Abstract
Background. Mucormycosis is a destructive invasive mold infection afflicting patients with diabetes and hematologic malignancies. Patients with diabetes are often treated with statins, which have been shown to have antifungal properties. We sought to examine the effects of statins on Rhizopus oryzae, a common cause of mucormycosis.
Methods. Clinical strains of R. oryzae were exposed to lovastatin, atorvastatin, and simvastatin and the minimum inhibitory concentrations (MICs) were determined. R. oryzae germination, DNA fragmentation, susceptibility to oxidative stress, and ability to damage endothelial cells were assessed. We further investigated the impact of exposure to lovastatin on the virulence of R. oryzae.
Results. All statins had MICs of >64 µg/mL against R. oryzae. Exposure of R. oryzae to statins decreased germling formation, induced DNA fragmentation, and attenuated damage to endothelial cells independently of the expression of GRP78 and CotH. Additionally, R. oryzae exposed to lovastatin showed macroscopic loss of melanin, yielded increased susceptibility to the oxidative agent peroxide, and had attenuated virulence in both fly and mouse models of mucormycosis.
Conclusions. Exposure of R. oryzae to statins at concentrations below their MICs decreased virulence both in vitro and in vivo. Further investigation is warranted into the use of statins as adjunctive therapy in mucormycosis.
Keywords: statins, apoptosis, virulence, Rhizopus oryzae, germination, murine model, fly model
Mucormycosis is a destructive invasive mold infection, afflicting predominantly patients with diabetes and those who are immunocompromised because of hematologic malignancies or organ/tissue transplantation [1]. Rhizopus species are the most common cause of mucormycosis [1]. Owing to the rapid growth rate and angio-invasive nature of the fungus, the prognosis of patients with mucormycosis remains poor, despite prompt antifungal therapy and aggressive surgery [1]. Development of effective antifungal strategies is a major unmet need for the treatment of mucormycosis [2].
Reported cases of mucormycosis have been increasing in all patient categories in developed countries. Comparing prevalence data between patients with hematologic malignancies and patients with diabetes is challenging owing to both ascertainment and publication biases. However, it has been hypothesized that widespread use of statins in patients with diabetes could be, in part, responsible for protection against mucormycosis [3, 4].
Statins are inhibitors of mevalonate synthesis and are widely used for the treatment of hypercholesterolemia [5]. Statins display modest antifungal activity in vitro against pathogenic yeasts, such as Candida albicans [6] and Cryptococcus neoformans [7], and molds, such as Aspergillus fumigatus [8] and Mucor racemosus [9]. In addition, statins were shown to induce apoptosis-like cell death in M. racemosus [9] and to enhance the activity of voriconazole against Mucorales in vivo [10]. However, the effects of statins on Rhizopus species specifically have not been studied. We sought to determine whether statins have pleiotropic effects on R. oryzae biology that may impact the virulence of R. oryzae infections.
METHODS
R. oryzae Isolates and Growth Conditions
Three clinical R. oryzae isolates (RO-969, RO-275, and RO-749) from patient with cancer who received a diagnosis of mucormycosis at MD Anderson Cancer Center (Houston, Texas) were used. For susceptibility testing, germination assay, and fluorometric assays investigating apoptosis, strains were grown on yeast extract agar glucose (YAG) plates for 72 hours at 37°C. For fruit fly and mouse infection, strains were cultured in lovastatin (LOV)–containing YAG plates (8 µg/mL). In all cases, after 72 hours of incubation at 37°C, R. oryzae spores were collected, washed in sterile phosphate-buffered saline (PBS), and counted using a hemocytometer.
Drugs
LOV (Merck, Sharp and Dohme Research Laboratories, Rahway, New Jersey), atorvastatin (ATO; Sigma Chemical, St Louis, Missouri), and simvastatin, (SIM; Sigma) were used at concentrations ranging from 16 to 256 µg/mL. Fluconazole (FLU; Pfizer, New York, New York) was used as a negative control at a concentration of 64 µg/mL. Amphotericin B (AMB; Sigma) was used as a positive control at a concentration of 1 µg/mL.
Susceptibility Testing
Minimum inhibitory concentrations (MICs) were determined as recommended by the Clinical Laboratory Standard Institute (guideline M38-A2) [11]. Briefly, 2-fold serial drug dilutions were prepared in round-bottomed microtiter plates. Each well was inoculated with 100 µL of freshly isolated R. oryzae spores (103 spores/mL). After 24 hours of incubation at 37°C, the MICs for LOV, SIM, and ATO were determined visually.
Germination Assay
To determine whether statins affect spore germination, we suspended 105 spores/mL in drug-containing Roswell Park Memorial Institute (RPMI) medium and 0.15% wt/vol Junlon (Nihon Junyaku, Tokyo, Japan). Junlon was added to the culture medium to avoid formation of hyphae clumps [12]. After 6 hours, an aliquot was centrifuged at 13 000g for 5 minutes. The formation of germlings was determined by bright field microscopy at 40× original magnification.
Terminal Deoxynucleotidyl Transferase-dUTP Nick-End Labeling (TUNEL) Assay
For the TUNEL assay [13], R. oryzae germlings pretreated with ATO, SIM, or LOV (16–256 µg/mL) for 3 hours at 37°C were fixed with 3.7% formaldehyde for 30 minutes on ice. Germlings were then rinsed twice with PBS and incubated in a permeabilization solution for 2 minutes on ice. Next, germlings were rinsed with PBS and incubated with 50 µL of a DNA-labeling solution (Biovision, Milpitas, California) for 60 minutes at 37°C. After incubation, the germlings were rinsed with PBS and incubated with 100 µL of anti-BrdU fluorescein (Biovision) for 30 minutes at room temperature, as per the manufacturer's instructions. The cells were then observed for fluorescence, with excitation wavelengths of 488 nm and excitation wavelengths of 520 nm.
Peroxide Disk Diffusion Assay
We compared the susceptibility of R. oryzae cultured in LOV-containing YAG plates with that of R. oryzae grown on drug-free YAG plates to the oxidative agent peroxide (Sigma), using disk diffusion testing. Briefly, 200 µL of a standardized spore suspension (105 spores/mL) of R. oryzae was plated on LOV-containing YAG plates (8 or 32 µg/mL) and on drug-free YAG plates. After the plates were allowed to dry, a sterile 0.25-inch paper disk (Scheicher and Schuell, Keene, New Hampshire) swollen with 20 µL of 30% peroxide solution was placed on the center of the agar surface. The plates were incubated at 37°C, and the radius of the zone of growth inhibition was measured in millimeters after 48 hours of incubation. The experiment was performed in triplicate on different days.
Growth Assay
To determine whether statins affect R. oryzae growth, we incubated a suspension of 103 spores/mL R. oryzae for 24 hours at 37°C in the presence of LOV (0, 4, 8, 16, 32, or 64 µg/mL) in U-bottomed 96-well microtiter plates. We used spectrophotometry (600-nm absorbance) to assess the growth rate every 4 hours for 24 hours [14].
Endothelial Cell–R. oryzae Interactions
Endothelial cells were collected from human umbilical veins, as previously described [15, 16]. We determined the number of microorganisms endocytosed by endothelial cells, using a fluorescence assay, as previously described [15]. Briefly, 12-mm glass coverslips in a 24-well cell culture plate were coated with fibronectin for at least 4 hours and seeded with endothelial cells until confluence was reached. The cells were infected with 105 spores of R. oryzae that had been incubated for 2 hours at 37°C in RPMI medium containing LOV (16–256 µg/mL). Following 2 hours of incubation, the cells were fixed in 3% paraformaldehyde and stained for 1 hour with 1% Uvitex (Ciba-Geygi, Greensboro, North Carolina). The total number of cell-associated microorganisms was determined using a Leica phase-contrast microscope. The number of endocytosed fungi was calculated by subtracting the number of fluorescent organisms from the number of total visible organisms. The experiment was performed in triplicate on different days. Endothelial cell damage induced by R. oryzae exposed to LOV was quantified using a chromium (51Cr) release assay [16]. Each experimental condition was tested in triplicate, and the experiment was repeated twice.
Quantification of Glucose-Regulated Protein (GRP) 78 and Cell Surface Protein Spore Coat Protein Homolog (CotH) Expression
Recent studies showed that GRP78 is a receptor for Mucorales in endothelial cells, mediating host cell adherence and invasion [15]. To determine GRP78 expression in endothelial cells, we performed real-time polymerase chain reaction (PCR) analysis as described previously [15]. The cell surface protein CotH in Mucorales acts as a fungal ligand, mediating attachment to GRP78 during host cell invasion [16]. To study the effect of LOV on the expression of CotH, we extracted RNA from flash-frozen R. oryzae after it was incubated with 16 µg/mL LOV for 2 hours by using TRI Reagent solution (Ambion, Grand Island, New York). Each experimental condition was tested in triplicate, and the experiment was repeated twice.
Fruit Fly Infection Model
We infected wild-type female fruit flies (7–10 days old, with 22 flies per test group, 3 independent experiments, and 66 flies per experimental group) with a spore suspension of R. oryzae grown either on LOV-containing YAG plates (8 µg/mL) or drug-free YAG plates. We injected the thoraxes of flies with a thin sterile needle that had been dipped into a spore suspension containing 5 × 107 spores per milliliter, as described previously [10]. Virulence was assessed by comparing survival rates between both conditions up to 7 days after infection. Infection was made on different days at the same time of the day (3:00 pm) to avoid circadian rhythm–associated variability.
Mouse Infection Model
Female BALB/c mice (8 weeks old; Harlan Laboratories, Indianapolis, Indiana) weighing 18–20 g were used in all experiments. All procedures were performed according to the standards for humane handling care and treatment of research animals approved by the MD Anderson Institutional Animal Care and Use Committee. The mice were housed in the MD Anderson biohazard barrier in sterile, individually ventilated cages with automated delivery of reverse osmosis chlorinated drinking water. Each cage housed 5 animals, and sterile rodent food was provided daily.
The mice were immunosuppressed by intraperitoneal injections of cyclophosphamide (100 mg/kg body weight) 4 days and 1 day prior to injection of R. oryzae spores and 2 and 6 days after the injection of spores. In addition, 250 mg/kg cortisone acetate was injected subcutaneously 1 day before injection of R. oryzae spores [17]. Mice were anesthetized by inhalation of 2% isoflurane and were infected intranasally with a 35-µL droplet of 2.5–5 × 107 spores (per milliliter) of R. oryzae grown on LOV-containing YAG plates (8 µg/mL) or drug-free YAG plates [18]. Animals were observed daily, and survival was assessed during the 7-day period after infection. Animals that appeared to be moribund were euthanized by CO2 asphyxiation, and their lungs were harvested for quantification of fungal burden. On day 7 after infection, all remaining mice were euthanized by CO2 asphyxiation, and their lungs were harvested for quantification of fungal burden. All experiments were done in triplicate, with 10 mice per test group and 30 mice per experimental group.
Quantification of Fungal Burden in Mouse Lungs
Mouse lungs were removed after the mice were euthanized and were stored at −80°C until quantitative PCR (qPCR) was performed to quantify the fungal burden. DNA was extracted from lung homogenates by using the DNeasy tissue kit (Qiagen, Valencia, California), and DNA samples were analyzed in duplicate, using the ABI PRISM 7000 sequence detection system (Applied Biosystems). Primers and dual-labeled fluorescent hybridization probes specific for R. oryzae 18S ribosomal RNA were designed using ABI PRISM SeqScape software, version 2 (Applied Biosystems). The sequence of the oligonucleotides was as follows: (1) sense amplification primer, 5′-AGGGCTTTGCTGCACGAC-3′; (2) antisense amplification primer, 5′-TCCACCTCGGGACTGTTTG-3′; (3) and hybridization probe, 5′-FAM-TGCTACTGTTGCAGACCGCCGCT-TAMRA-3′ [18]. The threshold cycle of each sample was interpolated from a 6-point standard curve of threshold cycle values prepared by spiking uninfected mouse lung tissue with 102–108 R. oryzae spores. PCR results were reported as spore equivalents of R. oryzae DNA.
Histopathologic Analysis
We obtained whole-lung tissue samples from euthanized mice in each of the infection groups. These samples were fixed in 10% formaldehyde, processed, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin and Grocott methenamine silver and examined under a bright-field microscope.
Statistical Analysis
The Kruskal–Wallis test was used to compare measurements of the radius of the zone of growth inhibition for the peroxide disk diffusion assay. Differences in CotH/GRP78 expression and fungi-endothelial cell interaction and cell damage were compared using the nonparametric Wilcoxon rank sum test. Survival curves for the mouse and fly infection models were analyzed using Mantel–Cox analysis in GraphPad Prism software (version 5.0; GraphPad Software, La Jolla, California). Pulmonary fungal load was compared between groups, using analysis of variance followed by the Tukey honest significant difference test. For all comparisons, P values <.05 were considered statistically significant.
RESULTS
Statins Do Not Inhibit R. oryzae Growth but Decrease Germination in a Dose-Dependent Manner
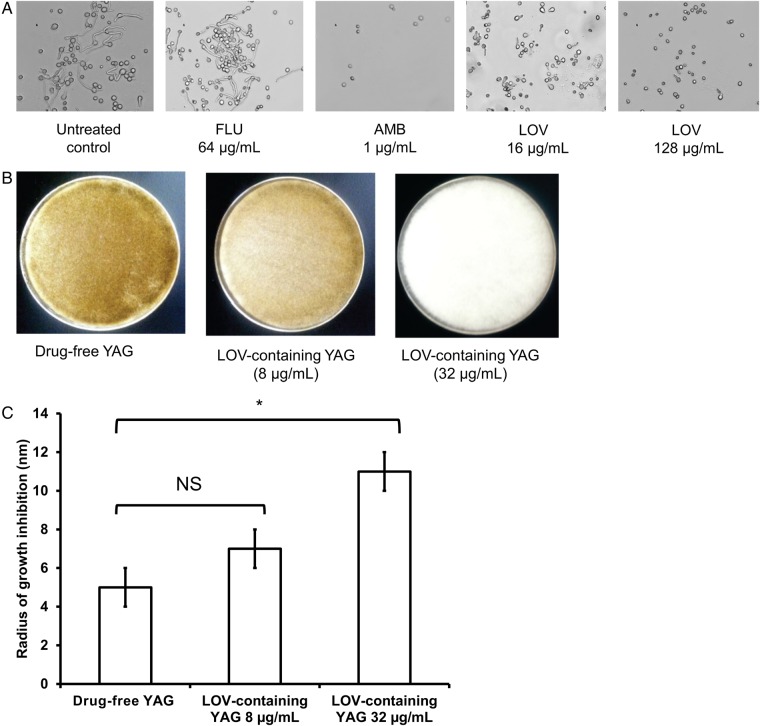
The MICs of statins were high for all 3 R. oryzae isolates (Table 1). Despite a lack of in vitro susceptibility, exposure of RO-969, RO-275, and RO-449 to LOV resulted in a dose-dependent decrease in germling formation, with germination inhibited by a mean (±SD) of 26% ± 2 at the lowest statin concentration tested (16 µg/mL) and by 62% ± 4% at the highest concentration tested (128 µ/mL; 3 experiments for each strain; Supplementary Table 1). Examples of what was observed are presented in Figure 1A.
Table 1.
Susceptibility of 3 Rhizopus oryzae Isolates (RO-969, RO-275, and RO-449) to Simvastatin (SIM), Atorvastatin (ATO), and Lovastatin (LOV)
| Isolate | Minimum Inhibitory Concentration, µg/mLa |
||
|---|---|---|---|
| SIM | ATO | LOV | |
| RO-969 | 256 | 64 | 128 |
| RO-275 | 256 | 64 | 64 |
| RO-449 | 256 | 128 | 64 |
a Determined according to Clinical and Laboratory Standards Institute guidelines.
Figure 1.
Effects of statins on Rhizopus oryzae growth. A, Germination of the R. oryzae isolate RO-969 with no treatment (control) and after treatment with fluconazole (FLU; 64 µg/mL), amphotericin B (AMB; 1 µg/mL), and 2 concentrations of lovastatin (LOV; 16 µg/mL and 128 µg/mL). Magnification ×40. B, Growth of R. oryzae after 48 hours of incubation at 37°C on drug-free and LOV-containing (8 µg/mL and 32 µg/mL) yeast extract agar glucose (YAG) plates. C, Growth inhibition of R. oryzae grown on LOV-containing YAG plates (0, 8, and 32 µg/mL) after exposure to peroxide. Significantly higher susceptibility to peroxide was observed in R. oryzae grown on the YAG plates containing 32 µg/mL LOV, compared with that grown on drug-free YAG plates (*P < .05, by the Kruskal–Wallis test). Data represent mean values (SD) of 3 experiments. Abbreviation: NS, not significant.
Treatment With Statins Induces Apoptosis and Increases the Susceptibility of R. oryzae to Oxidative Stress
Treatment with statins induced DNA fragmentation, as shown by positive TUNEL assay results (Supplementary Figure 2). Similar results were observed with RO-275 and RO-449 when exposed to each statin at the same concentration (64 µg/mL). A dose-dependent macroscopic loss of melanin was observed when R. oryzae was grown on LOV-containing YAG plates (Figure 1B). Because melanin is a recognized scavenger of oxidative species [19], we also analyzed the susceptibility of R. oryzae to the oxidative agent peroxide when grown on LOV-containing YAG plates or drug-free YAG plates. A significant increase in susceptibility to peroxide was observed in R. oryzae grown on YAG plates containing 32 µg/mL LOV (mean radius [±SD] of inhibited growth, 11 ± 1 mm; 3 experiments), compared with those grown on drug-free YAG plates (5 ± 1 mm; P < .05, by the Kruskal–Wallis test; Figure 1C).
Exposure of R. oryzae to Statins Decreases the Ability of R. oryzae to Damage Endothelial Cells, and This Effect Is Independent of GRP78 and CotH Expression
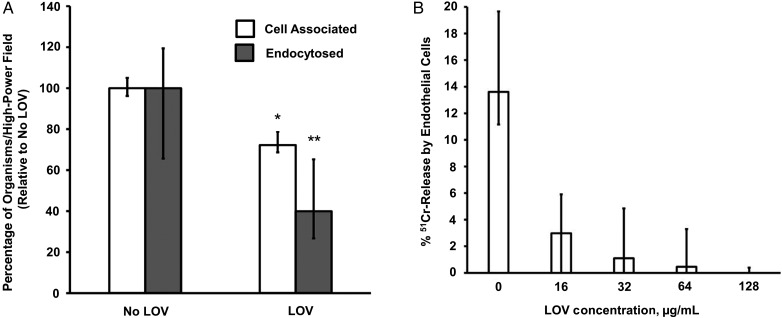
Exposure to LOV significantly decreased the ability of R. oryzae to adhere to (P = .005) and invade (P = .045) endothelial cells (Figure 2A). Consequently, R. oryzae exposed to LOV showed a significantly decreased ability to induce damage to endothelial cells (P < .05; Figure 2B). This effect was dose dependent. However, we found that neither expression of GRP78 nor expression of CotH was significantly modified after exposure of R. oryzae to LOV (Supplementary Figure 3).
Figure 2.
Ability of Rhizopus oryzae to adhere to, invade, and damage endothelial cells. A, R. oryzae exposed to lovastatin (LOV) showed decreased ability to adhere to and invade endothelial cells (*P = .005 and **P = .045, by the Wilcoxon rank sum test). B, R. oryzae exposed to LOV showed decreased ability to induce damage in endothelial cells (P < .05, by the Wilcoxon rank sum test). Data represent mean values (±SD) of 3 experiments. Abbreviation: 51Cr, chromium.
Exposure to LOV Decreases R. oryzae Virulence in Fly and Murine Models of Mucormycosis
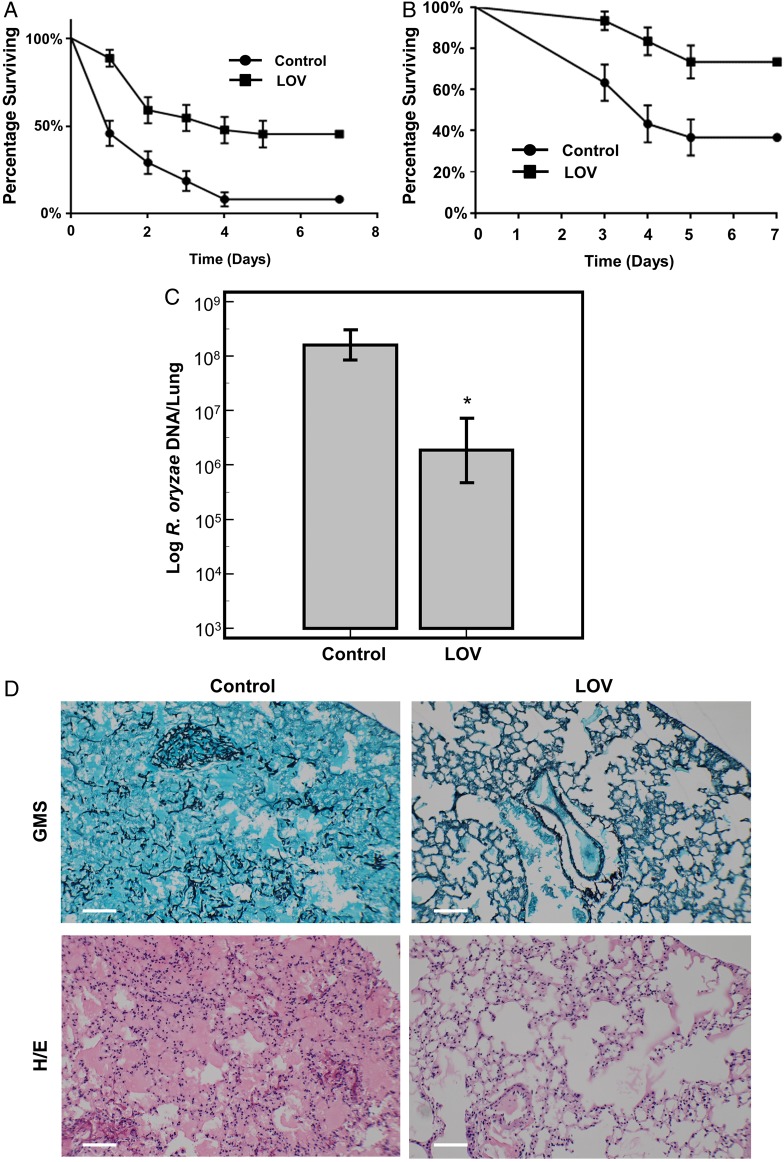
Wild-type fruit flies infected with R. oryzae grown on LOV-containing YAG plates (8 µg/mL) had significantly better overall survival rates than those infected with the same strain grown on drug-free YAG plates (50% compared with 8%; P < .0001, by the Mantel–Cox test; Figure 3A). Similarly, immunosuppressed mice had significantly better survival rates when infected with R. oryzae grown on LOV-containing YAG plates than when infected with the same strain grown on drug-free YAG plates (80% compared with 40%; P = .0021, by the Mantel–Cox test; Figure 3B). Additionally, qPCR of mouse lung tissue showed that the lung tissue infected with R. oryzae grown on LOV-containing YAG plates had a significantly smaller fungal burden than that infected with R. oryzae grown on drug-free YAG plates (P < .05, by analysis of variance followed by the Tukey honest significant difference test; Figure 3C). Finally, histopathologic analysis with Grocott methenamine silver and hematoxylin and eosin staining showed numerous hyphae and spore infiltration within the lung tissue of mice infected with R. oryzae grown on drug-free YAG plates. In contrast, in mice infected with R. oryzae grown on LOV-containing YAG plates, no hyphae invasion or fungal sporulation was observed (Figure 3D). This absence of fungal invasion was found in all mice infected with R. oryzae grown on LOV-containing YAG plates.
Figure 3.
Effects of statins on Rhizopus oryzae virulence in vivo. A, Overall survival of female wild-type fruit flies infected with R. oryzae grown on drug-free yeast extract agar glucose (YAG) plates or lovastatin (LOV)–containing YAG plates (8 µg/mL). Fruit flies had better overall survival rates when infected with R. oryzae grown on LOV-containing YAG plates (P < .0001, by the Mantel–Cox test). There were 22 flies per test group, with 66 flies per experimental group (3 independent experiments performed). B, Overall survival of immunosuppressed female BALB/c mice infected with R. oryzae (RO-969) grown on either drug-free YAG plates or LOV-containing YAG plates (8 µg/mL; P = .0021, by the Mantel–Cox test). All experiments were done in triplicate, with 10 mice per test group and 30 mice per experimental group. C, Quantitative polymerase chain reaction analysis of fungal burden in lung tissue from mice infected with RO-969 grown on either drug-free YAG plates or LOV-containing YAG plates (8 µg/mL). *P < .05, by analysis of variance. There were 20 tissue samples per group. D, Bright-field microscopy of lung tissues from mice infected with R. oryzae grown on drug-free YAG plates (left) or LOV-containing YAG plates (8 µg/mL; right), stained with Grocott methenamine silver (GMS; top) or hematoxylin and eosin (H/E; bottom). Scale bar = 100 µm. Lung tissue from mice infected with R. oryzae grown without exposure to LOV showed numerous hyphae and spore infiltration within the tissue, which is absent in tissue from mice infected with R. oryzae exposed to LOV. Data represent mean values (±SD) of 3 experiments.
DISCUSSION
Several studies have reported modest antifungal activity of statins against yeasts and molds [6–9]. We have previously hypothesized that the widespread use of statins in patients with diabetes mellitus may be a protective factor against mucormycosis [4]. In the present study, we demonstrate that exposure of R. oryzae to statins at concentrations below their MICs induces, in a dose-dependent manner, (1) inhibition of germling formation; (2) DNA fragmentation, a key apoptotic event; (3) loss of melanin and an increase in susceptibility to oxidant species; (4) prevention of endothelial cell damage; and (5) attenuation of virulence in 2 disparate in vivo models of mucormycosis.
Statins are competitive inhibitors of HMG-CoA reductase and are widely used to treat hypercholesterolemia [5]. Ergosterol, a critical component of the fungal cell wall, is synthesized via the acetate-mevalonate pathway, in which HMG-CoA reductase is a key enzyme [20]. ATO and SIM were previously shown to inhibit the growth of Candida species and A. fumigatus [8]. This effect was reversible when ergosterol was added to the culture, suggesting that statin-induced inhibition of growth in A. fumigatus and Candida species was linked to inhibition of ergosterol biosynthesis [8]. Furthermore, a correlation between developmental stage (hyphal growth) and triggering of death has been reported in M. racemosus [9]. This has led to speculation that statins may interfere with prenylation of a number of proteins that control morphogenesis, resulting in a shift to a proapoptotic state. In the present study, using similar statin concentrations, we showed that exposure to statins induced apoptosis and impaired germling development in R. oryzae. This demonstrates that the antifungal effects of statins also extend to Rhizopus species that are the most frequent cause of mucormycosis.
Pigments are cell wall components in melanized fungi that play an important role in virulence and pathogenicity, although pigments are not necessary for growth and development [21]. Melanin, a type of pigment, is thought to play an important role in evasion of host immune responses. Melanin was shown to block the effects of hydrolytic enzymes on the cell wall and to scavenge on the free radicals that phagocytic cells release during the oxidative burst in black molds [21]. We observed a macroscopic loss of brown pigmentation in R. oryzae cultured in LOV-containing YAG plates, suggesting that melanin deposition loss occurred in the cell wall.
Because melanin exerts a protective role against reactive oxygen species in molds [21], the susceptibility of R. oryzae exposed to a high concentration of LOV (32 µg/mL) to peroxide was consistent with lack of protection against oxidative stress. The loss of melanin and the subsequent susceptibility to oxidative stress may explain, at least in part, the decreased virulence of the R. oryzae exposed to LOV that we observed in both models of mucormycosis. The significantly reduced fungal burden, quantified by qPCR, in the lungs of mice infected with R. oryzae exposed to LOV further confirmed that statin exposure induced attenuated virulence in R. oryzae. Our histopathologic findings also supported this result: neither spores nor hyphae invaded lung tissue in mice inoculated with R. oryzae exposed to LOV. One explanation of this absence of fungal invasion could be the consequence of lack of fungal sporulation and decreased ability of R. oryzae to invade the tissue. However, other explanations might be plausible. For example, other major components of the fungal cell wall, such as chitin, chitosan, or glycoproteins [22], could also be affected by statin exposure and result in the observed decreased virulence. The clinical relevance of our findings is supported by the fact that we obtained a good agreement between the 2 disparate models of invasive mucormycosis. As both systems have their specific limitations, comparing data from different models has more impact and may reveal functional insight into the infection process [23].
To better elucidate the mechanisms involved in the effects of statins on R. oryzae, we further investigated the consequences of exposure of R. oryzae to statins in terms of endothelial cell invasion and damage. Recent studies have demonstrated that R. oryzae adheres to human endothelial cells in vitro and invades these cells by inducing endocytosis, using the host endothelial cell receptor GRP78 [15]. Additionally, CotH cell surface proteins were identified as fungal ligands that mediate attachment of R. oryzae to GRP78 receptor during host cell invasion [16]. Our results showed that R. oryzae exposed to LOV had significantly decreased ability to adhere to, invade, and damage endothelial cells. However, the expression of GRP78 and CotH was not modified by statin exposure. These results suggest that the modification of R. oryzae interactions with endothelial cells and attenuated virulence of R. oryzae exposed to LOV is mediated by a mechanism independent of GRP78/CotH interactions.
Our experimental results demonstrate that statins clearly impact the biology of R. oryzae in a variety of ways that could limit its virulence, although the exact mechanistic explanation of R. oryzae virulence modulation induced by statin exposure needs to be further elucidated. It is also important to note that most of the in vitro effects we observed were at concentrations below the MICs, which are still substantially higher than the mean serum concentrations measured in treated patients (usually around 10–20 ng/mL) [24, 25]. Future studies should address the minimum statin concentration needed to attenuate the virulence of Mucorales species. The effect of fungal inoculum and the optimum growth media needed to attain the observed pleiotropic changes described here should be further explored. In addition, we tested only strains of R. oryzae in the present study. Therefore, the generalizability of our findings would be strengthened by testing other Mucorales species. Moreover, analysis of an animal model involving statin prophylaxis should be addressed in a future study toward clinical translation.
Defining the minimum statin concentrations at which antifungal effects occur might be important; a previous study demonstrated that pharmacologically low statin concentrations could be used in combination with an antifungal protein from Penicillium marneffei to maintain a significant antifungal effect while avoiding the adverse effects associated with statins [26]. Our results support the idea that using statins as adjunctive therapy for mucormycosis, in combination with conventional antifungal agents, warrants further exploration. Finally, studying not only the fungus-specific effects of statin preexposure on Rhizopus virulence, but also the impact of statin prophylaxis of the host in regards to Rhizopus lethality, should be an interesting direction for further investigation.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. D. P. K. thanks the Frances King Black Endowment for Cancer Research.
Financial support. This work was supported by the Public Health Service (grants R01 AI063503 and 1R41 AI115907-01 to A. S. I.) and the National Center for Advancing Translational Sciences (through UCLA CTSI grant UL1TR000124).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lewis RE, Kontoyiannis DP. Epidemiology and treatment of mucormycosis. Future Microbiol 2013; 8:1163–75. [DOI] [PubMed] [Google Scholar]
- 2.Kontoyiannis DP, Lewis RE, Lortholary O et al. Future directions in mucormycosis research. Clin Infect Dis 2012; 54:S79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden MM, Zaoutis TE, Buchanan WL et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41:634–53. [DOI] [PubMed] [Google Scholar]
- 4.Kontoyiannis DP. Decrease in the number of reported cases of zygomycosis among patients with diabetes mellitus: a hypothesis. Clin Infect Dis 2007; 44:1089–90. [DOI] [PubMed] [Google Scholar]
- 5.Minder CM, Blaha MJ, Home A, Michos ED, Kaul S, Blumenthal RS. Evidence-based use of statins for primary prevention of cardiovascular disease. Am J Med 2012; 125:440–6. [DOI] [PubMed] [Google Scholar]
- 6.Gyetval A, Emri T, Takacs K et al. Lovastatin possesses a fungistatic effect against Candida albicans but does not trigger apoptosis in this opportunistic human pathogen. FEMS Yeast Res 2006; 6:140–8. [DOI] [PubMed] [Google Scholar]
- 7.Chin NX, Weitzman I, Della-Latta P. In vitro activity of fluvastatin, a cholesterol-lowering agent and synergy with fluconazole and itraconazole against Candida species and Cryptococcus neoformans. Antimicrob Agents Chemother 1997; 41:850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macreadie IG, Johnson G, Schlosser T, Macreadie PI. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol Lett 2006; 262:9–13. [DOI] [PubMed] [Google Scholar]
- 9.Roze LV, Linz JE. Lovastatin triggers an apoptosis-like cell death process in the fungus Mucor racemosus. Fungal Genet Biol 1998; 25:119–33. [DOI] [PubMed] [Google Scholar]
- 10.Chamilos G, Lewis RE, Kontoyiannis DP. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob Agents Chemother 2006; 50:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi, approved standard. 2nd ed Wayne, PA: Clinical and Laboratory Institute, 2008. [Google Scholar]
- 12.Barbu EM, Shirazi F, McGrath DM et al. An antimicrobial peptidomimetic induces Mucorales cell death through mitochondria-mediated apoptosis. PLoS One 2013; 8:e76981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez MM, Reif RD, Pappas D. Detection of apoptosis: a review of conventional and novel techniques. Anal Methods 2010; 2:996–1004. [Google Scholar]
- 14.Bellanger AP, Minteos YD, Albert N, Shirazi F, Walsh TJ, Kontoyiannis DP. Glucocorticosteroids do not impact directly growth rate and biomass of Rhizopus arrhizus (syn. R. oryzae) in vitro. Virulence 2015; 6:441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Spellberg B, Phan QT et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest 2010; 120:1914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebremariam T, Liu M, Lio G et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest 2014; 124:237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis RE, Albert ND, Kontoyiannis DP. Comparative pharmacodynamics of posaconazole in neutropenic murine models of invasive pulmonary aspergillosis and mucormycosis. Antimicrob Agents Chemother 2014; 58:6767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamaris GA, Ben-Ami R, Lewis RE, Chamilos G, Samonis G, Kontoyiannis DP. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis 2009; 199:1399–406. [DOI] [PubMed] [Google Scholar]
- 19.Brakhage AA, Liebmann B. Aspergillus fumigatus conidial pigment and cAMP signal transduction: significance for virulence. Med Mycol 2005; 43(suppl 1):S75–82. [DOI] [PubMed] [Google Scholar]
- 20.Maciejak A, Leszczynska A, Warchol I et al. The effects of statins on the mevalonic acid pathway in recombinant yeast strains expressing human HMG-CoA reductase. BMC Biotechnol 2013; 13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinekamp T, Thywissen A, Macheleidt J, Keller S, Valiante V, Brakhage AA. Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence. Front Microbiol 2013; 3:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Free SJ. Fungal cell wall organization and biosynthesis. Adv Genet 2013; 81:33–82. [DOI] [PubMed] [Google Scholar]
- 23.Brunke S, Quintin J, Kasper L et al. Of mice, flies-and men? Comparing fungal infection models for large-scale screening efforts. Dis Model Mech 2015; 8:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Clin Pharmacokinet 1997; 32:403–25. [DOI] [PubMed] [Google Scholar]
- 25.Desager JP, Horsmans Y. Clinical pharmacokinetics of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Clin Pharmacokinet 1996; 31:348–71. [DOI] [PubMed] [Google Scholar]
- 26.Galgóczy L, Papp T, Lucacs G, Leiter E, Pocsi I, Vagvolgyi C. Interactions between statins and Penicillium chrysogenum antifungal protein (PAF) to inhibit the germination of sporangiospores of different sensitive Zygomycetes. FEMS Microbiol Lett 2007; 270:109–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.