Abstract
The migration of dendritic cells (DCs) to secondary lymphoid organs depends on chemoattraction through the interaction of the chemokine receptors with chemokines. However, the mechanism of how lymphoid chemokines attract DCs to lymphoid organs remains unclear. Here, we demonstrate the mechanism of DC migration in response to the lymphoid chemokine CCL21. CCL21-mediated DC migration is controlled by the regulation of sarcoplasmic reticulum Ca2+ ATPase 2 (SERCA2) expression rather than through the activation of mitogen-activated protein kinases CCL21-exposed mature DCs (mDCs) exhibited decreased SERCA2 expression but not decreased phospholamban (PLB) or Hax-1 expression, which are known to be SERCA2-interacting proteins. In addition, CCL21 did not affect the mRNA levels of SERCA2 or its interacting protein Hax-1. Interestingly, SERCA2 expression was inversely related to DC migration in response to chemokine stimulation. The migratory capacity of CCL21-treated mDCs was decreased by the phospholipase C inhibitor U73122 and by the protein kinase C inhibitor BAPTA-AM. The migratory capacities of mDCs were increased in response to SERCA2 siRNA expression but were decreased by SERCA2 overexpression. In addition, DCs treated with a SERCA2-specific inhibitor (cyclopiazonic acid) had significantly increased migratory capacities as mDCs regardless of SERCA2 expression. Moreover, SERCA2 expression was dependent on DC maturation induced by cytokines or Toll-like receptor agonists. Therefore, the migratory capacities differed in differentially matured DCs. Taken together, these results suggest that SERCA2 contributes to the migration of CCL21-activated DCs as an important feature of the adaptive immune response and provide novel insights regarding the role of SERCA2 in DC functions.
Introduction
Dendritic cells (DCs) can be used as potent immunotherapeutic vaccines for cancer because they are the most effective antigen-presenting cells involved in regulating in vivo immune responses.1, 2 Unlike other antigen-presenting cells, DCs are specialized for homing to the T cell zones of lymphoid organs for the sensitization of T lymphocytes.3, 4 The migration of DCs toward T cell zones requires the upregulation of CCR7 in response to its ligands, CCL19 and CCL21, which are expressed by stromal cells in the T cell zones of lymph nodes.5, 6, 7, 8
Chemokine signals are regulated by their cognate receptors, G-protein-coupled cell-surface receptors. Consistent with these findings, Forster et al.9 demonstrated that CCR7-knockout mice show severe morphological alterations and defective DC migration. Moreover, mice deficient in CCL19 and CCL21 show defects in leukocyte migration and altered immune responses.10, 11 Chemokine receptors allow DCs to migrate toward a chemotactic stimulus and regulate the migratory speed of the cells.12, 13, 14 Chemokine receptors relay intracellular signals that lead to the inhibition of adenyl cyclases through the Gαi subfamily of G proteins, which regulate a variety of signaling molecules, especially mitogen-activated protein kinase (MAPK) family members such as MAPKs, ERK1/2, JNK and p38.14, 15, 16, 17, 18, 19 These receptors may also lead to the activation of phospholipase C (PLC) and phosphatidylinositol-3-kinases (PI3Ks) in response to Gβγ proteins released from Gαi proteins, followed by the diacylglycerol (DAG)-mediated activation of protein kinase C (PKC) and the release of calcium from intracellular stores, leading to the activation of protein kinase B (PKB, Akt) and ERK-2.20, 21, 22 Other signaling pathways involving chemokine receptors include the GTPase/Rho-dependent pathway that activates proline-rich tyrosine kinase 2 (Pyk2), which regulates leukocyte motility.14, 21, 23 Interestingly, MAPK- and PLC-mediated signals regulate CCR7-dependent chemotaxis, whereas Rho/Pyk2-mediated signals regulate migratory speed.14 However, the PI3K/Akt signaling pathway does not regulate the CCR7-dependent chemotaxis or migratory speed of DCs.14 It is difficult to dissect the importance of each event in relation to a specific biological end-point. Various signaling pathways, rather than a single pathway, acting through the chemokine receptor CCR7 appear to orchestrate and regulate distinct DC migratory responses, such as chemotaxis and migratory speed.
Clinical vaccine studies in patients with several types of cancer have shown that human in vitro-generated DCs are the most potent cancer vaccines among the various types examined, including peptides, viruses, tumor cells, heat shock proteins and DCs.24 Most protocols involve the in vitro loading of tumor antigens on DCs, followed by DC maturation and injection of the DC vaccine. The important variables that impact T cell priming are the number of DCs injected, and ultimately, the number of DCs that migrate to the T cell zone. An understanding of the mechanism of DC migration in response to lymphoid chemokines will facilitate the development of more potent DC vaccines.
In our previous study, we demonstrated that pre-stimulating mature DCs (mDCs) with the lymphoid chemokine SLC/CCL21 dramatically enhanced the cytotoxic T lymphocyte-inducing functions of DCs by increasing cytolytic activity without any significant alterations in the expression of cell surface markers or the production of cytokines.25 Furthermore, we recently reported that mDCs treated with IFN-γ, IL-1β and polyI:C, out of six different maturation cocktails, showed a lower expression of SERCA2 and a higher expression of p-cofilin, and consequently, an increased migratory capacity relative to cells treated with the other cocktails.26 Along this line, this study provides cellular and molecular clues in regard to DC migration with a focus on the lymphoid chemokine SLC/CCL21 and sarcoplasmic reticulum Ca2+ ATPase 2 (SERCA2).
Therefore, we investigated the regulatory mechanism of DC migration in response to the pre-stimulation of maturing DCs with chemokine CCL21 and demonstrated that SERCA2, which is located in the sarcoplasmic reticulum and is involved in calcium influx from the cytosol to the sarcoplasmic reticulum, is associated with the capacity of DCs to migrate to lymph nodes in response to the lymphoid chemokine CCL21. SERCA2 expression was decreased by CCL21 and was inversely associated with DC migratory capacity, which was supported by the results from tests using adenovirus-mediated SERCA2 siRNA expression and SERCA2 overexpression. Moreover, mDCs treated with a SERCA2-specific inhibitor, but not mDCs treated with a MAPK-specific inhibitor, had an increased migratory capacity in response to CCL21. SERCA2 was found to be more related to DC migration than were MAPKs and cofilin. Therefore, we present the novel observation that SERCA2 is involved in DC migration and that this relationship may be used to develop potent DC vaccines.
Materials and methods
Reagents
The DC culture medium used was Iscove's modified Dulbecco's medium (IMDM) from Gibco-BRL (Grand Island, New York, USA) containing 10% FBS from PAA Laboratories Inc. (Toronto, Canada). IL-4, IL-1β, TNFα and IFN-γ were obtained from Peprotech (Rocky Hill, New Jersey, USA). IFN-α was provided from LG Life Sciences (Chonbuk, Korea) and GM-CSF was from LG Biochemicals (Daejeon, Korea). CCL21 was purchased from R&D Systems (Minneapolis, MN, USA). Ficoll-Hypaque was purchased from Axis-SHIELD PoC AS (Lymphoprep, Oslo, Norway). All monoclonal antibodies (mAb) used for flow cytometry were obtained from BD Biosciences (Pharmingen, San Diego, CA, USA), except the mAb for CCR7 (R&D Systems, Minneapolis, MN, USA). CD14-conjugated microbeads were purchased from Miltenyi Biotec (Auburn, CA, USA). MAPK inhibitors were from Cell Signaling Technology (Boston, MA, USA) for U0126, Tocris Bioscience (Bristol, UK) for SP600125 and Calbiochem (Darmstadt, Germany) for SB203580. Wortmannin, LY294002, U73122 and BAPTA-AM were obtained from Dr Han's Laboratory (Chonnam National University, Gwangju, Republic of Korea).
Generation and maturation of monocyte-derived DCs
Peripheral blood samples were collected from healthy donors and melanoma patients after obtaining informed consent according to a protocol approved by the Chonnam National University Hwasun Hospital institutional review board. Peripheral blood mononuclear cells were isolated using density gradient centrifugation with Ficoll-Hypaque, and monocytes were isolated by positive selection with CD14-conjugated microbeads and a magnetic-activated cell sorter. Monocytes of at least 95% purity were cultured at a concentration of 1 × 106 cells ml−1 in a six-well plate (Becton Dickinson, Franklin Lakes, New Jersey, USA) in IMDM containing 10% FBS and 1% penicillin/streptomycin and supplemented with GM-CSF (50 ng ml−1) and IL-4 (20 ng ml−1). On day 6, immature DCs were matured with α-type 1-polarizing cocktail (IL-1β (10 ng ml−1), TNFα (50 ng ml−1), IFNα (3000 U ml−1), IFNγ (100 ng ml−1) and polyI:C (20 μg ml−1)) for 2 days. mDCs were pre-treated with the chemokine CCL21 in a dose- and time-dependent manner before getting collected. CCL21 treatments were added at 2 h before collecting mDCs. In addition, mDCs were treated with various inhibitors—including those of the MAPK-specific signaling pathway and the Ca2+ signaling pathway—for 2 h before collection.
Production of adenovirus-mediated SERCA2 siRNA and SERCA2-overexpressing adenoviruses
DNA templates for the synthesis of silencing RNAs were cloned into pRNAT-H1.1 plasmids under control of the H1 promoter (GenScript, NJ, USA). The coding sequence for human SERCA2 siRNA was from the homologous region, as described previously.27, 28 SERCA2 siRNA was synthesized with flanking sequences recognized by the restriction enzymes XhoI and HindIII (5′-TCGAGGACTTGCTAGTTAGGATTTTTGCGGCCGCAAAATCCTAACTAGCAAGTCTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAGACTTGCTAGTTAGGATTTTGCGGCCGCAAAAATCCTAACTAGCAAGTCC-3′) (Genotech, Korea). The oligonucleotides were annealed in the presence of 50 mM Tris–HCl (pH 8.0) and 100 mM NaCl while being heated at 95 °C for 2 min, followed by cooling to room temperature. The annealed oligonucleotides were ligated with pRNAT-H1.1 plasmids (digested with XhoI/HindIII) at 16 °C for 2 h. The ligated DNA was then transformed into competent DH5α cells, and the cells were selected for ampicillin resistance. The pRNAT/SERCA2 siRNA plasmids were linearized with PmeI and transformed into competent BJ5138 cells by electroporation to produce recombinant adenoviral plasmids. The cells were selected for kanamycin resistance. The recombinant plasmids were selected by digestion with PacI (insert size should be 4.5 or 3 kb) and amplified by transformation into competent DH5α cells. The pAd/SERCA2 siRNA plasmids were linearized with PacI and then transfected into Ad-293 cells to produce adenovirus-containing SERCA2 siRNA. The scrambled adenovirus was a gift from Dr Keesook Lee (Chonnam National University, Gwangju, Korea).
The SERCA2 ORF region was cloned into the pCL20C-MSCV-IRES-GFP retroviral vectors via PCR amplification. Retroviruses were produced in 293T cells via the transient transfection of SERCA2 or GFP expressing vectors and packaging plasmids expressing the env, gag and pol genes according to the manufacturer's protocol using Fugene 6 (Roche, IN, USA).
Adenovirus or retrovirus-mediated gene transfer
Immature DCs (1.5 × 106 cells) were collected, washed with 1 × phosphate-buffered saline, resuspended in 100 μl of serum-free IMDM, and combined with adenoviruses containing SERCA2 siRNA or scrambled siRNA, or with retroviruses containing SERCA2 ORF or GFP. The suspension was gently mixed and incubated for 2 h at 37 °C. After incubation, the cells were centrifuged, the supernatant was removed, and the cells were resuspended at 0.3 × 106 per ml in complete IMDM containing DC-maturation cocktails. Cells were then incubated at 37 °C for 48 h before analysis of GFP expression using fluorescence microscopy.
Migration assay
DC migration toward CCL21 (R&D Systems) was measured in 24-well transwell plates fitted with polycarbonate filters with a 5-μm pore size (Corning Costar, New York, NY, USA). A total of 600 μl of culture medium (IMDM with 10% FBS) with CCL21 (250 ng ml−1) was added to the bottom of the chambers. The mDCs (5 × 104 cells/100 μl) were added to the upper chamber. After 3 h of incubation at 37 °C, the migrated cells (500 μl) in the bottom chamber were collected and counted using a FACSAria cell sorter for 60 s.
Co-immunoprecipitation and western blot analysis
Total proteins were prepared from mDCs in a lysis buffer (PRO-PREP, iNtRON Biotechnology, Daejeon, Korea) supplemented with phosphatase inhibitor cocktail 1 and 2 (Sigma, St Louis, MO, USA) and were separated via SDS-PAGE. Proteins on the gels were transferred to a PVDF membrane (Millipore Corporation, Billerica, MA, USA), subjected to western blotting with anti-SERCA2, anti-PLB, anti-Hax-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ERK1/2, anti-p-ERK1/2, anti-p38, anti-p-p38, anti-JNK, anti-p-JNK, and anti-tubulin (Cell Signaling Technology, Beverly, MA, USA), and then detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore). Immunoreactive bands were analyzed and quantitated using an LAS-3000 imaging system and Multi Gauge version 3.0 (Fuji film, Fuji photo film Co, Ltd., Tokyo, Japan), respectively.
Real-time reverse transcription (RT)-PCR
Total RNA was extracted from monocytes, immature DC (imDC) and mDCs cultured in the presence or absence of CCL21. Quantitative RT-PCR was performed using a real-time PCR machine (Corbett Research, Sydney, Australia) and a QuantiTect SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. PCRs were performed using the human SERCA2 primer set (forward primer: 5′-GGTGGTTCATTGCTGCTGAC-3′, reverse primer: 5′-TTTCGGACAAGCTGTTGAGG-3′) or the human Hax-1 primer set (forward primer: 5′-TCCAGATAGTCACCAGCCCA-3′, reverse primer: 5′-GGGAAACCTGGGAATCAAGA-3′) and the β-actin primer set (forward primer: 5′-CCCTCCATCGTCCACCGCAAATGCTTC-3′, reverse primer: 5′-CGACTGCTGTCACCTTCACCGTTCCAG-3′) as a quantitative control. Signals were analyzed using Rotor Gene 2000 (RG-2000) software (Corbett Research).
Statistical analysis
Unpaired, two-tailed Student's t-tests were used to evaluate differences in means between two groups. P-values were considered statistically significant at P<0.05, and significant differences are indicated with an asterisk (*).
Results
MAPKs are not involved in the migration of CCL21-treated DCs
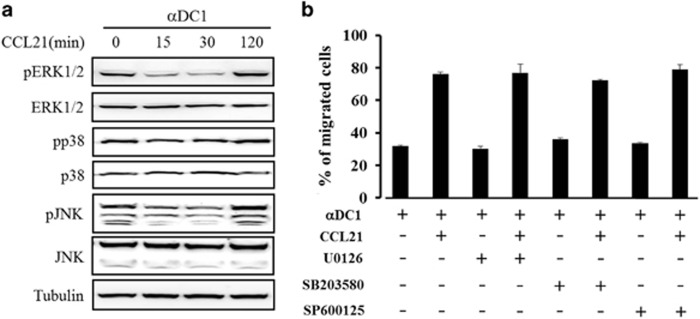
It is known that CCL21 triggers DC migration via its cognate receptor, CCR7. The signaling pathway involving CCR7 includes MAPK activation via the Gi-protein signaling cascade.14 However, the mechanism of DC migration in response to CCL21 remains unclear. We examined the effect of CCL21 pretreatment on DC migration at different time points (0, 15, 30, 60 and 120 min) and at different concentrations of CCL21 (5, 10, 25 and 250 ng ml−1). In this preliminary experiment, the DCs showed an enhanced migration capacity, without any alteration of CCR7 and CD38 expression on the DCs, in response to 25 ng ml−1 of CCL21 on 2 h treatment before DC collection, as described in Supplementary Figures 1A–C. Thus, we investigated whether the activation of MAPKs was associated with the DC migration stimulated by CCL21. Whole-cell lysates were prepared from mDCs with or without CCL21 exposure (0, 15, 30 and 120 min) before collection. Total proteins were analyzed using mAbs against MAPKs and phospho-MAPKs. The levels of phospho-MAPKs—including pERK1/2, pp38 and pJNK—were transiently decreased at 15–30 min after exposure to CCL21, but were restored at 120 min after CCL21 exposure to levels similar to the phospho-proteins in untreated control mDCs (Figure 1a). The levels of total MAPKs were constant, regardless of CCL21-exposure time. Consistent with this result, CCL21-exposure-enhanced DC migratory capacity was not inhibited by the MAPK inhibitors U0126 (for ERK1/2), SB203580 (for p38), or SP600125 (for JNK) (Figure 1b). In addition, MAPK inhibitors did not affect the migratory capacity of DCs not exposed to CCL21.
Figure 1.
DC migration induced by CCL21 treatment was not mediated by MAPKs. (a) At the indicated time points (0, 15, 30 and 120 min) before DC collection, CCL21 (25 ng ml−1)-treated DCs were prepared and analyzed via western blotting using antibodies against phospho ERK1/2, ERK1/2, p38, p38, pJNK and JNK. An anti-tubulin antibody was used as the internal control. The data are representative of three independent experiments. (b) DCs were treated with CCL21 (25 ng ml−1) for 2 h before getting collected in the absence or presence of the indicated MAPK inhibitors (U0126, SB203580 and SP600125), and the migratory capacities of mDCs were evaluated. The data are presented as the means±s.d. of three independent experiments.
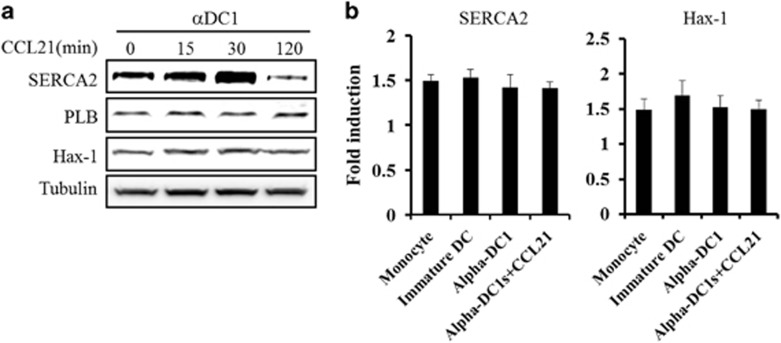
CCL21 reduces the protein level of SERCA2 but not of PLB or Hax-1
On the basis of our results, other signaling pathways may be involved in the migratory capacity of DCs exposed to CCL21. Previous studies have shown that CCL21-triggered signaling in DC migration requires the calcium signaling cascade through the PLC and PKC signaling pathways in mature DCs.19 Interestingly, the migration of myocytes was found to be associated with the calcium signaling cascade through SERCA2 regulation via protein tyrosine kinase 2 (PYK2).29 Thus, we first examined SERCA2 as a downstream signaling molecule of the intracellular calcium signaling pathway. In contrast to MAPK phosphorylation, the level of SERCA2 expression increased at 15 and 30 min after CCL21 exposure, and it was significantly decreased at 2 h (Figure 2a). This decrease in SERCA2 expression was consistent with the enhancement of DC migration caused by CCL21 exposure (Figure 1b and data not shown). However, the expression of phospholamban (PLB) and HCLS1-associated protein X-1 (Hax-1), which are SERCA2-associated proteins, was unaffected by CCL21 exposure (Figure 2a). The mRNA level of SERCA2 was not in concordance with its decreased protein level (Figure 2b), suggesting that the enhanced DC migration caused by CCL21 exposure occurred due to effects in the post-transcriptional stage.
Figure 2.
SERCA2 protein expression was regulated in a time-dependent manner in CCL21-treated DCs. (a) At the indicated time points (0, 15, 30 and 120 min) before DC collection, CCL21 (25 ng ml−1)-treated DCs were prepared and analyzed via western blotting using antibodies against SERCA2, PLB and Hax-1. An anti-tubulin antibody was used as the internal control. The data are representative of three independent experiments. (b) RT-PCR analysis was performed using DCs treated with CCL21 for 2 h before getting collected. The data are presented as the means±s.d. of three independent experiments.
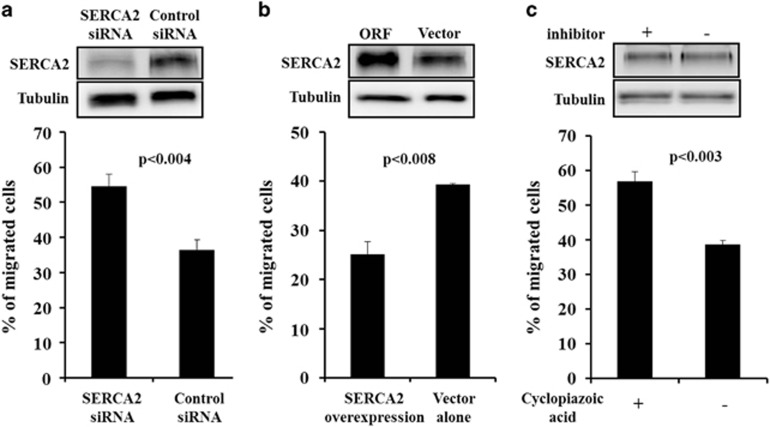
SERCA2 is directly associated with DC migration
To explore the role of SERCA2 in DCs, we investigated whether SERCA2 proteins were associated with DC migration. First, after 6 days of culturing, we infected imDCs with adenovirus-mediated SERCA2 siRNA or scrambled siRNA for 2 h before initiating DC maturation. After 2 h of infection, imDCs were matured with fresh, complete IMDM medium supplemented with a DC-maturation cocktail. Whole-cell lysates were prepared and analyzed via western blotting using mAb against SERCA2. Adenovirus infection efficiency was determined based on GFP expression following SERCA2 protein expression (~70%, data not shown). SERCA2 siRNA expression led to lower SERCA2 protein levels compared with the protein levels associated with scrambled siRNA expression (Figure 3a). In vitro migration assays using SERCA2 siRNA-infected DCs showed increased DC migration in response to the chemokine CCL21 (Figure 3a). SERCA2 was then overexpressed in DCs and their in vitro migratory capacity was determined. In contrast to SERCA2 siRNA expression, the overexpression of SERCA2 decreased DC migration in response to the chemokine CCL21 (Figure 3b). Interestingly, the SERCA2 inhibitor cyclopiazonic acid increased DC migration but did not suppress SERCA2 expression (Figure 3c), suggesting that SERCA2 activity is directly associated with DC migration.
Figure 3.
The levels of SERCA2 expression determined the migratory capacities of DCs. (a) Immature monocyte-derived DCs were transduced with adenovirus-mediated SERCA2 siRNA or scrambled siRNA and then matured using the cytokine cocktails described in the Materials and methods. mDCs were collected and analyzed via Western blot and migration assays. (b) Immature monocyte-derived DCs were transduced with SERCA2- or GFP-overexpressing adenoviruses and then matured using the cytokine cocktails described in the Materials and methods. (c) Cyclopiazonic acid was administered during DC maturation. Western blot results are representative of three independent experiments, and results of the migration assays are presented as the means±s.d. of three independent experiments. P-values indicate significant differences compared with the controls.
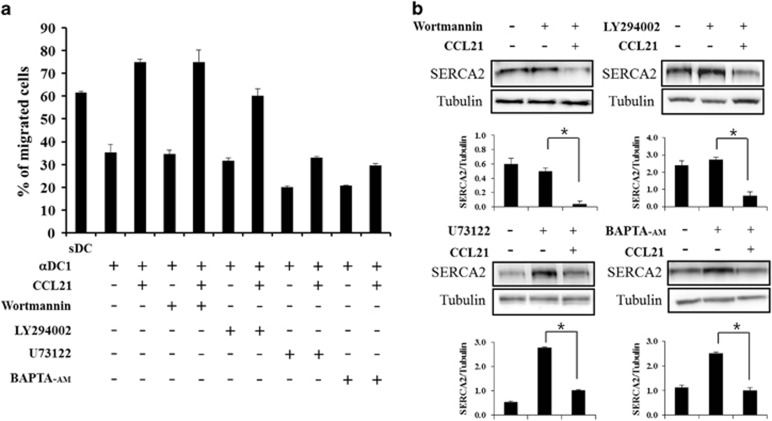
CCL21 inhibits SERCA2 expression through PLC and PKC
Previous studies have shown that DC migration is mediated by intracellular calcium influx via the PLC and PKC signaling pathways but not by phosphatidylinositol-3 (PI3) kinase.19 To explore whether the enhanced migratory capacity of CCL21-exposed DCs was associated with the Ca2+ signaling and the SERCA2 axis, we analyzed whether CCL21-treated DCs were regulated by inhibitors, such as wortmannin, LY294002, U73122 and BAPTA-AM. DCs were incubated with the inhibitors and with or without CCL21 for 2 h before collection. Consistent with previous results, the addition of the specific PI3-kinase inhibitor wortmannin or LY294002 to maturing DCs had no effect on DC migration, but the PLC inhibitor U73122 and the intracellular Ca2+ chelator and PKC inhibitor BAPTA-AM inhibited DC migration in response to CCL21 (Figure 4a). Interestingly, wortmannin and LY294002 did not affect the CCL21-induced decrease in SERCA2 expression, but U73122 and BAPTA-AM counteracted the decrease in SERCA2 level caused by CCL21 exposure and returned it to the level of the control cells that were not treated with the inhibitors (Figure 4b).
Figure 4.
SERCA2 regulation by CCL21 was mediated by the PLC and PKC pathways. CCL21-stimulated mDCs were treated with or without a PI3K inhibitor (wortmannin or LY294002), PLC inhibitor (U73122) or PKC inhibitor (BAPTA-AM) for 2 h before collection. (a) Migration assays were performed as described in the Materials and methods. The data are presented as the means±s.d. of three independent experiments. (b) Western blot analysis was performed as described in the Materials and methods. The data are representative of three independent experiments. The quantitative SERCA2/tubulin expression levels are shown using bar graphs, with statistical significances determined using Multi Gauge version 3.0, as described in the Materials and methods (*P<0.05).
DC migration is coordinated by SERCA2 expression and cofilin phosphorylation
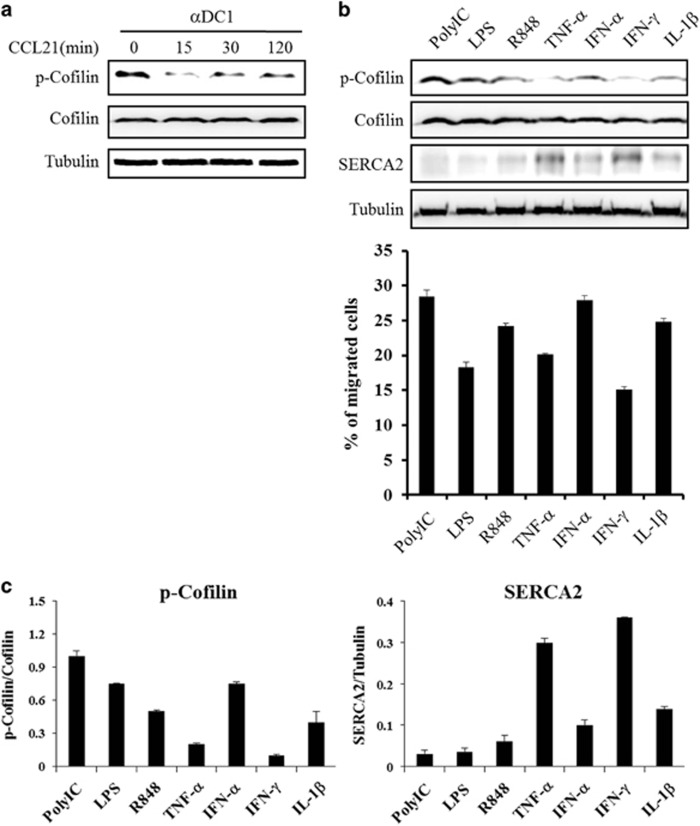
Previous studies have shown that the reorganization of the actin cytoskeleton is a central event in cell migration, and the actin-binding protein cofilin has an essential role in controlling the actin cytoskeleton through the depolymerization and severing of filamentous actin.30, 31 In addition, the phosphorylation of cofilin has a critical role in IL-8-dependent chemotaxis.32 Thus, we examined cofilin expression after CCL21 exposure to explore whether CCL21 affects cofilin expression. CCL21 exposure caused a decrease in the phosphorylation of cofilin compared to phosphorylation in unexposed DCs but did not affect total cofilin levels (Figure 5a). This result suggests that the phosphorylation of cofilin is associated with DC migration, which differs from the results for SERCA2 expression during CCL21 exposure.
Figure 5.
CCL21 differentially regulated SERCA2 expression and cofilin phosphorylation in the presence of various maturation cytokines or TLR agonists. (a) At the indicated time points, CCL21-treated mDCs were collected and a western blot analysis was performed as described in the Materials and methods. (b) Immature DCs were stimulated with various cytokines or TLR agonists for 48 h, after which, mature DCs were collected for western blot and migration assays. (c) The immunoreactive bands were analyzed and quantitated using an LAS-3000 imaging system and Multi Gauge version 3.0, respectively. Western blot results are representative of three independent experiments, and migration data are presented as the means±s.d. of three independent experiments.
The migratory capacity of differentially matured DCs seemed to be different. Thus, we examined SERCA2 and cofilin expression to explore the role of cytokines and Toll-like receptor (TLR) agonists in the migration of differentially matured DCs (Figure 5b). Cofilin phosphorylation and SERCA2 expression were differentially regulated by DC maturation cytokines and TLR agonists. Interestingly, SERCA2 expression was inversely associated with the phosphorylation of cofilin.
To examine the effect of each protein on DC migration, we measured in vitro the migratory capacity of differentially matured DCs in response to CCL21. As shown in Figure 5c, SERCA2 expression was very low in highly migrated polyI:C-matured DCs, whereas it was very high in minimally migrating IFN-γ- and TNFα-matured DCs. The phosphorylation of cofilin was high in highly migrated polyIC-matured DCs, whereas it was relatively low in minimally migrating IFN-γ- and TNFα-matured DCs compared to the results in other cytokine-matured DCs. Although lipopolysaccharides (LPS)-matured DCs expressed low levels of SERCA2 and showed lower levels of cofilin phosphorylation than polyIC-matured DCs, DC migratory capacity was lower in LPS-matured DCs than in polyIC-matured DCs. In addition, IFNα-matured DCs expressed higher levels of SERCA2 and had lower levels of cofilin phosphorylation than polyIC-matured DCs. However, the DC migratory capacity of these cells was similar to that of polyIC-matured DCs. These results suggest that the regulation of DC migration is orchestrated by SERCA2 expression and cofilin phosphorylation.
Discussion
In DC-based cancer immunotherapy, vaccine efficiency is dependent on the potency of an injected vaccine to activate naive T lymphocytes in lymph nodes. To improve the clinical capability of a DC vaccine, potent mDCs must migrate toward lymph nodes in response to lymphoid chemokines. DC migration starts with the expression of CCR7 during the maturation of imDCs,5 which appears to be expressed at different levels in the presence of various maturation cocktails in response to lymphoid chemokines, which may attract maturing DCs to the lymph nodes with different migratory efficiencies.33, 34, 35 Previous studies have shown that the signaling pathway triggered by the engagement of the lymphoid chemokines CCL19 and CCL21 with their cognate receptor CCR7 has a central role in DC migration to lymph nodes.14, 36 Indeed, because DC migration occurs as a simultaneous event with DC maturation and because DCs mature in response to lymphoid chemokines,25 it is difficult to identify the intrinsic function of lymphoid chemokines in attracting mDCs to the lymph node and to determine the specific mechanism by which DC-based cancer vaccines move to the lymph node after injection. Therefore, it remains challenging to identify specific intracellular target proteins involved in DC migration and in the underlying molecular signaling pathways through the interaction of a lymphoid chemokine and its cognate receptor. Here, we demonstrate that the functional role of lymphoid chemokine is to control intracellular calcium levels by regulating the SERCA2 protein, which is located in the sarcoplasmic and endoplasmic reticulum, and is involved in the influx of calcium ions from the cytosol.
Although previous studies have demonstrated that the signaling pathways involving CCR7 are coupled to G proteins of the Gαi type,36 and include PI3-kinase and PKB phosphorylation, MAPK activation and PLC activation,14, 19 the migration of CCL21-exposed mDCs is independent of the activation of MAPKs and PI3-kinase because it was insensitive to the MAPK inhibitors U0126, SB203580 and SP600125 (Figure 1b), and the PI3-kinase inhibitors wortmannin and Ly294002 (Figure 4a). Moreover, CCL21 exposure for 2 h before the collection of mDCs did not affect the phosphorylation of MAPKs (Figure 1a), although ERK activation is dependent on G proteins and is required for cell migration.37, 38 These results indicate that the DC migration signaling pathway stimulated by CCL21 is different from LPS-induced DC migration, which involves KCa3.1 activation via TLR4.39 Therefore, several maturation signals may induce the migration of DCs to secondary lymphoid organs via different signaling cascades.
Intracellular Ca2+ acts as a key regulator of actin assembly, thereby affecting the migratory activity of DCs.40 For example, exposure of DCs to Gram-negative bacteria or LPS increases cytosolic Ca2+ levels through two mechanisms: the entry of extracellular Ca2+ and the release of Ca2+ from intracellular stores.41, 42 Elevated Ca2+ in turn causes extensive actin-based cytoskeletal rearrangement, including the loss of podosomes, thereby facilitating the conversion of DCs to a migratory phenotype.43 CCL21-mediated migration of DCs may be regulated by the SERCA2 protein, which regulates the influx of Ca2+ from the cytosol to the sarcoplasmic reticulum. Blocking Ca2+ stores from the sarcoplasmic reticulum may maintain or cause high-cytosolic Ca2+ levels during DC maturation, thereby increasing DC migration to target organs. Interestingly, during DC maturation, SERCA2 expression was increased within 30 min and reduced at 2 h after CCL21 treatment (Figure 2a). At the same time, ERK and JNK were less activated within 30 min compared with their activation in cells at 2 h (Figure 1a). These results suggest that SERCA2 has several functions in response to lymphoid cytokines. Moreover, SERCA2 expression was regulated by the Ca2+ signaling pathway, especially through the PLC and PKC signaling cascades (Figure 4). However, how PLC and PKC signals regulate SERCA2 expression remains unclear.
In summary, the present data demonstrate that the migratory properties of CCL21-treated monocyte-derived DCs are mediated by Ca2+ influx from the cytosol to the sarcoplasmic reticulum via SERCA2 regulation. These results provide novel insights into the role of the SERCA2 protein in DCs in vitro and suggest a novel mechanism of DC migration that is dependent on the presence of maturation-inducing cytokines.
Acknowledgments
This study was financially supported by grants (2015R1D1A1A09057809) from the National Research Foundation of Korea and from the Leading Foreign Research Institute Recruitment Program (2011-0030034) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
Supplementary Material
References
- Engleman EG. Dendritic cell-based cancer immunotherapy. Semin Oncol 2003; 30: 23–29.2. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 2005; 5: 296–306. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392: 245–252. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ et al. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18: 767–811. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 1998; 28: 2760–2769. [DOI] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A 2000; 97: 12694–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A 1998; 95: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med 2001; 193: 1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med 1999; 189: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Gunn MD. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol 2001; 166: 361–369. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol 1996; 134: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol 1999; 162: 3859–3864. [PubMed] [Google Scholar]
- Riol-Blanco L, Sanchez-Sanchez N, Torres A, Tejedor A, Narumiya S, Corbi AL et al. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol 2005; 174: 4070–4080. [DOI] [PubMed] [Google Scholar]
- Thelen M. Dancing to the tune of chemokines. Nat Immunol 2001; 2: 129–134. [DOI] [PubMed] [Google Scholar]
- Wong MM, Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol 2003; 15: 5–14. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol 1997; 137: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature 2003; 424: 219–223. [DOI] [PubMed] [Google Scholar]
- Scandella E, Men Y, Legler DF, Gillessen S, Prikler L, Ludewig B et al. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood 2004; 103: 1595–1601. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang CK, Jiang H. Roles of phospholipid signaling in chemoattractant-induced responses. J Cell Sci 2000; 113: 2935–2940. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol 2008; 26: 293–316. [DOI] [PubMed] [Google Scholar]
- Tilton B, Ho L, Oberlin E, Loetscher P, Baleux F, Clark-Lewis I et al. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J Exp Med 2000; 192: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganju RK, Brubaker SA, Chernock RD, Avraham S, Groopman JE. Beta-chemokine receptor CCR5 signals through SHP1, SHP2, and Syk. J Biol Chem 2000; 275: 17263–17268. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CY, Lee HJ, Kim HJ, Lee JJ. The lymphoid chemokine CCL21 enhances the cytotoxic T lymphocyte-inducing functions of dendritic cells. Scand J Immunol 2014; 79: 173–180. [DOI] [PubMed] [Google Scholar]
- Choi NR, Lee HJ, Jung SH, Hong CY, Vo MC, Hoang MD et al. Generation of potent dendritic cells with improved migration ability via p-cofilin and sarco/endoplasmic reticulum Ca2+ transport ATPase 2 (SERCA2) regulation. Cytotherapy 2015; 17: 1421–1433. [DOI] [PubMed] [Google Scholar]
- Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A et al. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci U S A 2004; 101: 16683–16688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Cornatzer E, Cornatzer W, Shin DM, Pittelkow MR, Hovnanian A et al. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier's disease. Mol Biol Cell 2006; 17: 4446–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidkamp MC, Scully BT, Vijayan K, Engman SJ, Szotek EL, Samarel AM. PYK2 regulates SERCA2 gene expression in neonatal rat ventricular myocytes. Am J Physiol Cell Physiol 2005; 289: C471–C482. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Glogauer M. Cytoskeletal remodeling in leukocyte function. Curr Opin Hematol 2004; 11: 15–24. [DOI] [PubMed] [Google Scholar]
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 1999; 15: 185–230. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Adachi R, Otani S, Kasahara T, Suzuki K. Cofilin plays a critical role in IL-8-dependent chemotaxis of neutrophilic HL-60 cells through changes in phosphorylation. J Leukoc Biol 2007; 81: 720–728. [DOI] [PubMed] [Google Scholar]
- Jongmans W, Tiemessen DM, van Vlodrop IJ, Mulder PF, Oosterwijk E. Th1-polarizing capacity of clinical-grade dendritic cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J Immunother 2005; 28: 480–487. [DOI] [PubMed] [Google Scholar]
- Auray G, Facci MR, van Kessel J, Buchanan R, Babiuk LA, Gerdts V. Differential activation and maturation of two porcine DC populations following TLR ligand stimulation. Mol Immunol 2010; 47: 2103–2111. [DOI] [PubMed] [Google Scholar]
- Larange A, Antonios D, Pallardy M, Kerdin-Romer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J Leukoc Biol 2009; 85: 673–683. [DOI] [PubMed] [Google Scholar]
- Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 2002; 100: 1354–1361. [DOI] [PubMed] [Google Scholar]
- Cambien B, Pomeranz M, Millet MA, Rossi B, Schmid-Alliana A. Signal transduction involved in MCP-1-mediated monocytic transendothelial migration. Blood 2001; 97: 359–366. [DOI] [PubMed] [Google Scholar]
- Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A 1997; 94: 3052–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobner S, Lukowski R, Autenrieth IB, Ruth P. Lipopolysaccharide induces cell volume increase and migration of dendritic cells. Microbiol Immunol 2014; 58: 61–67. [DOI] [PubMed] [Google Scholar]
- Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol 2001; 280: F739–F747. [DOI] [PubMed] [Google Scholar]
- Matzner N, Zemtsova IM, Nguyen TX, Duszenko M, Shumilina E, Lang F. Ion channels modulating mouse dendritic cell functions. J Immunol 2008; 181: 6803–6809. [DOI] [PubMed] [Google Scholar]
- Salter RD, Watkins SC. Dendritic cell altered states: what role for calcium? Immunol Rev 2009; 231: 278–288. [DOI] [PubMed] [Google Scholar]
- van Helden SF, van den Dries K, Oud MM, Raymakers RA, Netea MG, van Leeuwen FN et al. TLR4-mediated podosome loss discriminates gram-negative from gram-positive bacteria in their capacity to induce dendritic cell migration and maturation. J Immunol 2010; 184: 1280–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.