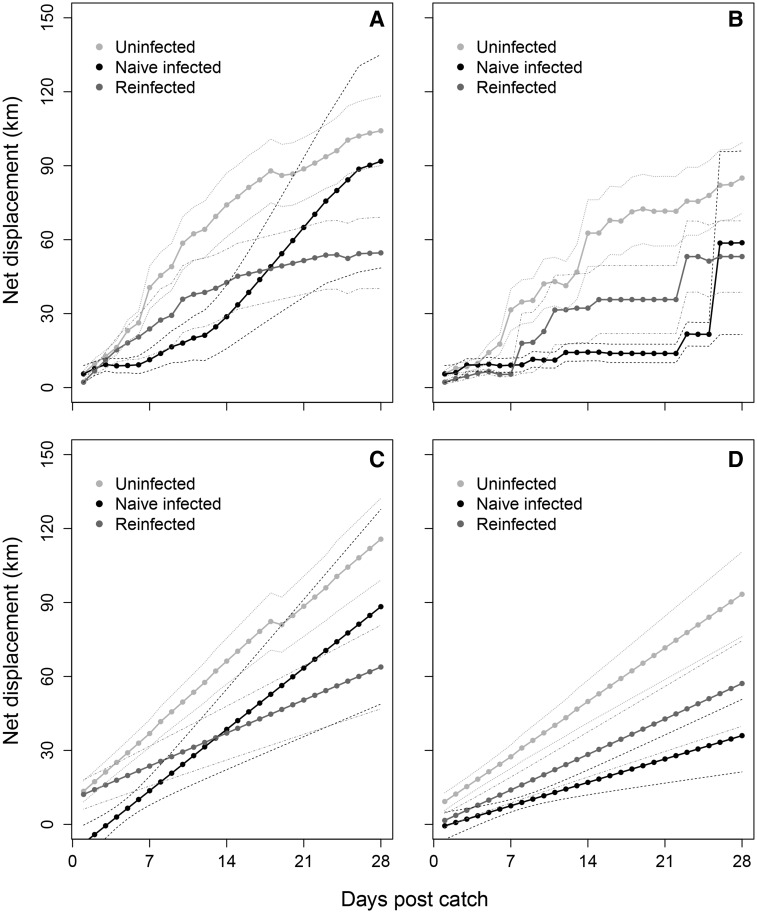
Over the first 4 weeks following capture, swans in the naïve-infected and re-infected categories were seen to disperse from the catch site at a similar rate to uninfected birds on the basis of the “average” displacement interpolation (t = −0.77; P = 0.44 and t = −1.35; P = 0.18, respectively; Fig. 3A and C; Supplementary Table S11), with the most parsimonious model also including effects of days post capture, as well as random effects to account for repeated observations on each individual (Supplementary Table S10). There was no significant effect of age, scaled mass index, year, sex, or date of capture in any of the competing models. Near identical results were seen using the “maximum” displacement interpolation (data not shown). However, the “minimum” displacement interpolation suggested individuals in the naïve-infected category displaced shorter distances and were more delayed in their movements (Fig. 3B and D), with the most parsimonious model that included infection status containing a significant effect of naïve infection (t = −2.05; P = 0.044; Supplementary Table S13), but not re-infection (t = −0.88; P = 0.38; Supplementary Table S13), as well as significant effects of days post capture, date of capture, and random effects of individual. The model containing a (non-significant) effect of sex was also well supported (ΔAIC = 1.83), with a similar, significant effect of naïve infection (t = −2.25; P = 0.028) but not re-infection (t = −0.81; P = 0.42). However, the model with the lowest AIC, although not significantly better supported (ΔAIC = 1.07), was the null model that did not include an effect of infection (Supplementary Table S12).
Fig. 3.
Average of net displacement (km; ± SE) of Bewick’s swans for the first 4 weeks after capture, from raw values (A and B), and predicted values from the most parsimonious model (C and D; Supplementary Tables S11 and S13) including effects of infection category (uninfected: light gray; re-infected: dark gray; naïve-infected: black). Displacement was interpolated from GPS data and observer resightings as remaining at the former site until seen elsewhere (“minimum”; B and D); or intervening days at linear average positions between resighting locations (“average”; A and C). All values represent mean (filled circles) ± SE (dashed lines).
Breeding success
As Bewick’s swans do not reach sexual maturity until at least their third year, breeding status was only assessed in adults. All but one of the adults infected with AIV were in the re-infected category, and hence comparisons are between infected and uninfected birds rather than between infection categories. In the year of capture, roughly half of the adult birds that were infected had arrived with young (4/7 known breeding status adults); however, of the birds whose breeding status could be assessed in the winter after capture, none of the infected adults (0/5) were seen to return with young, compared with 28% of the adult birds who were uninfected in the year of capture (9/32) although these apparent differences were not statistically significant (Fisher’s exact test: P = 0.31). Of the birds whose breeding status could be assessed in the winter after capture, 24% (8/33) of those that were seropositive in the year of capture (had preciously experienced infection) were seen to return with young.
Return
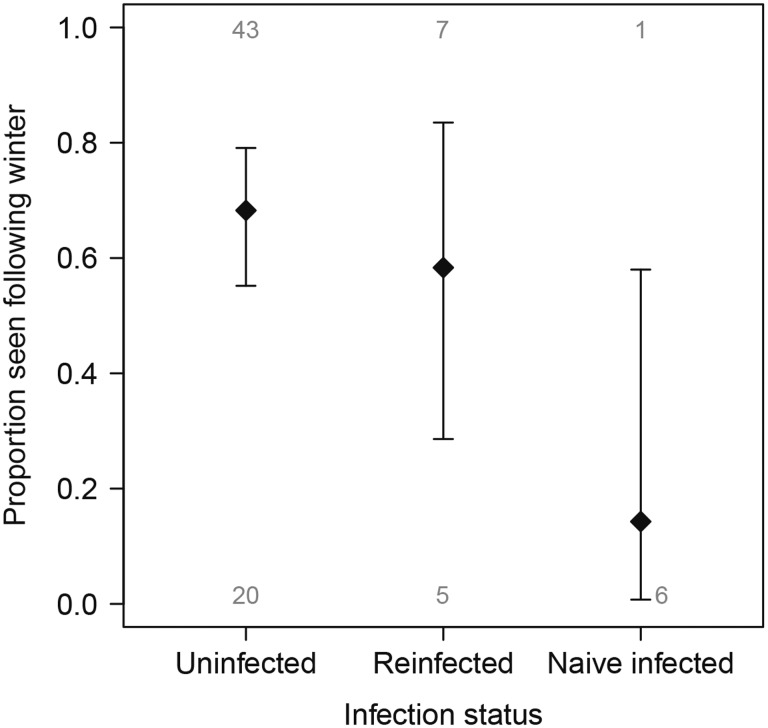
Sixty-two percent of marked swans were resighted the following winter. All birds that were recorded as absent in the first winter after catch were never sighted in subsequent winters (2–4 years after capture), suggesting that any bird that returned was likely to have been detected. Individuals in the naïve-infected category were 12.99 times less likely to be seen the following winter compared to uninfected birds (95% CI: 2.02–252.3; P = 0.022; n = 7; Supplementary Table S15; Fig. 4). Only one naïve-infected individual—the adult—was resighted in the year after capture; all naïve-infected hatch-year birds were not seen after the year of capture. Return rates of hatch-year birds in the re-infected (3 of 5) and uninfected (10 of 16) categories were similar to re-infected (4 of 7) and uninfected (33 of 47) adults. Naïve infection was a significant factor (P < 0.05) in all candidate models tested to explain probability of return, with no significant effect of scaled mass, year, sex, or date of capture in any of the competing models, and the most parsimonious model including an effect of infection status alone (Supplementary Table S14). Age was not included due to its collinearity with infection status. Individuals in the re-infected category were no less likely to be seen the following winter than uninfected individuals (P = 0.51; Fig. 4; Supplementary Table S16).
Fig. 4.
Proportion of Bewick’s swans resighted in the winter after capture (black diamonds; ± 95% CI). Those in the naïve-infected category were less likely to be seen the following winter than birds in the uninfected or re-infected categories. Numerals indicate the number of individuals swans.
Discussion
Some of our results suggest that naturally-acquired LPAIV infection may have a pernicious effect on the performance of migratory Bewick’s swans, both within and between seasons. Without manipulation of or assignment to infection categories, and in the absence of repeated assessment of infection status following capture, these results may suffer from misclassification biases such that individuals in the uninfected category may actually have been re-infected or naïve-infected during the winter (Kuiken 2013). There is also potential for misclassification as a result of individuals not seroconverting following infection (as recently described in experimentally infected gulls (Verhagen et al. 2015); in contrast to 100% seroconversion in experimentally infected mallards (Fereidouni et al. 2010; Jourdain et al. 2010) and Bewick’s swans (Hoye 2011)), or seroreverting before the current infection. In addition, it is possible that seropositive birds may simply have started their current infection several days earlier than seronegative birds; however, we found no evidence of decreased viral shedding in these birds as has been demonstrated with the onset of antibody responses in laboratory infected mallards (Jourdain et al. 2010), and signal-to-noise ratios from the antibody ELISA showed a clear distinction between antibody positive and antibody negative birds refuting suggestion that seropositive hatch-year birds were misclassified. We, therefore, assume that antibody-positive birds have indeed survived a previous infection. Moreover, each of these scenarios would be expected to dilute our observations of the true effects of naïve infection. That we find significant differences in spite of any such misclassifications suggests the effects we describe may in fact be conservative estimates.
Critically, significant effects of infection were only seen in birds that were infected but lacked antibodies indicative of prior infection—those individuals assumed to be naïve to AIV. Both adult and hatch-year birds that were infected but also had detectable antibodies, and therefore had survived a previous infection, were indistinguishable from uninfected birds in each of the ecological performance metrics. Infection history may, therefore, play an important role in shaping both the consequences of infection for host populations, and the transmission potential of individuals.
Individuals in the naïve-infected category showed reduced foraging intake, similar to the two infected swans in van Gils et al. (2007). However, we found no increase in the number of droppings produced. Although frequency of defecation relative to intake may have changed, as would be expected if LPAIV infection reduced the function of the digestive tract and induced diarrhea (Kuiken 2013), simultaneous observation of bite and dropping rate were rare. Increased dropping rates may not have been detected over the 4 weeks post capture as such modifications are likely to be transient and limited to the 3- to 8-day duration of infection. Differences in intake throughout the overwintering period did not culminate in a reduction of accumulated body stores (API index) prior to departure on spring migration; however, birds in the naïve-infected category had a higher scaled mass at capture. Although viral infection has been associated with poorer “body condition” in some studies, invoking suggestions of negative feedback cycles between infection and reduced condition (Beldomenico and Begon 2010), LPAIV infection in Bewick’s swans represent more of a “catch-22” phenomenon. Bewick’s swans foraging in aquatic habitats throughout fall migration and early overwintering show significant benefits in the form of higher body mass prior to spring migration and increased breeding success in previously unsuccessful adults (Hoye et al. 2012b). Yet, individuals preferentially foraging in aquatic habitats also experience a higher risk of infection (Hoye et al. 2012a). This link, between nutritionally superior foraging and increased risk of LPAIV infection, may explain the higher scaled mass of infected individuals. Together with the lack of any effects of re-infection, these findings also cast doubt on the notion that infected individuals show hampered performance because they were simply “poor performers” before becoming infected (sensu Beldomenico and Begon 2010; Kuiken 2013).
Movement behavior was highly sensitive to the method used to interpolate between known resighting positions. Swans in the naïve-infected category appeared to disperse slower and over shorter distances in the first 4 weeks after capture on the basis of the minimum movement interpolation, similar to the infected birds in van Gils et al. (2007). However, infection status was not a significant factor explaining movement behavior based on the average or maximum interpolation methods. Unfortunately, we were only able to retrieve daily movements for 9 of the 82 swans in our study, none of whom were in the naïve-infected category. Moreover, we were not able to monitor movements throughout migration. At best, our movement results suggest that far more detailed measurements are required to assess the long-term impact of infection on the movement of migrants. Ideally, monitoring would extend beyond the site of infection (e.g., the overwintering period, as reported here and in van Gils et al. 2007) to include detailed assessment of the migratory behavior of individuals (Brown and O'Brien 2011). In addition, our study suggests that remote monitoring of the movements of hatch-year individuals, a group traditionally neglected in migration studies, would prove especially illuminating both in terms of impact on host performance and transmission potential.
Infection with LPAIV also appeared to have certain carry-over effects on subsequent seasons. Strikingly, individuals in the naïve-infected category were unlikely to be resighted 1 year after infection. Only one naïve-infected individual was resighted in the winter after infection. Notably, this individual was an adult who, given the high seroprevalence in the population and the fact that antibody responses become undetectable over time in other species (Fereidouni et al. 2010; Hoye et al. 2011), may have been infected previously and seroreverted before acquiring the current infection, although this bird did record the highest signal-to-noise ratio in the antibody ELISA. None of the six hatch-year birds in the naïve-infected category were ever seen again. Definitive estimates of survival and return probability require multi-state mark-recapture modeling to estimate differences in survival while accounting for imperfect detection of marked animals. However, estimation of these parameters requires more data than are currently available. In particular, none of the birds deemed absent 1 year after capture were ever seen in subsequent winters, hindering our ability to estimate the probability of detection. For our comparison of resighting rates between infection categories to be valid, infection itself must not alter the probability that a bird uses this wintering site or its detection probability while overwintering given that the bird survived. Because NW Europe is the predominant wintering area for Bewick’s swans, and in the 7 years of marking Bewick’s swans, only 1 of over 150 individuals has been sighted on an alternate flyway, we are reasonably confident that birds that were not resighted had not returned to any known overwintering sites.
Curiously, hatch-year birds with detectable antibodies were resighted at the same rate as re-infected and uninfected adults, indicating that age and migratory experience alone could not explain the low resighting rate of individuals in the naïve-infected category, and perhaps also that some hatch-year birds have been “lost” from the population through AIV infection earlier on migration. The apparent disappearance of naïve-infected individuals may imply cumulative effects of subtle changes to foraging and movement behavior long after infection has cleared, potentially amplified by the physiological demands of migration. Migratory behaviors have been shown to undergo prolonged development and improvement with age (e.g., Sergio et al. 2014). Because hatch-year birds are also more likely to become infected (Hoye et al. 2012a; van Dijk et al. 2014; Verhagen et al. 2015), they may be doubly disadvantaged by infections as they experience greater physiological stress during migration, as well as being immunologically naïve to infection. Yet, without detailed tracking and/or observation of individuals throughout the full annual cycle, the mechanisms by which LPAIV infection might reduce survival remain unknown.
Down-regulation of current reproduction to increase the probability of survival, and future reproduction, has previously been suggested as an evolutionarily optimum for hosts with acute infections (Telfer et al. 2005). Although none of the infected adults were seen to return with young, our sample size lacked the power to substantiate a statistically significant effect and a larger number of individuals is required to investigate the consequences of LPAIV for breeding success in adults.
Taken together, our results suggest that acute and superficially harmless infection with LPAIV may have indirect effects on individual performance and recruitment of hatch-year individuals in migratory Bewick’s swans. While it remains uncertain whether this association is causal, it raises the possibility that complex interactions between LPAIV, migration, host immune development, and potentially other co-infecting pathogens can have significant fitness consequences for migratory birds. Our study, therefore, adds to a growing body of evidence indicating gradual, subtle, detrimental effects of endemic pathogens on the performance of natural host species in the wild (Booth et al. 1993; Burthe et al. 2008; Knowles et al. 2012; Asghar et al. 2015). Although considerably more research is required to understand the relationship between LPAIV and its endemic hosts, our observations of natural infections highlight the need to account for both infection history and current infection status when examining ecological constraints throughout the annual cycle.
Supplementary Material
Acknowledgments
We would like to thank Anna Duden, Andrea Vos, Wouter Wietses, Symen Deuzeman, Thijs de Boer, Jacintha van Dijk, Erik Kleyheeg, and Marie Lopez-Salez for their dedicated field work, as well as Pascal Lexmond, Oanh Vuong, Christa Mateman, and Judith Guldemeester for excellent laboratory assistance. We are also grateful for the invaluable input from the volunteer resighting community and the cooperation of farmers who allowed us to work on their fields. BJH’s participation in the symposium “Are migratory animals superspreaders of infection?” was supported by SICB Division of Animal Behavior and Division of Ecoimmunology and Disease Ecology as well as a scientific meeting grant from the Company of Biologists.
Funding
This study was supported by the Research Council for Earth and Life Sciences (ALW) with financial aid from the Netherlands Organization for Scientific Research (NWO) [grant number 851.40.073]; EU Framework six program NewFluBird [grant number 044490]; National Institute of Allergy and Infectious Diseases, National Institutes of Health [contract number HHSN266200700010C]; and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health [to V.J.M.].
Supplementary data
Supplementary Data available at ICB online.
References
- Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331:296–302. [DOI] [PubMed] [Google Scholar]
- Altizer S, Hobson KA, Davis AK, De Roode JC, Wassenaar LI. 2015. Do healthy monarchs migrate farther? tracking natal origins of parasitized vs. uninfected monarch butterflies overwintering in mexico. PLoS One 10:e0141371.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer SM, Oberhauser KS, Brower LP. 2000. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol Entomol 25:125–39. [Google Scholar]
- Anderson RM, May RM. 1978. Regulation and stability of host-parasite population interactions. 1. Regulatory processes. J Anim Ecol 47:219–47. [Google Scholar]
- Asghar M, Hasselquist D, Bensch S. 2011. Are chronic avian haemosporidian infections costly in wild birds? J Avian Biol 42:530–7. [Google Scholar]
- Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347:436–8. [DOI] [PubMed] [Google Scholar]
- Bartel RA, Oberhauser KS, de Roode JC, Altizer SM. 2011. Monarch butterfly migration and parasite transmission in eastern North America. Ecology 92:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344:1242552.. [DOI] [PubMed] [Google Scholar]
- Bauer S, Lisovski S, Hahn S. 2016. Timing is crucial for consequences of migratory connectivity. Oikos. 125:605–12. [Google Scholar]
- Beldomenico PM, Begon M. 2010. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol Evol 25:21–7. [DOI] [PubMed] [Google Scholar]
- Bengtsson D, Safi K, Avril A, Fiedler W, Wikelski M, Gunnarsson G, Elmberg J, Tolf C, Olsen B, Waldenström J. 2016. Does influenza A virus infection affect movement behaviour during stopover in its wild reservoir host? R Soc Open Sci 3:150633 DOI: 10.1098/rsos.150633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DT, Clayton DH, Block BA. 1993. Experimental demonstration of the energetic cost of parasitism in free-ranging hosts. Proc R Soc Lond B Biol Sci 253:125–9. [Google Scholar]
- Bowler J. 1994. The condition of Bewick’s Swana Cygnus columbianus bewickii in winter as assessed by their abdominal profile. Ardea 82:241–8. [Google Scholar]
- Bradley CA, Altizer S. 2005. Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol Lett 8:290–300. [Google Scholar]
- Brown CR, O’Brien VA. 2011. Are wild birds important in the transport of arthropod-borne viruses? Ornithological Monographs No. 71. American Ornithologists' Union, p. 1–64.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information theoretic approach. 2nd edNew York (NY: ): Springer-Verlag. [Google Scholar]
- Burthe S, Telfer S, Begon M, Bennett M, Smith A, Lambin X. 2008. Cowpox virus infection in natural field vole Microtus agrestis populations: significant negative impacts on survival. J Anim Ecol 77:110–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroote LW, Rodewald PG. 2010. Blood parasites in migrating wood-warblers (Parulidae): effects on refueling, energetic condition, and migration timing. J Avian Biol 41:147–53. [Google Scholar]
- Fellous S, Quillery E, Duncan AB, Kaltz O. 2011. Parasitic infection reduces dispersal of ciliate host. Biol Lett 7:327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereidouni SR, Grund C, Haeuslaigner R, Lange E, Wilking H, Harder TC, Beer M, Starick E. 2010. Dynamics of specific antibody responses induced in mallards after infection by or immunization with low pathogenicity avian influenza viruses. Avian Dis 54:79–85. [DOI] [PubMed] [Google Scholar]
- Fritzsche McKay A, Hoye BJ. 2016. Are migratory animals superspreaders of infection? Int Comp Biol. [DOI] [PubMed] [Google Scholar]
- Galsworthy SJ, ten Bosch QA, Hoye BJ, Heesterbeek JAP, Klaassen M, Klinkenberg D. 2011. Effects of infection-induced migration delays on the epidemiology of avian influenza in wild mallard populations. PLoS One 6:e26118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier R, Ramos R, Staszewski V, Militão T, Lobato E, González-Solís J, Boulinier T. 2012. Maternal antibody persistence: a neglected life-history trait with implications from albatross conservation to comparative immunology. Proc R Soc Lond B Biol Sci 279:2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80:4–18. [DOI] [PubMed] [Google Scholar]
- Hawley DM, DuRant SE, Wilson AF, Adelman JS, Hopkins WA. 2012. Additive metabolic costs of thermoregulation and pathogen infection. Funct Ecol 26:701–10. [Google Scholar]
- Hénaux V, Samuel MD, Bunck CM. 2010. Model-based evaluation of highly and low pathogenic avian influenza dynamics in wild birds. PLoS One 5:e10997.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NJ, Runstadler JA. 2016. A bird’s eye view of influenza transmission: challenges with characterising both sides of a co-evolutionary dynamic. Int Comp Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoye BJ. 2011. Host-pathogen interactions on the move: migratory waterfowl and avian influenza viruses [PhD thesis]. [Utrecht, the Netherlands]: Utrecht University.
- Hoye BJ, Fouchier RAM, Klaassen M. 2012a. Host behaviour and physiology underpin individual variation in avian influenza virus infection in migratory Bewick’s swans. Proc R Soc Lond B Biol Sci 279:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoye BJ, Hahn S, Nolet BA, Klaassen M. 2012b. Habitat use throughout migration: linking individual consistency, prior breeding success and future breeding potential. J Anim Ecol 81:657–66. [DOI] [PubMed] [Google Scholar]
- Hoye BJ, Munster VJ, Nishiura H, Fouchier RAM, Madsen J, Klaassen M. 2011. Reconstructing an annual cycle of interaction: natural infection and antibody dynamics to avian influenza along a migratory flyway. Oikos 120:748–55. [Google Scholar]
- Hudson P, Dobson AP, Newborn D. 1998. Prevention of population cycles by parasite removal. Science 282:2256–8. [DOI] [PubMed] [Google Scholar]
- Jansen BD, Krausman PR, Heffelfinger JR, Noon TH, Devos JC. 2007. population dynamics and behavior of bighorn sheep with infectious keratoconjunctivitis. J Wildl Manag 71:571–5. [DOI] [PubMed] [Google Scholar]
- Jeffries KM, Hinch SG, Gale MK, Clark TD, Lotto AG, Casselman MT, Li S, Rechisky EL, Porter AD, Welch DW, et al. 2014. Immune response genes and pathogen presence predict migration survival in wild salmon smolts. Mol Ecol 23:5803–15. [DOI] [PubMed] [Google Scholar]
- Jourdain E, Gunnarsson G, Wahlgren J, Latorre-Margalef N, Brojer C, Sahlin S, Svensson L, Waldenstrom J, Lundkvist A, Olsen B. 2010. Influenza virus in a natural host, the mallard: experimental infection data. PLoS One 5:e8935.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio ER, Voutilainen L, Vapalahti O, Vaheri A, Henttonen H, Koskela E, Mappes T. 2007. Endemic hantavirus infection impairs the winter survival of its rodent host. Ecology 88:1911–16. [DOI] [PubMed] [Google Scholar]
- Kleijn D, Munster VJ, Ebbinge BS, Jonkers DA, Muskens G, Van Randen Y, Fouchier RAM. 2010. Dynamics and ecological consequences of avian influenza virus infection in greater white-fronted geese in their winter staging areas. Proc R Soc Lond B Biol Sci 277:2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SC, Fenton A, Pedersen AB. 2012. Epidemiology and fitness effects of wood mouse herpesvirus in a natural host population. J Gen Virol 93:2447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Stallknecht DE, Negovetich NJ, Niles LJ, Webby RJ, Webster RG. 2010. Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological “hot spot” for influenza viruses. Proc R Soc Lond B Biol Sci 277:3373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T. 2013. Is low pathogenic avian influenza virus virulent for wild waterbirds? Proc R Soc Lond B Biol Sci 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon BC. 2011. Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J Anim Ecol 80:1196–206. [DOI] [PubMed] [Google Scholar]
- Lafferty KD, Kuris AM. 2002. Trophic strategies, animal diversity and body size. Trends Ecol Evol 17:507–13. [Google Scholar]
- Latorre-Margalef N, Gunnarsson G, Munster VJ, Fouchier RAM, Osterhaus A, Elmberg J, Olsen B, Wallensten A, Haemig PD, Fransson T, et al. 2009. Effects of influenza A virus infection on migrating mallard ducks. Proc R Soc Lond B Biol Sci 276:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre-Margalef N, Gunnarsson G, Munster VJ, Fouchier RAM, Osterhaus A, Elmberg J, Olsen B, Wallensten A, Haemig PD, Fransson T, et al. 2009b. Does influenza A affect body condition of wild mallard ducks, or vice versa? A reply to Flint and Franson. Proc R Soc Lond B Biol Sci 276:2347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom KM, Van der Veen IT, Legault BA, Lundstrom JO. 2003. Activity and predator escape performance of Common Greenfinches Carduelis chloris infected with Sindbis virus. Ardea 91:103–11. [Google Scholar]
- Martinez-de la Puente J, Merino S, Tomas G, Moreno J, Morales J, Lobato E, Garcia-Fraile S, Belda EJ. 2010. The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 6:663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzal A, Bensch S, Reviriego M, Balbontin J, de Lope F. 2008. Effects of malaria double infection in birds: one plus one is not two. J Evol Biol 21:979–87. [DOI] [PubMed] [Google Scholar]
- Marzal A, de Lope F, Navarro C, Møller AP. 2005. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–5. [DOI] [PubMed] [Google Scholar]
- Maxted AM, Luttrell MP, Goekjian VH, Brown JD, Niles LJ, Dey AD, Kalasz KS, Swayne DE, Stallknecht DE. 2012a. Avian influenza virus infection dynamics in shorebird hosts. J Wildl Dis 48:322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxted AM, Porter RR, Luttrell MP, Goekjian VH, Dey AD, Kalasz KS, Niles LJ, Stallknecht DE. 2012b. Annual survival of ruddy turnstones is not affected by natural infection with low pathogenicity avian influenza viruses. Avian Dis 56:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, De Lope F, Saino N. 2004. Parasitism, immunity, and arrival date in a migratory bird, the barn swallow. Ecology 85:206–19. [Google Scholar]
- Munster VJ, Baas C, Lexmond P, Bestebroer TM, Guldemeester J, Beyer WEP, de Wit E, Schutten M, Rimmelzwaan GF, Osterhaus A, et al. 2009. Practical considerations for high-throughput influenza a virus surveillance studies of wild birds by use of molecular diagnostic tests. J Clin Microbiol 47:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolet BA, Drent RH. 1998. Bewick’s Swans refuelling on pondweed tubers in the Dvina Bay (White Sea) during their spring migration: first come, first served. J Avian Biol 29:574–81. [Google Scholar]
- O”Brien VA, Brown CR. 2012. Arbovirus infection is a major determinant of fitness in house sparrows (Passer domesticus) that invade cliff swallow (Petrochelidon pyrrhonota) colonies. Auk 129:707–15. [Google Scholar]
- Ostfeld RS. 2008. Parasites as weapons of mouse destruction. J Anim Ecol 77:201–4. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Greives TJ. 2008. The interaction of parasites and resources cause crashes in a wild mouse population. J Anim Ecol 77:370–7. [DOI] [PubMed] [Google Scholar]
- Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–91. [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ratti O, Dufva R, Alatalo RV. 1993. Blood parasites and male fitness in the pied flycatcher. Oecologia 96:410–14. [DOI] [PubMed] [Google Scholar]
- Reed TE, Daunt F, Hall ME, Phillips RA, Wanless S, Cunningham EJA. 2008. Parasite treatment affects maternal investment in sons. Science 321:1681–2. [DOI] [PubMed] [Google Scholar]
- Rees E. 2006. Bewick’s swan. London: T&AD Poyser. [Google Scholar]
- Ricklefs R, Fallon S, Latta S, Swanson B, Bermingham E. 2005. Migrants and their parasites In: Greenburg R, Marra P, editors. Birds of two worlds: the ecology and evolution of migration. Baltimore: John Hopkins University Press; p. 210–21. [Google Scholar]
- Santiago-Alarcon D, Mettler R, Segelbacher G, Schaefer HM. 2013. Haemosporidian parasitism in the blackcap Sylvia atricapilla in relation to spring arrival and body condition. J Avian Biol 44:521–30. [Google Scholar]
- Satterfield DA, Maerz JC, Altizer S. 2015. Loss of migratory behaviour increases infection risk for a butterfly host. Proc R Soc Lond B Biol Sci 282:20141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanz LE. 2006. Schistosome infection in deer mice (Peromyscus maniculatus): impacts on host physiology, behavior and energetics. J Exp Biol 209:5029–37. [DOI] [PubMed] [Google Scholar]
- Schwanz LE. 2008. Chronic parasitic infection alters reproductive output in deer mice. Behav Ecol Sociobiol 62:1351–8. [Google Scholar]
- Sergio F, Tanferna A, De Stephanis R, Jimenez LL, Blas J, Tavecchia G, Preatoni D, Hiraldo F. 2014. Individual improvements and selective mortality shape lifelong migratory performance. Nature 515:410–13. [DOI] [PubMed] [Google Scholar]
- Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T, Begon M. 2005. Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos 109:317–22. [Google Scholar]
- Tersago K, Crespin L, Verhagen R, Leirs H. 2012. Impact of puumala virus infection on maturation and survival in bank voles: a capture-mark-recapture analysis. J Wildl Dis 48:148–56. [DOI] [PubMed] [Google Scholar]
- Tolf C, Latorre-Margalef N, Wille M, Bengtsson D, Gunnarsson G, Grosbois V, Hasselquist D, Olsen B, Elmberg J, Waldenström J. 2013. Individual variation in influenza A virus infection histories and long-term immune responses in mallards. PLoS One 8:e61201.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk JGB, Fouchier RAM, Klaassen M, Matson KD. 2015a. Minor differences in body condition and immune status between avian influenza virus-infected and noninfected mallards: a sign of coevolution? Ecol Evol 5:436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk JGB, Hoye BJ, Verhagen JH, Nolet BA, Fouchier RAM, Klaassen M. 2014. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. J Anim Ecol 83:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk JGB, Kleyheeg E, Soons MB, Nolet BA, Fouchier RAM, Klaassen M. 2015b. Weak negative associations between avian influenza virus infection and movement behaviour in a key host species, the mallard Anas platyrhynchos. Oikos 124:1293–303. [Google Scholar]
- van Gils JA, Munster VJ, Radersma R, Liefhebber D, Fouchier RAM, Klaassen M. 2007. Hampered foraging and migratory performance in swans infected with low-pathogenic avain influenza A virus. PLoS One 2:e184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift KJ, Raffel TR, Hudson PJ. 2008. Parasites prevent summer breeding in white-footed mice, Peromyscus leucopus. Ecology 89:2251–8. [DOI] [PubMed] [Google Scholar]
- Verhagen JH, Höfle U, van Amerongen G, van de Bildt M, Majoor F, Fouchier RAM, Kuiken T. 2015. Long-term effect of serial infections with H13 and H16 low pathogenic avian influenza viruses in black-headed gulls. J Virol 89:11507–11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorinks N, Atkinson CT. 2000. Effects of malaria on activity budgets of experimentally infected juvenile Apapane (Himatione sanguinea). Auk 117:731–8. [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York: Springer Science+Business Media. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.