Abstract
Background
The repurposing of existing agents may accelerate TB drug development. Recently, we reported that the lipid-lowering drug simvastatin, when added to the first-line antitubercular regimen, reduces the lung bacillary burden in chronically infected mice.
Objectives
We investigated whether the addition of simvastatin to the first-line regimen (isoniazid/rifampicin/pyrazinamide) shortens the duration of curative TB treatment in mice.
Methods
Mycobacterium tuberculosis-infected THP-1 cells were exposed to simvastatin to determine the effect of statins on the activity of first-line anti-TB drug activity and intracellular rifampicin concentration. Single-dose and steady-state pharmacokinetic studies guided optimized simvastatin dosing in vivo. BALB/c mice were aerosol-infected with M. tuberculosis H37Rv and drug treatment was initiated 6 weeks post-infection. Separate groups of mice received standard TB treatment with or without simvastatin. Relapse rates were assessed 3 months after discontinuation of each treatment regimen. MALDI-MS imaging was used to image the cholesterol content of mouse lung lesions.
Results
Simvastatin significantly enhanced the bactericidal activity of first-line drugs against intracellular M. tuberculosis without altering intracellular rifampicin concentrations. Adjunctive treatment with 60 mg/kg simvastatin shortened the time required to achieve culture-negative lungs from 4.5 to 3.5 months. Following 2.5, 3.5 and 4.5 months of treatment, relapse rates were 100%, 50% and 0%, respectively, in the control group and 50% (P = 0.03), 20% and 0%, respectively, in the statin group. Simvastatin did not alter plasma or lung lesion cholesterol levels.
Conclusions
Statins are attractive candidates for host-directed, adjunctive TB therapy. Further preclinical studies are needed to define the optimal statin and dosing.
Introduction
New regimens with the potential to shorten the duration of curative treatment of active TB are urgently needed.1 Since Mycobacterium tuberculosis is known to subvert immune responses to establish infection and cause disease, host-directed, adjunctive therapies have generated considerable interest.2,3 3-Hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase) inhibitors (statins), which are among the most commonly prescribed drugs in developed countries,4 have lipid-lowering,5 immunomodulatory and anti-inflammatory properties.6–9 We and others have shown that, while lacking a direct antimicrobial effect on M. tuberculosis in axenic cultures, statins reduce M. tuberculosis growth and infection-induced lipid accumulation in macrophages,10,11 and promote phagosomal maturation and autophagy.12 Additionally, simvastatin and atorvastatin increase rifampicin-mediated killing of M. tuberculosis in macrophages.13 We have shown that adjunctive therapy with simvastatin enhances the bactericidal activity of the first-line anti-TB regimen in a standard mouse model of chronic TB infection.10
In the present study, we sought to characterize the effect of simvastatin on the killing of intracellular M. tuberculosis by first-line TB drugs and on the intracellular concentration of rifampicin. Single-dose and steady-state pharmacokinetic studies were performed in mice to determine plasma exposures to simvastatin and its metabolite, as well as any drug interactions with the standard regimen. The bactericidal and sterilizing activity of statin adjunctive therapy was then assessed in the standard murine model of chronic TB.14,15 Primary study outcomes included lung bacillary counts during treatment and the proportion of mice with lung culture-positive relapse 3 months after treatment discontinuation. MALDI-MS imaging (MALDI-MSI) was used to measure lung cholesterol content in control and experimental mice.
Materials and methods
M. tuberculosis strains
M. tuberculosis H37Rv expressing the lux operon, which can be monitored in real time using a luminometer by relative light units (RLU), was used for macrophage studies. RLU serve as a reliable surrogate for cfu under the experimental conditions described by Andreu et al.16 M. tuberculosis H37Rv, which was passaged twice in mice,14,15 was used for in vivo efficacy studies.
Infection of THP-1 cells
To determine the 50% cytotoxic concentration (CC50),17 50% effective concentration (EC50)18,19 and intracellular activities of simvastatin, ex vivo infections were performed with M. tuberculosis H37Rv and human monocytic THP-1 cells (ATCC TIB-202). Briefly, cells were differentiated overnight with 40 nM phorbol 12-myristate 13-acetate (PMA) (Sigma, MO, USA) and seeded at a density of 5 × 105 cells/well in RPMI-1640 supplemented with 2% FBS (Seradigm, PA, USA) and 4 mM l-glutamine (Corning, NY, USA). Following differentiation, the cells were infected with a frozen stock of M. tuberculosis H37Rv at an moi of 1:20. The wells were washed three times with culture medium 4 h post-infection to remove extracellular bacteria. The cells were incubated with RPMI medium containing solvent only (untreated, negative control), simvastatin (NorthStar RX, TN, USA) alone (0.1 μM = 0.04 mg/L) or simvastatin paired with isoniazid (0.011 μM = 0.001 mg/L), rifampicin (0.012 μM = 0.009 mg/L) or pyrazinamide (162.5 μM = 20 mg/L). For these assays, sub-MIC concentrations of each drug were selected that reduced RLU by 50%. When simvastatin was paired with the three-drug combination, each antibiotic in the combination was used at half the concentration required to reduce RLU by 50% when dosed individually. Culture medium, containing antibiotics as needed, was replenished 3 days post-infection. THP-1 cell viability and total cell numbers were determined using trypan blue staining. Cells were lysed 6 days post-infection with 0.05% SDS and serial dilutions of the lysates were plated onto 7H10 agar plates. Bacterial cfu were enumerated after 3 weeks of incubation at 37°C and normalized to the number of viable THP-1 cells in each well.
Drug accumulation assay in human THP-1 cells
The assay was performed using our previously published method with minor modifications.20 Briefly, PMA-treated THP-1 cells were seeded into 96-well plates at a density of 1 × 105, 5 × 104, 3 × 104 and 1 × 104 cells/well to create a cell number standard curve of fluorescence to determine the number of cells per well using the PicoGreen Assay (Molecular Probes Inc., Eugene, OR, USA) according to the manufacturer's specifications. After overnight incubation, drugs (simvastatin and rifampicin) dissolved in culture medium were added at the indicated final concentrations to wells containing 1 × 105 cells. After 30 min of incubation, the culture medium was removed and the cells were gently washed twice with an equal volume of ice-cold PBS to remove any extracellular drug residuals. Cells were lysed with an equal volume of deionized water for 1 h at 37°C. Cell lysates were transferred to 1.5 mL centrifuge tubes and stored at −20°C or analysed immediately by LC-coupled MS (LC-MS).
Pharmacokinetic studies
In single-dose studies, groups of three mice were given one dose of simvastatin alone (20 or 60 mg/kg) or 60 mg/kg simvastatin together with 10 mg/kg isoniazid, 10 mg/kg rifampicin and 150 mg/kg pyrazinamide (isoniazid/rifampicin/pyrazinamide refers to the three-drug combination). In steady-state studies, drugs were given once daily at the above concentrations for 6 successive days. Rifampicin doses were separated from the accompanying drugs by 1 h to minimize drug–drug interactions at the absorption stage.21,22 During the efficacy study, additional blood samples were collected at various time intervals (0, 0.5, 1.5 and 4 h) between week 2 and week 6. Blood was collected in lithium heparin tubes (BD Microtainer®). Whole lung tissue and plasma at each timepoint were frozen at −20°C and later analysed for concentrations of each drug by LC-MS/MS. Plasma and lung drug concentration data were entered into a WinNonlin worksheet (WinNonlin Version 4.0, 2002, Pharsight, Mountain View, CA, USA) and analysed using standard non-compartmental techniques.14
Animals
Female BALB/c mice aged 4–6 weeks were purchased from Charles River Laboratories (Wilmington, MA, USA). All animals were maintained in an animal biosafety level-3 facility under pathogen-free conditions and fed water and chow ad libitum. All procedures involving animals were performed in compliance with the US Animal Welfare Act regulations and Public Health Service Policy according to protocols approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University (JHU protocol approval number MO13M298).
Drug treatment
Drugs were either dissolved or suspended in distilled water and administered daily (five times weekly) by oesophageal gavage. The following doses were used: 10 mg/kg isoniazid; 10 mg/kg rifampicin; 150 mg/kg pyrazinamide; and 60 mg/kg simvastatin. Six weeks after M. tuberculosis infection, separate groups of mice were left untreated (negative controls) or were treated once daily with the standard regimen isoniazid/rifampicin/pyrazinamide (positive control) or isoniazid/rifampicin/pyrazinamide + simvastatin (experimental regimen) for up to 4.5 months. Rifampicin doses were separated from the accompanying drugs by 1 h to minimize drug–drug interactions.
Aerosol infections and study endpoints
A total of 140 female BALB/c mice were aerosol-infected with M. tuberculosis H37Rv using the Inhalation Exposure System (Glas-Col, Terre Haute, IN, USA) calibrated to deliver ∼102 cfu per lung. The mice were randomized into different treatment groups and five mice from each group were sacrificed at predefined timepoints. Primary study endpoints included the bactericidal activity of each regimen at defined timepoints and relapse rates upon discontinuation of treatment. Bacteriological relapse was defined as a positive culture upon plating the entire undiluted lung homogenate, with a theoretical detection limit of 1 bacillus/lung. To assess the efficacy of each treatment group, the animals were sacrificed 3 days after the last drug dose to minimize drug carryover onto culture plates. Lung homogenates were spread onto Middlebrook 7H11 selective agar plates for cfu enumeration.14,15
MALDI-MSI and relative quantification of cholesterol
Tissue sections (12 μm thick) for haematoxylin/eosin staining and MALDI-MSI analysis were prepared as previously described.20 MALDI-MSI analysis was performed using a MALDI LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) with a resolution of 60 000 [at m/z 400, full width half maximum (FWHM)]. Spectra were acquired in positive mode and with a mass window of m/z 200–500. A laser energy of 7.5 μJ was applied and 20 laser shots were fired at each position (total of one microscan per position). The laser step size was set at 75 μm, at which lesions could easily be resolved from surrounding tissue. Data visualization was performed using Thermo Image Quest software. Normalized ion images of dehydrated cholesterol ([M + H–H2O]+) were generated by dividing the [M + H–H2O]+ signal (m/z 369.351 ± 0.003) by the total ion current (TIC). Relative quantification was performed by determining the mean dehydrated cholesterol signal in regions of interest (lesions and whole tissue) using MSiReader software.23,24
Determination of cholesterol in mouse plasma
Plasma was collected in heparin-containing tubes directly from the heart at the time of sacrifice. Total-cholesterol and LDL-cholesterol were measured using the Roche Cobas® Cholesterol Gen.2 (catalogue number 05168538190) and the LDL-Cholesterol plus 2nd generation (catalogue number 05171369190) assays and a Roche Cobas® c701 analyser. Both analytes were measured photometrically following enzymatic conversion of substrates.
Statistical analysis
Comparisons of data among experimental groups were performed by Student's t-test. Group means were compared by one-way analysis of variance (ANOVA) with Dunnett's post test (day 0 or untreated controls versus treatment groups) or Bonferroni comparison (all treatment groups), using GraphPad Prism version 4 (GraphPad, San Diego, CA, USA). Group relapse proportions were compared using Fisher's exact test. P values < 0.05 were considered to be statistically significant.
Results
Simvastatin significantly enhances the killing of M. tuberculosis in macrophages by first-line antitubercular drugs
In order to determine whether simvastatin can enhance the activity of anti-TB drugs against intracellular bacilli, we used a bioluminescent M. tuberculosis strain expressing luciferase16 to infect THP-1 cells. First, we determined the concentration of isoniazid, rifampicin or pyrazinamide required to reduce bacterial RLU by 50%. This concentration of each anti-TB drug was used alone and with simvastatin against M. tuberculosis-infected THP-1 cells. Next, we tested multiple concentrations of simvastatin (ranging from 0.05 to 5 μM) and determined that, while cell viability was similarly affected by the doses tested (<20% reduction after 6 days of treatment), 0.1 μM simvastatin exhibited the greatest activity against intracellular M. tuberculosis (Figure S1, available as Supplementary data at JAC Online). Simvastatin given alone at 0.1 μM showed significant (P < 0.01) antitubercular activity compared with the no-drug control and equivalent activity to 0.011 μM isoniazid (Figure 1a). Simvastatin at 0.1 μM significantly increased the antitubercular activity of isoniazid, rifampicin or pyrazinamide relative to each of these drugs alone (P < 0.05). Furthermore, M. tuberculosis killing by the three-drug combination of isoniazid (0.006 μM), rifampicin (0.0055 μM) and pyrazinamide (81.23 μM) was significantly increased following simvastatin exposure (P = 0.001; Figure 1a). Similarly, the metabolite of simvastatin, simvastatin acid, significantly increased M. tuberculosis killing in THP-1 cells by the combination regimen (P < 0.05) (Figure 1b). Together, these data show that statin exposure enhances the activity of the first-line drugs against intracellular M. tuberculosis.
Figure 1.
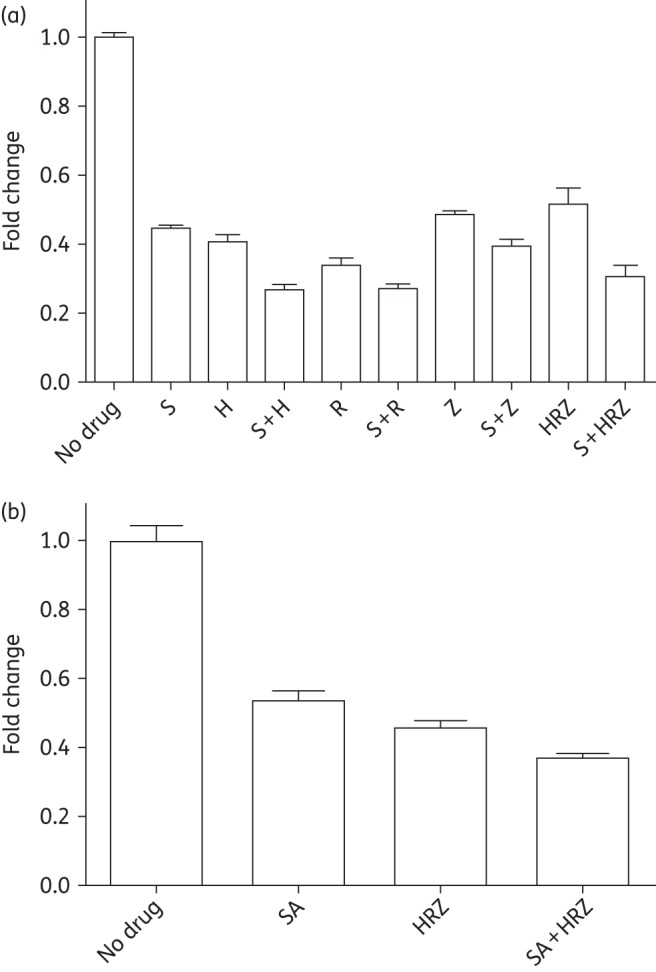
Effects of simvastatin alone and in combination with anti-TB drugs against M. tuberculosis residing in THP-1 macrophages. Data are presented as inhibition of M. tuberculosis growth in macrophages at 6 days post-infection in the presence of the indicated drug treatment, relative to the corresponding solvent (no drug) control. Drug doses yielding 50% reduction of RLU were used: 0.1 μM simvastatin; 0.011 μM isoniazid; 0.012 μM rifampicin; and 162.5 μM pyrazinamide. In the combination drug studies, one-half of the above concentrations of antitubercular drugs were used to study the potential adjunctive activity of simvastatin. S, simvastatin; SA, simvastatin acid; H, isoniazid; R, rifampicin; Z, pyrazinamide.
In parallel testing of the efficacy and toxicity of simvastatin and simvastatin acid alone at 0.1 μM, simvastatin showed (P = 0.0005) greater activity than simvastatin acid without any significant (P = 0.54) difference in toxicity profiles in THP-1 cells after 6 days of treatment (data not shown).
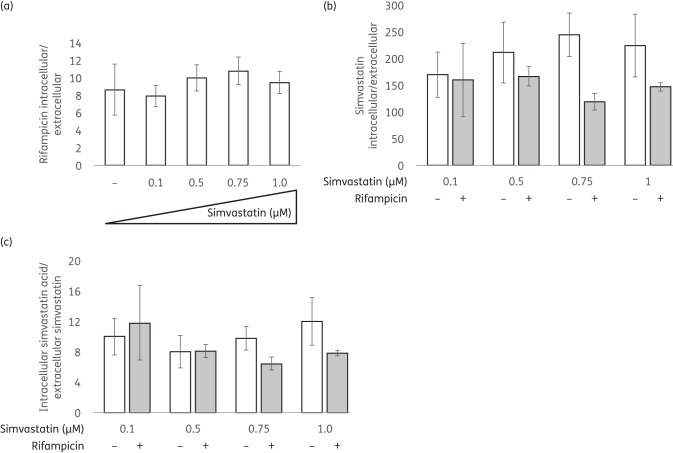
Intra-macrophage concentration of rifampicin following exposure to statins
Rifampicin is a key sterilizing drug and its incorporation into the first-line regimen reduced the duration of TB treatment from 18 to 9 months.25 Given our data and previous reports indicating potential synergy of statins with rifampicin against M. tuberculosis in macrophages, we next sought to determine whether simvastatin exposure increases intracellular accumulation of rifampicin within THP-1 cells. As shown in Figure 2(a), the intracellular/extracellular ratio of rifampicin was not altered following incubation of THP-1 cells with simvastatin at concentrations ranging from 0.1 to 1 μM. Next, we measured the intracellular concentration of simvastatin in macrophages, as well as the extent of bioconversion into its active metabolite, simvastatin acid. Simvastatin showed >150-fold intracellular accumulation, with a non-significant increase in intracellular levels accompanying increasing concentrations of the parent drug, but not following exposure to rifampicin, in the medium (Figure 2b). Within THP-1 cells, 5%–7% of simvastatin was converted into the acid metabolite, resulting in an intracellular/extracellular ratio (acid/parent) ranging from 8 to 12. The intracellular acid/extracellular parent ratio was independent of the initial extracellular concentration of simvastatin. Similarly, rifampicin co-administration did not influence the intracellular accumulation of simvastatin acid (Figure 2c).
Figure 2.
Intracellular accumulation of simvastatin with and without rifampicin exposure within THP-1 cells. THP-1 cells were treated with a range of simvastatin doses (0.1–1 μM) given alone or with 0.224 μM rifampicin. After a 30 min exposure at 37°C, the intracellular concentrations of simvastatin and rifampicin were measured using LC-MS/MS. Data represent the mean of three biological replicates with standard deviations.
Pharmacokinetic evaluation of simvastatin pro-drug and metabolite and effect on anti-TB drug exposure
Prior to undertaking the in vivo drug efficacy studies, we sought to characterize plasma exposures of simvastatin pro-drug and metabolite in mice at steady-state and to determine potential drug–drug interactions between simvastatin and the standard anti-TB drugs (particularly rifampicin) given as a combination. The plasma concentrations of simvastatin acid after dosing simvastatin at 60 mg/kg/day yielded a Cmax of 0.7 mg/L, which was ∼16-fold higher than the stated target of growth inhibition in the whole-cell assays in macrophages (0.1 μM). Further, a dose–response curve in lipid-lowering activity of simvastatin was observed in mice up to 1.6 mg per day,26 justifying the selection of a dose of 60 mg/kg/day for the efficacy studies.
On co-administration of 60 mg/kg simvastatin with human-equivalent doses of isoniazid/rifampicin/pyrazinamide, simvastatin exposure in mouse plasma increased ∼2-fold (Figure 3). In contrast, a 3-fold reduction in simvastatin exposure was observed in humans during concomitant dosing with rifampicin, likely due to CYP3A4 (where CYP stands for cytochrome P450) induction by the latter.27 The plasma pharmacokinetic profiles of each drug in the standard anti-TB regimen remained largely unchanged in the presence of simvastatin (Table 1). Targeted blood sampling in infected mice on treatment at steady-state provided drug levels comparable to those measured in uninfected mice (data not shown).
Figure 3.
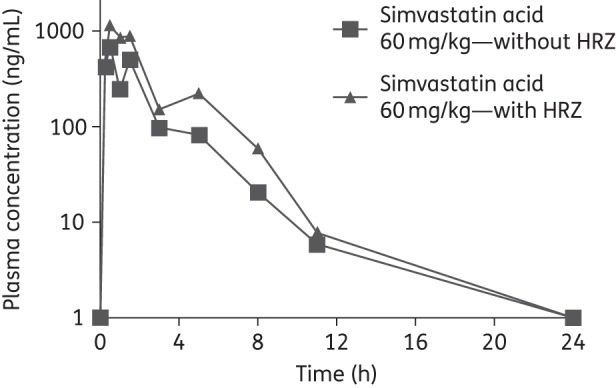
Pharmacokinetic profile of simvastatin acid (dosed as 60 mg/kg simvastatin) with and without the first-line antitubercular drugs at steady-state. Average concentrations are shown (n = 3 at each timepoint) with standard deviations (error bars). HRZ, 10 mg/kg isoniazid + 10 mg/kg rifampicin + 150 mg/kg pyrazinamide.
Table 1.
Pharmacokinetic parameters of simvastatin and the first-line TB drugs in BALB/c mice following a single oral dose (SD) or at steady-state (SS)
| Drug | Dose (mg/kg) | SD or SS | Co-drugs | Cmax (mg/L) | AUC (mg·h/L) |
|---|---|---|---|---|---|
| Simvastatin acid (dosed as simvastatin) | 20 | SS | none | 0.3 | 0.4 |
| 60 | SS | none | 0.7 | 1.5 | |
| 60 | SS | isoniazid/rifampicin/pyrazinamide | 1.5 | 3.0 | |
| Isoniazid | 10 | SD | rifampicin/pyrazinamide | 3.2 | 4.5 |
| 10 | SS | rifampicin/pyrazinamide | 5.1 | 5.4 | |
| 10 | SS | rifampicin/pyrazinamide + simvastatin | 2.0 | 4.1 | |
| Rifampicin | 10 | SD | isoniazid/pyrazinamide | 9.5 | 102 |
| 10 | SS | isoniazid/pyrazinamide | 10.7 | 132 | |
| 10 | SS | isoniazid/pyrazinamide + simvastatin | 10.1 | 118 | |
| Pyrazinamide | 150 | SD | isoniazid/rifampicin | 180 | 306 |
| 150 | SS | isoniazid/rifampicin | 192 | 232 | |
| 150 | SS | isoniazid/rifampicin + simvastatin | 179 | 394 |
Data represent average values for three animals.
The drug concentrations in the lungs of uninfected mice closely mirrored those in the plasma (data not shown).
Simvastatin adjunctive therapy reduces the time to lung culture negativity in mice chronically infected with M. tuberculosis
Simvastatin at 60 mg/kg was well tolerated in mice, as normalized mean body weights increased steadily over time, similar to those of control mice (data not shown). All mice survived until the scheduled study endpoints.
An average of 2.44 ± 0.02 log10 M. tuberculosis bacilli were implanted into mouse lungs and the organisms multiplied to a peak burden of 6.55 ± 0.02 log10 cfu at treatment initiation. Bacillary growth was controlled in the lungs of untreated mice, which maintained a relatively stable plateau ranging from 5.7 to 5.9 log10 cfu up to 4.5 months post-treatment (Figure 4). Relative to untreated mice, those receiving isoniazid/rifampicin/pyrazinamide experienced a reduction of 3, 4.7 and 5.8 log10 lung cfu following treatment for 1.5, 2.5 and 4.5 months, respectively. The addition of simvastatin enhanced the bactericidal activity of isoniazid/rifampicin/pyrazinamide, reducing bacterial burden further by 1.4 log10 (P = 0.001), 0.64 log10 (P = 0.04) and 0.5 log10 following treatment for 1.5, 2.5 and 3.5 months, respectively. Consistent with our previous findings, no remarkable difference was observed in the number or size of grossly visible lung lesions between the treatment groups at any timepoint (data not shown). Similarly, morphometric analysis of lung histology revealed no discernable differences between the two treatment groups at 1.5 or 2.5 months (Figure 5a).
Figure 4.
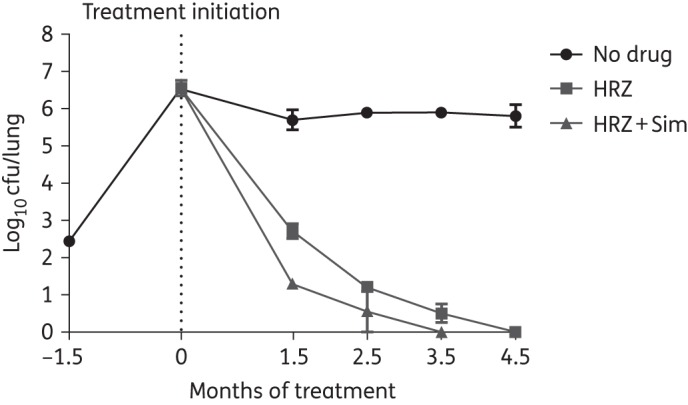
Adjunctive treatment with simvastatin reduces the time required to achieve culture-negative lungs in M. tuberculosis-infected BALB/c mice. HRZ, 10 mg/kg isoniazid + 10 mg/kg rifampicin + 150 mg/kg pyrazinamide; Sim, 60 mg/kg simvastatin.
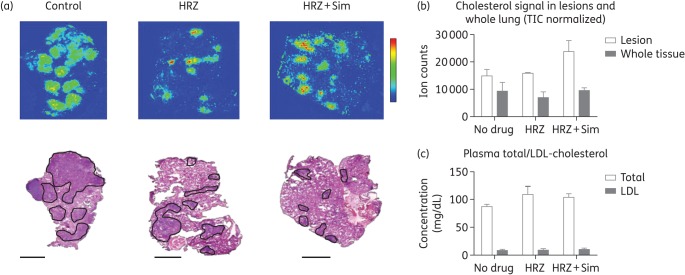
Figure 5.
Simvastatin adjunctive therapy does not alter cholesterol content of lungs or plasma of M. tuberculosis-infected mice. (a) MALDI-MS images showing the distribution of cholesterol ([M + H–H2O]+) in control, isoniazid/rifampicin/pyrazinamide-treated and isoniazid/rifampicin/pyrazinamide + simvastatin-treated mouse lung sections. A haematoxylin/eosin (H&E)-stained reference tissue is shown for identification of tissue structures. Isoniazid/rifampicin/pyrazinamide- and isoniazid/rifampicin/pyrazinamide + simvastatin-treated lungs have smaller lesions containing concentrated levels of cholesterol. Lesions are outlined in black in the corresponding H&E image. Scale bar = 5 mm. (b) Comparative quantification of cholesterol ions in the lesions of control, isoniazid/rifampicin/pyrazinamide-treated and isoniazid/rifampicin/pyrazinamide + simvastatin-treated mice. Relative quantification of cholesterol ions in mouse lesion sections was performed by determining the mean dehydrated cholesterol signal in regions of interest using MSiReader software.23,24 (c) Plasma cholesterol levels. n = 3 mice; values represent median ± SD. Data are from tissue collected at 1.5 months post-treatment. HRZ, 10 mg/kg isoniazid + 10 mg/kg rifampicin + 150 mg/kg pyrazinamide; Sim, 60 mg/kg simvastatin. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
TB relapse rates are improved following statin adjunctive therapy
Relapse rates were assessed 3 months after discontinuation of treatment to determine the sterilizing activity of each combination regimen. In mice treated with isoniazid/rifampicin/pyrazinamide, relapse rates of 100%, 50% and 0% were observed after completion of treatment for 2.5, 3.5 and 4.5 months, respectively. On the other hand, mice treated with isoniazid/rifampicin/pyrazinamide + simvastatin showed relapse rates of 50% (P = 0.03), 20% (P = 0.34) and 0% after completion of treatment for 2.5, 3.5 and 4.5 months, respectively (Table 2).
Table 2.
Sterilizing activity of simvastatin in combination with the first-line regimen against chronic TB in mice
| Regimen | Percentage relapse assessed 12 weeks after the completion of treatment for |
||
|---|---|---|---|
| 2.5 months | 3.5 months | 4.5 months | |
| Isoniazid/rifampicin/pyrazinamide | 100 (10/10) | 50 (5/10) | 0 (15/15) |
| Isoniazid/rifampicin/pyrazinamide + simvastatin | 50 (5/10) | 20 (2/10) | 0 (15/15) |
Relapse is defined as having culture-positive lungs at the time of assessment.
Simvastatin does not alter cholesterol levels in lung lesions or plasma of M. tuberculosis-infected mice
Using MALDI-MSI, we imaged cholesterol and cholesterol esters in the lungs of mice (n = 3) receiving isoniazid/rifampicin/pyrazinamide only, isoniazid/rifampicin/pyrazinamide + simvastatin or vehicle alone (Figure 5a). To compare cholesterol abundance in the three dosing groups, cholesterol ions were quantified in regions of interest drawn on MALDI-MSI maps to encompass lesion clusters in which mean dehydrated cholesterol signals were quantified with MSiReader software. No significant differences in ion counts were observed between the three groups (Figure 5b). Additionally, no significant difference in plasma cholesterol levels were found between groups (Figure 5c). These results suggest that cholesterol depletion is unlikely to be the major mechanism by which simvastatin potentiates anti-TB drugs.
Discussion
Statins are among the most promising candidates for adjuvant, host-directed therapy against TB for a variety of reasons.2,3 Although they lack direct antimicrobial activity, statins are effective against M. tuberculosis in macrophages and enhance the activity of the first-line anti-TB regimen in mice.10,12 Additionally, statins are widely used worldwide, having a highly favourable safety profile.28 Several statins can be co-administered with antiretroviral drugs,29 an important consideration for patients with HIV/TB coinfection. Finally, the widespread availability of less costly generics facilitates their potential use in resource-limited settings. In the present study, we have shown that simvastatin enhances the sterilizing activity of the first-line regimen against drug-susceptible TB in a murine model, shortening the time required to eradicate infection.
M. tuberculosis infection induces intracellular lipid accumulation in macrophages in the form of lipid droplets, quasi-organelles consisting of cholesterol esters and triglycerides surrounded by a phospholipid monolayer. Lipid-laden macrophages acquire a ‘foamy’ phenotype, which is associated with delayed phagolysosomal maturation, enhanced IL-10 induction, alternative macrophage polarization and blunting of innate immunity.30 Although their precise role remains poorly characterized, lipid droplets may serve as a food source for intracellular bacilli.30 In addition, their accumulation has been associated with slowed M. tuberculosis division and phenotypic tolerance to front-line drugs.31 Therefore, foamy macrophages appear to represent a stable reservoir for M. tuberculosis, facilitating persistence of the organism in the host. Previous studies have suggested a correlation between the antimycobacterial and lipid-lowering activities of statins, since exposure of M. tuberculosis-infected macrophages to statins reduces bacterial burden and lipid droplet formation.10,12 However, in the current study we observed potent adjunctive activity of simvastatin without any detectable change in cholesterol in the plasma or lungs of infected mice. It is possible that the anti-mycobacterial effect of simvastatin may indeed be mediated through its cholesterol-lowering properties, albeit at the cellular level, which is beyond the resolution of MALDI. Future studies will examine the antimycobacterial activity of specific inhibitors of each branch of the cholesterol synthesis pathway to distinguish between these possibilities.
The target enzyme of statins is HMG-CoA reductase, which catalyses the conversion of HMG-CoA into mevalonate in the rate-limiting step of the cholesterol biosynthesis pathway.32 The mevalonate pathway is a multi-branched pathway leading to the biosynthesis of cholesterol, lipid hormones, including vitamin D, isoprenoids and isoprenoid intermediates, such as farnesylpyrophosphate and geranylgeranylpyrophosphate, which are required for protein prenylation. The prenylation state of proteins, including the Rho, Rac and Ras protein families,33 results in their subcellular redistribution and membrane anchoring. In particular, Rac transduction signalling modulates the generation of reactive oxygen species, which is an important mechanism by which macrophages control M. tuberculosis infection.34 Due to the multiple functions of the mevalonate pathway, statins possess anti-inflammatory activities in addition to their well-known lipid-lowering properties. For example, statins reduce expression of pro-inflammatory chemokines and cytokines in animal models of atherosclerosis and in patients with rheumatic diseases.35–40 Indeed, the cardioprotective effect of statins is likely related to their immunomodulatory, rather than lipid-lowering, properties.33 In addition, statins appear to activate peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ in inflammatory cells and other cell types. PPAR-γ regulates lipid uptake, adipogenesis and glucose metabolism, and has been implicated in the pathology of obesity, diabetes, atherosclerosis and cancer.41 In the mouse efficacy studies, we used a dose of 60 mg/kg simvastatin based on our pharmacokinetic studies, which showed a Cmax value 16-fold greater than the concentration required for bacillary growth inhibition in macrophages and in a published dose–response study of cholesterol lowering showing a linear increase in efficacy up to this dose in mice.26 An important limitation of this exploratory proof-of-concept study is that the drug exposure in mice was significantly higher than in humans prescribed typical doses of simvastatin as a cholesterol-lowering agent. The simvastatin prodrug was entirely converted into the active acid metabolite in mice, in contrast to humans, where the prodrug/acid exposure ratio is ∼1 over the course of a single dosing interval.42 In fact, the THP-1 cells converted only a small fraction of simvastatin into the acid form. The conversion of simvastatin into simvastatin acid is both non-enzymatic and catalysed by carboxylesterases and paraoxonases in the liver, small intestine and plasma. Most statins are substrates of CYP3A4 or CYP2C9, and of p-glycoproteins, with the exception of pravastatin.43,44 CYP enzymes, including CYP3A4, which is induced by rifampicin, metabolize both simvastatin and simvastatin acid, and the open acid forms of some of these metabolites are active.27 In healthy volunteers, 5 days of pretreatment with rifampicin at a dose of 600 mg daily following a single 40 mg dose of simvastatin led to reduced Cmax and AUC, but had no significant effect on the Tmax or t1/2 of simvastatin or simvastatin acid. On the other hand, no interaction was observed in mice between 60 mg/kg simvastatin and 10 mg/kg rifampicin at steady-state and neither drug altered the intracellular concentration of the other following exposure of THP-1 cells. Therefore, it is difficult to make projections of an efficacious dose of simvastatin in humans, given the complex and species-specific metabolism of this drug. Future preclinical studies will focus on the relationship between dose of prodrug and concentration of simvastatin acid in mouse plasma and lungs, and antitubercular activity. In addition, pharmacokinetic studies in other animal models with more human-like pathology will be required to understand the interactions of statins with first-line TB drugs and recommend adequate dose adjustment.
The statins are an important class of drugs, which can be further developed for adjunctive TB treatment. Additional preclinical studies are required to identify the statin with the most potent antimycobacterial activity, as well as optimal dosing with first-line drugs. In addition, the activity of statins should be assessed in an animal model of cell-mediated immune deficiency in order to simulate AIDS, as well as in combination with second-line agents for MDR TB. As with any host-directed therapy, clinical parameters to determine the efficacy of statins as adjunctive therapy in a clinical trial should include measures of lung function, including pulmonary function testing and respiratory questionnaires, as well as standard microbiological and clinical outcomes.
Funding
This work was supported by BMGF grant OPP 1066499 to V. D., R01 HL106788 to M. L. G. and ACTG grant 110007, CFAR supplement P30AI094189, R01 HL106786 and UH2AI122309 to P. C. K.
Transparency declarations
None to declare.
Disclaimer
The funding sources had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
References
- 1.Zumla AI, Gillespie SH, Hoelscher M et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis 2014; 14: 327–40. [DOI] [PubMed] [Google Scholar]
- 2.Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015; 15: 255–63. [DOI] [PubMed] [Google Scholar]
- 3.Mahon RN, Hafner R. Immune cell regulatory pathways unexplored as host-directed therapeutic targets for Mycobacterium tuberculosis: an opportunity to apply precision medicine innovations to infectious diseases. Clin Infect Dis 2015; 61 Suppl 3: S200–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 2002; 346: 539–40. [DOI] [PubMed] [Google Scholar]
- 5.Davidson MH, Robinson JG. Lipid-lowering effects of statins: a comparative review. Expert Opin Pharmacother 2006; 7: 1701–14. [DOI] [PubMed] [Google Scholar]
- 6.Kwak B, Mulhaupt F, Myit S et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000; 6: 1399–402. [DOI] [PubMed] [Google Scholar]
- 7.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 2009; 203: 325–30. [DOI] [PubMed] [Google Scholar]
- 8.Khurana V, Bejjanki HR, Caldito G et al. Statins reduce the risk of lung cancer in humans: a large case–control study of US veterans. Chest 2007; 131: 1282–8. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell C, Lebreton A, Young Ng C et al. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology 2009; 389: 8–19. [DOI] [PubMed] [Google Scholar]
- 10.Skerry C, Pinn ML, Bruiners N et al. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother 2014; 69: 2453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guler R, Brombacher F. Host-directed drug therapy for tuberculosis. Nat Chem Biol 2015; 11: 748–51. [DOI] [PubMed] [Google Scholar]
- 12.Parihar SP, Guler R, Khutlang R et al. Statin therapy reduces the Mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis 2014; 209: 754–63. [DOI] [PubMed] [Google Scholar]
- 13.Lobato LS, Rosa PS, Ferreira Jda S et al. Statins increase rifampin mycobactericidal effect. Antimicrob Agents Chemother 2014; 58: 5766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta NK, Pinn ML, Karakousis PC. Reduced emergence of isoniazid resistance with concurrent use of thioridazine against acute murine tuberculosis. Antimicrob Agents Chemother 2014; 58: 4048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta NK, Pinn ML, Karakousis PC. Sterilizing activity of thioridazine in combination with the first-line regimen against acute murine tuberculosis. Antimicrob Agents Chemother 2014; 58: 5567–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreu N, Fletcher T, Krishnan N et al. Rapid measurement of antituberculosis drug activity in vitro and in macrophages using bioluminescence. J Antimicrob Chemother 2012; 67: 404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods 1990; 131: 165–72. [DOI] [PubMed] [Google Scholar]
- 18.Tsang AW, Oestergaard K, Myers JT et al. Altered membrane trafficking in activated bone marrow-derived macrophages. J Leukoc Biol 2000; 68: 487–94. [PubMed] [Google Scholar]
- 19.Salamon H, Bruiners N, Lakehal K et al. Cutting edge: vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. J Immunol 2014; 193: 30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prideaux B, Via LE, Zimmerman MD et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med 2015; 21: 1223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosset J, Truffot-Pernot C, Lacroix C et al. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 1992; 36: 548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhillon J, Dickinson JM, Sole K et al. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob Agents Chemother 1996; 40: 552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller NL, Franquet T, Lee KS et al. Imaging of Pulmonary Infections. Philadelphia: Lippincott Williams & Wilkins, 2007. [Google Scholar]
- 24.Robichaud G, Garrard KP, Barry JA et al. MSiReader: an open-source interface to view and analyze high resolving power MS imaging files on Matlab platform. J Am Soc Mass Spectrom 2013; 24: 718–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutta NK, Karakousis PC. Can the duration of tuberculosis treatment be shortened with higher dosages of rifampicin? Front Microbiol 2015; 6: 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Steeg E, Kleemann R, Jansen HT et al. Combined analysis of pharmacokinetic and efficacy data of preclinical studies with statins markedly improves translation of drug efficacy to human trials. J Pharmacol Exp Ther 2013; 347: 635–44. [DOI] [PubMed] [Google Scholar]
- 27.Kyrklund C, Backman JT, Kivisto KT et al. Rifampin greatly reduces plasma simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 2000; 68: 592–7. [DOI] [PubMed] [Google Scholar]
- 28.Mihaylova B, Emberson J, Blackwell L et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380: 581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham PA, Flexner CW. HIV Drug Interaction Guide: DDI: The Companion Guide to MMHIV: Medical Management of HIV Infection. Knowledge Source Solutions, 2012. [Google Scholar]
- 30.Mahajan S, Dkhar HK, Chandra V et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARγ and TR4 for survival. J Immunol 2012; 188: 5593–603. [DOI] [PubMed] [Google Scholar]
- 31.Daniel J, Maamar H, Deb C et al. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 2011; 7: e1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari V, Khokhar M. Mechanism of action of anti-hypercholesterolemia drugs and their resistance. Eur J Pharmacol 2014; 741: 156–70. [DOI] [PubMed] [Google Scholar]
- 33.Antonopoulos AS, Margaritis M, Lee R et al. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des 2012; 18: 1519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamichhane G. Mycobacterium tuberculosis response to stress from reactive oxygen and nitrogen species. Front Microbiol 2011; 2: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng SM, Lai JH, Yang SP et al. Modulation of human T cells signaling transduction by lovastatin. Int J Cardiol 2010; 140: 24–33. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu K, Aikawa M, Takayama K et al. Direct anti-inflammatory mechanisms contribute to attenuation of experimental allograft arteriosclerosis by statins. Circulation 2003; 108: 2113–20. [DOI] [PubMed] [Google Scholar]
- 37.Rezaie-Majd A, Maca T, Bucek RA et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 2002; 22: 1194–9. [DOI] [PubMed] [Google Scholar]
- 38.Bruegel M, Teupser D, Haffner I et al. Statins reduce macrophage inflammatory protein-1α expression in human activated monocytes. Clin Exp Pharmacol Physiol 2006; 33: 1144–9. [DOI] [PubMed] [Google Scholar]
- 39.Leung BP, Sattar N, Crilly A et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol 2003; 170: 1524–30. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Liu P, Liang L et al. RhoA-mediated, tumor necrosis factor α-induced activation of NF-κB in rheumatoid synoviocytes: inhibitory effect of simvastatin. Arthritis Rheum 2006; 54: 3441–51. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Qi Y, Huang TH et al. Pomegranate flower: a unique traditional antidiabetic medicine with dual PPAR-α/-γ activator properties. Diabetes Obes Metab 2008; 10: 10–7. [DOI] [PubMed] [Google Scholar]
- 42.Jemal M, Ouyang Z, Powell ML. Direct-injection LC-MS-MS method for high-throughput simultaneous quantitation of simvastatin and simvastatin acid in human plasma. J Pharm Biomed Anal 2000; 23: 323–40. [DOI] [PubMed] [Google Scholar]
- 43.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004; 109: III50–7. [DOI] [PubMed] [Google Scholar]
- 44.Kyrklund C, Backman JT, Neuvonen M et al. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br J Clin Pharmacol 2004; 57: 181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.