Abstract
Scaffold-free cell sheet engineering (CSE) is a new technology to regenerate injured or damaged tissues, which has shown promising potential in tissue regeneration. CSE uses a thermosensitive surface to form a dense cell sheet that can be detached when temperature decreases. The detached cell sheet can be stacked on top of one another according to the thickness of cell sheet for the specific tissue regeneration application. One of the key challenges of tissue engineering is vascularization. CSE technique provides excellent microenvironment for vascularization since the technique can maintain the intact cell matrix that is crucial for angiogenesis. In this review paper, we will highlight the principle technique of CSE and its application in tissue regeneration.
Keywords: : bone tissue engineering, cell sheet engineering, vascularization
Since Langer and Vacanti proposed the concept of tissue engineering (TE) in the 1980s [1–4], TE strategy has been widely used in many tissue regeneration fields. This approach was developed in order to restore damaged tissues and organs by implanting synthetic tissue-engineered constructs. TE came about to do away with the challenge of having an organ transplant list. The transplant list has the challenge of the patient's body rejecting the organ or there being a lack of a donor [1]; to bypass this system it was proposed to instead grow organs using regenerative tissue-engineered implants [1]. The goal of TE is to establish a new clinical technology that makes medical treatments possible for diseases that have been too challenging to be cured by conventional methods [5].
There are many challenges that TE techniques encounter that need to be improved on such as cell growth [5], vascularization [6,7], biodegradation rate and stress-shielding caused by scaffolds, cellular phenotype changes [8], biomaterial's immunogenicity [9] and many more. The major challenge in thick tissue regeneration depends on rapid and sufficient vascularization in tissue-engineered constructs, which is a prerequisite for optimal cell survival and integration of implants [7,10,11]. The creation of blood vessels is a major obstacle in TE because the vascularization of an organ is important in the survival of that particular organ, by allowing the organ to acquire nutrients and oxygen [6]. When there is poor vascularization, the tissue can undergo hypoxia, nutrient insufficiency and waste accumulation [6]. The other problem that has been encountered is an immunological response to the biodegradable scaffolds. In specifics, the key challenge of bone TE is vascularization. The lack of vascularization causes necrosis and limited nutrients in the implants [7,10,11]. The vascularization of the bone implants is highly required to promote the formation of new bone tissue in vivo, which this challenge can be overcome with the development of new techniques and more research [12].
Cell sheet engineering
Cell sheet engineering (CSE) is a promising technique used in TE. This technique was founded by Yamato and Okano [1,13]. The CSE process is used to keep the entire cell and adhesion of the extracellular matrix (ECM) together. By using the temperature-responsive polymer, cell sheets can be harvested noninvasively as an undamaged sheet without disrupting the cell-to-cell connection [6]. CSE is a bottom-up technique in which cells are formed in an ECM sheet. In cell sheets, we have superior cell density and higher transplantation efficiency; therefore the regenerating function will be increased [14]. As proteolytic enzymes cause ECM damage and loss of intact cell-to-cell interaction, CSE uses temperature-responsive surfaces so cells can be collected easily without using any enzymes [15].
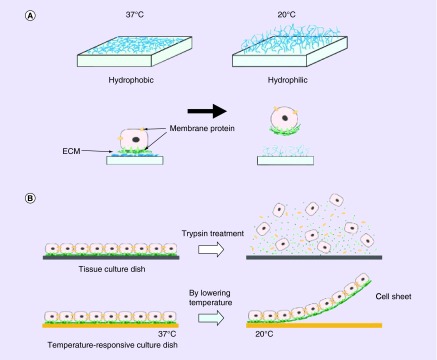
The cell sheet technique is based on a temperature-responsive culture dish that is covalently grafted with poly(N-isopropylacrylamide) (PIPAAm) [1,16,17]. This polymer is a temperature-responsive polymer that allows the cells to attach at 37°C and proliferate. After cells form a layer, it can be detached from the culture dish when temperature decreases to 32°C [18]. The cells can grow on the modified culture dish because at 37°C the PIPAAm is hydrophobic and once the temperature is changed to 32°C the surface will be changed to hydrophilic, which cells are unable to adhere to the surface because of rapid hydration [19]. PIPAAm is used regularly in this technique as the temperature-responsive polymer. The temperature-responsive culture dishes allowed for the maintenance of intact ECM whereas proteolytic enzymes are usually used to digest ECM for the removal of cells (Figure 1) [6]. These proteolytic enzymes, trypsin and dispase, would degrade cell-to-cell junction and the ECM [1]. The cell-to-cell junction and ECM help the cells communicate and grow, and therefore, communicate and grow in vivo. By avoiding the use of proteolytic enzymes, CSE also allows cells to be noninvasively harvested and have growth factors for implantation with the ECM [1,20]. In vivo, cells need to be able to engraft with the host's tissues for growth and tissue development. Cell sheets can be easy to shape into different structures for different applications of regenerating new tissues. The cell sheet could be made into many layers or only one layer and it can be flat or a 3D structure. CSE is capable of keeping long-term engraftment. It has been shown that there has been an ignorable effect on immunoreaction once it is transplanted [21]. The CSE approach has promising potential to overcome many of the obstacles that were shown in the cell microenvironment.
Figure 1. . Temperature-responsive culture dishes showing the attachment and detachment of cell sheet.
(A) At 37°C the cells attach to the surfaces that are hydrophobic and at lower than 32°C the cells detach from the hydrophilic surface. Below 32°C the poly(N-isopropylacrylamide) are rapidly hydrated and are nonadhesive because the surface changes from hydrophobic to hydrophilic. (B) Cells connect to each other by cell-to-cell junctions and ECM; when enzymatic enzymes are introduced to remove cells from the surface, these cell-to-cell junctions and ECM are disrupted. This is not the case for the temperature-responsive culture surface which is able to preserve the cell-to-cell junction and ECM.
ECM: Extracellular matrix.
Reprinted with permission from [6].
One current challenge of TE is the lack of vascularization causing necrosis [14,22,23]. Vascularization improves the overall function of organs. When layering the cell sheets it is important for the cell sheets to be able to successfully form capillaries and to continue to form capillaries where it is needed for the oxygen and nutrients to be provided for the host tissue [14]. CSE is an alternative way to improve a major issue of biodegradable scaffolds, which have no bioactive ECM sites for cell proliferation. For specific tissues, the cell sheets can be transplanted into the host with no need of sutures [1]. This is important in the technique not to need sutures because that can add other complications when implicated and the lack of sutures allows the cell sheet to have cell-to-cell communications undisrupted [6].
The ECM and cell sheets can be noninvasively harvested and directly transplanted because of the use of temperature responsive culture dishes [1,6,24]. CSE is a useful technique applied to many different tissues such as heart, liver, cornea, bladder, esophagus and bone to name a few that will be reviewed in this paper.
Applications of CSE
Heart
The heart is a vital organ in the body and can have many complications from heart failure or other heart diseases. The regeneration of cardiomyocytes is a major topic because the cardiomyocytes lose the ability to proliferate quickly after birth [19,25]. There are many types of cells that have been used in combination for the closest structure of a heart, for example skeletal myoblasts [26–31], mesenchymal stem cells (MSCs) from adipose tissue [32,33], and cardiomyocytes to name a few [19].
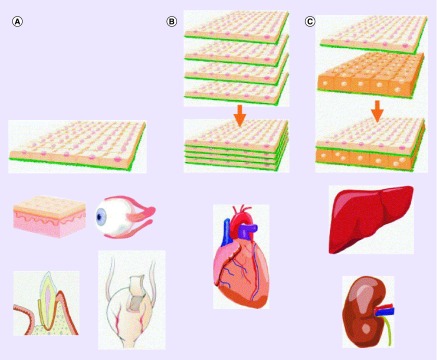
The cell sheets for the cardiac tissue are layered with multiple sheets and in a 3D fashion (Figure 2B) [1,13]. The cell-to-cell communication is highly important in the cardiac cell sheet because the sheets are thicker and, therefore, are needed to work at an optimal level. In another part, the ECM is an important part of the cell-to-cell connections to improve the connection in between the layers of cells [6]. This approach for cardiac cells is a more useful process because it allows for the cell sheets to be directly layered on top of each other and bind to the host directly and the cell sheet will create gap junctions through the whole cell sheet, which in the use of a scaffold cannot occur [1]. With the layering of cell sheets the focus is on being able to fully vascularize the cell sheets in implantation and have cell-to-cell connections. The vascularization of cell sheets on multiple sheet layers has been shown on vascularized beds but more research is continuing [19]. The challenge in this approach of multilayers with vascularization is that the cell sheet is fully dependent on the capillaries that are formed in cell sheet tissue [14,34]. Even though it was found that the endothelial cells form capillaries spontaneously, it was only shown in a single layer and in between cell sheets, but for improvement needs to be more in multilayered structure [14,35].
Figure 2. . Cell sheet engineering layers of the cell sheet.
(A) A single cell sheet layer is used to construct the cornea and the bladder and can be directly transplanted. (B) The heart is multihomotypic layer of cell sheets. (C) The liver is multiheterotypic layer of cell sheets.
Reprinted with permission from [1].
Liver
The liver is an important organ in the body by taking part in protein synthesis, metabolism and detoxification [19]. The liver cell sheet approach is developed as a multilayer and formed in a 3D structure (Figure 2C). The hepatocytes are the cells used in this technique and high oxygen demanding cells; therefore, the cells can undergo ischemia more often than other types of cells [19,36–38]. The vascularization of the hepatocytes is important to the cell sheet because of the need of the exponential amount of oxygen for cell growth. The basic FGF showed improved prevascularization in the hepatocyte sheets by layering the growth factor between the cell sheets and then transplanting the structure [19]. The challenge is not being able to apply the technique to a large-scale cell proliferation [19].
Cornea
The cornea is the visual part of the eye and if damaged, it can impair the vision of a person. This damage can occur in many ways from abrasions or other harmful substances in the eye. The oral mucosal epithelial cells are used for the cell sheet of the cornea epithelium because it is a better representation of the native corneal epithelium [1,39]. The CSE technique is useful to this tissue because the temperature responsive polymer is easier to manipulate the transfer of the culture dish than using proteolytic enzymes, which degrade the cells and the ECM [40,41]. The technique of CSE is made with a single cell sheet layer (Figure 2A) [1]. The single cell sheet is able to attach to the surface of the host cornea quickly without the use of sutures [1,14,41]. By using this technique, it makes the cell sheet less fragile because it is held with cell-to-cell junction and ECM proteins [40], and avoids the need for carrier substrates [1]. The technique has also shown that without having to use the proteases or scaffolds the cell density is still high [40]. The corneal epithelium cell sheet shows vascularization on the peripheral portion of the cornea but not in the center [42]. This is beneficial to the technique to improve the vascularization of the entire transplant. The technique has to focus on expanding the vascularization of the cornea to make the cell sheet more like the host.
Bladder
The bladder is a hollow and contractible muscular organ that is constructed of urothelial cells for the implantation of the bladder cell sheet. The cells are able to spontaneously attach and have adhesive properties, which is why there is no need for suturing or fixing [13,43]. For this technique a biopsy is taken from the bladder and grown in culture into the urothelial cell sheet. There are problems with the implantation of the cells with the side effects of lithiasis, urinary tract infection and electrolyte imbalance [13]. In another experiment using smooth muscle cell (SMCs) sheet for sheet engineering, SMCs were combined with a scaffold to increase the vascularization of the bladder. The use of SMCs is for the contraction of the bladder. On another note, the conventional enterocystoplasty showed complications in the body, which is the reason to find better techniques and the cell sheet technique was then applied to form a better bladder function. There are many complications that are involved in developing the function of the bladder with it being able to contract and stretch properly. The urothelium cell sheet is useful for the attachment because of the use of the ECM in combination with the SMCs [43].
Esophagus
Compared with the progress of heart, liver and cornea tissue regeneration, using cell sheets to regenerate injured or resected esophagus started recently. Currently surgical resection is a main method for esophageal cancer treatment. After tumor resection, damaged esophagus causes stricture, ulcerations and function loss in some patients [44].
CSE technology has shown promising ability to prevent stricture formation following endoscopic submucosal dissection and to improve patients’ quality of life [45]. Ohki and colleagues used patients’ autologous oral mucosal epithelial cells to produce tissue-engineered autologous oral mucosal epithelial cell sheets for preventing the formation of strictures after endoscopic submucosal dissection. They cultured epithelial cell sheets ex vivo for 16 days on temperature-responsive cell culture surfaces. After they detached the cell sheet by a reduction in temperature, they endoscopically transplanted directly to the ulcer surfaces of esophagus. Results showed that the damaged surface of esophagus obtained complete re-epithelialization after 3.5 weeks of implantation. No complications following the procedure occurred in the patients, which shows the promising potential of the tissue-engineered cell sheets [45]. Stratified epithelial cell sheet grafts from autologous oral mucosal epithelium showed the ability to stop stenosis and inflammation, and also promoted the reconstruction of esophageal luminal surface. These transplanted cell sheets could secrete cytokines and growth factors, which will dramatically promote wound healing and decrease host inflammatory responses [45–48].
On the other hand, the vascularization is one of the important factors for tissue regeneration after esophageal resection in cancer treatment. Currently long-gap esophageal defects are often repaired by the upper GI tract, gastric pull-up or interposition grafts using either jejunum or colon to restore organ continuity [49]. However, poor vascularization at the anastomosis site will decrease the cervical esophagogastrostomy healing. So, to repair the resected esophagus, the vascularization of the gastric tube should be improved. CSE technology provides the potential to solve this problem by building new muscle tissue around mucosa–submucosa cell sheet layers. The idea is to allow vascularization to occur at the region where the mucosa meets the smooth muscle. In this case, the ability of blood vessels to reach the basal layer of the submucosa should be at reasonable distance [50,51].
This new cell sheet technology integrated with endoscopic technology has created a new therapy for treating patients with esophageal disorders, which has brought tremendous potential for clinical application.
Bone
Bone is highly vascularized due to the osteoblasts calcified matrix and induces capillary formation [52,53]. Bone has to be highly adaptable for survival in the human body because it is exposed to dynamic external environments regularly [54,55]. The skeletal system could undergo birth defects, trauma, disease, and other problems and TE has played a role in bone regeneration or substitution [56]. Bone TE field faces challenges such as the lack of sufficient vascularization at the bone defect sites [12].
The cell sheet technique improves the challenges of vascularization that are faced with other techniques because thin layers in the cell sheet are able to spontaneously form capillaries and therefore it can be applied for the vascularization of tissue-engineered bone grafts. The vascularization of CSE is critical because without the vascularization of the tissue the implant will not be able to grow or last in the body. The problem with the approach, specifically with the scaffolding approach, is that the structure is unable to become as vascularized as the normal tissue. In the body most of the tissues have blood vessels that form into capillaries to supply the tissue with nutrients and oxygen [11]. Most tissues do not diffuse nutrients and oxygen across the blood vessels from a distance, which means the blood vessel system has to have an optimal distance to supply nutrients and oxygen to the tissue, this optimal distance is 200 μm [56]. This has shown that TE is successful with avascular tissue but for TE to be successful to larger tissues vascularization must occur in the implant [57]. Vascularization has a major effect on how the cells communicate, function and survive [58]. Without blood supply, cells will not survive without oxygen, other nutrients and the ability to disposal of waste, which will be a problem in large tissue-engineered constructs [6]. The major challenge to vascularization is the thickness of the tissue formed [7,10,11]. For optimal growth the tissue needs rapid and sufficient vascular networking [19].
A way to overcome the challenge of vascularization is to create prevascularized structure by having vessel-like microchannels in a scaffold-based matrix to facilitate vascularization which should improve the nutrient and oxygen supply to large synthetic scaffolding grafts [59–62]. There are many approaches that have been created to facilitate vascularization such as layer-by-layer assembly [59,63–66], 3D sacrificial molding [67–69], bioprinting [70] and photolithography [71,72]. As well as in the CSE technique, prevascularization has been proposed to circumvent vascularization of 3D grafts [73,74].
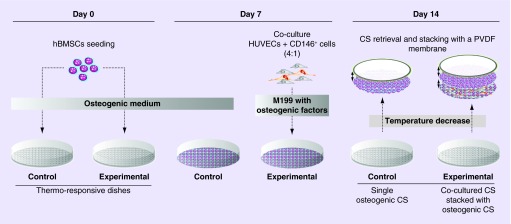
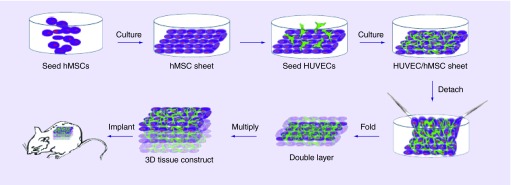
Mendes et al. experiment showed that the vascular network of osteogenic tissue was improved through the formation of prevascularized blood vessels with the combination of endothelial cells [74]. They used the technique of CSE. Their results showed that CD146-positive blood vessels have a higher blood vessel diameter, which helped the stability and maturation of the prevascularized cell sheet [74,75]. Prevascularization has helped the problem of vascularization after implantation by decreasing the amount of time for vascularization to occur in the implant (Figure 3) [74]. This technique was shown to improve the vasculature of the cell sheet. In addition to this experiment, Ren et al. prevascularized a 3D cell sheet [7]. The use of human umbilical vein endothelial cells (HUVECs) seeded on human bone marrow-derived MSCs (hMSCs) sheet promoted the prevascularization of the structure and the anastomosis with host blood vessels. It was shown that by prevascularizing the 3D structure the blood vessels formed a higher density in comparison to no prevascularization of the 3D structure [7]. The experiment showed that prevascularization of cell sheet structure is important to promote the vascularization when in vivo (Figure 4) [7].
Figure 3. . The experiment performed by Mendes et al., showing stacking of co-cultured cell sheets.
The hBMSCs were in thermoresponsive culture dishes for 7 days with the osteogenic medium. The cell sheets were co-cultured with human umbilical vein endothelial cells and CD146. For 7 more days, the co-cultured cells were supplemented with M199.
CS: Cell sheet; hBMSC: Human bone marrow-derived mesenchymal stem cell; HUVEC: Human umbilical vein endothelial cell; PVDF: Polyvinylidene difluoride.
Reprinted with permission from [74] © PLoS ONE (2012).
Figure 4. . The procedures of preparing a 3D prevascularized construct.
Forceps were used to fold the cell sheet three-times to form the eight-layer square construct.
HUVEC: Human umbilical vein endothelial cell; hMSC: Human bone marrow-derived mesenchymal stem cell.
Reprinted with permission from [7] © Hindawi Publishing Corporation (2014).
The connection of vascularization and bone formation is linked in bone healing [58]. In an experiment done by Zhang et al., the cell sheet used was based on the endothelial progenitor cells to test the vascularization and osteogenic effect. It was shown that with introducing the endothelial progenitor cells on the cell sheet, it improved both the vascularization and bone formation in vivo [76].
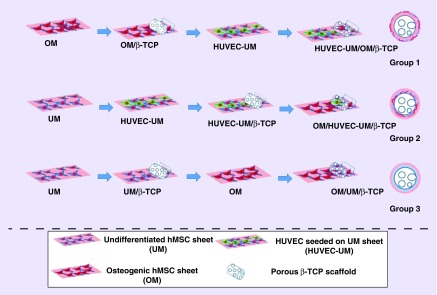
For the vascularization of tissues implanted, some groups have combined the two techniques of CSE and biodegradable scaffolds to improve the vascularization. The increase of vascularization is important and can be done in a unique way. The experiment Kang et al. performed was combining the two techniques, CSE and scaffolding, to produce a higher level of vascularization. The experiment was designed with three testing groups and one control group. The scaffolding used was a biodegradable porous beta-tricalcium phosphate which was present in all the groups tested. The first group was wrapped with osteogenic hMSC sheet and then with HUVEC seeded on to undifferentiated hMSC sheet. The second group was wrapped with HUVEC seeded on to undifferentiated hMSC sheet and then with osteogenic hMSC sheet. The third group was a nonprevascularized undifferentiated hMSC sheet and then the osteogenic hMSC sheet while the fourth group only contained the beta-tricalcium phosphate scaffolds (Figure 5) [77]. The grafts were implanted in mice and removed after 2, 4 and 8 weeks. The main point of this process was to find if cell sheets with the strength of the scaffold would be able to promote vascularization and have osteogenic potentials, because there are still challenges with the vascularization and osteoconductivity to produce healing on large bone defects [78–80]. The results of the experiment showed that the two layers of cell sheets added to the scaffold did promote vascularization, while they were prevascularized. The prevascularized cell sheets showed improvement of blood flow and the vascular system of the mouse in vivo. With the hematoxylin and eosin staining showed the results in the experiment of the prevascularized cell sheet showing blood vessel growth and formation compared with the group with only the scaffold, but all of the vascularized declined in all groups after a certain period of time.
Figure 5. . The step-by-step procedures for preparing cell sheet/β-TCP composite grafts.
The process is shown in stages for the three different cell sheet/β-TCP composite grafts.
HUVEC: Human umbilical vein endothelial cell; hMSC: Human bone marrow-derived mesenchymal stem cell; OM: Osteogenic mineralized cell sheet; UM: Undifferentiated hMSCs sheet.
Reprinted with permission from [77] © American Chemical Society (2014).
In addition to combining two techniques, Ueha et al. used scaffolding and the cell sheet technique to test the growth of bone in large bone defects and an application for the experiment included osteonecrosis [81]. Three groups were formed to test if cell sheets would promote bone formation and, therefore, the function of bone growth vascularization is required. The three groups were: the first group consisted of bone marrow stromal cell (BMSC) and tricalcium phosphate (TCP) scaffold, the second group encompassed TCP scaffold and osteogenic matrix cell sheet, and the third group consisted of TCP scaffold, osteogenic matrix cell sheet and bone marrow stromal cell. The results showed that the third group had a better osteogenic potential compared with the second group, then followed by the first group. The two groups with the cell sheet showed a higher osteogenic potential, which causes a greater bone formation and helps from forming osteonecrosis [81].
Prevascularization is a key process in CSE to promote vascularization of the synthetic bone tissue. A study was done by Ren et al. to promote vascularization of cell sheets. The purpose of the experiment was to use two different layers in the cell sheet approach. The inner layer was seeded with HUVECs on undifferentiated hMSC sheet and the outer layer was osteogenic by inducing osteogenic differentiation of hMSCs. These two layers were wrapped around a rod shape to mimic an induced membrane, prevascularized and nonprevascularized dual layers. The results to the experiment were as follows: that the prevascularized biomimetic-induced membrane quickly anastomosed with the host vasculature, while the nonprevascularized cell sheet did not. The prevascularized biomimetic-induced membrane is important in vascularization, functional anastomosis and osteogenesis in vivo, therefore shows promise to treat large bone defects [73].
As previously discussed the prevascularization of cell sheets is a supported technique and an advantage to the vasculature of the cell sheets in vivo. The other techniques of implementing growth factors also help support the vasculature growth in vivo. The ability to transfer nutrients, waste and ions which allows the bone to grow, develop and remodel is dependent on how vascularized the bone tissue is [82]. Recently, other technologies have been explored to further broaden the potential application of cell sheets for thick tissues. Sakaguchi et al. developed a perfusion bioreactor to corporate collagen microchannels or a vascular bed with stacked cell sheets for the vascularization of thick cardiac constructs [83,84]. They found that medium flew into the cell sheets through the microchannels to form new capillaries. These new technologies showed that 3D perfusable tissues could be established by CSE. CSE brings new potentials to construct 3D-vascularized tissues, which may be applied to treat diseases and tissue model construction [85].
Conclusion
The cell sheet technique has been applied to many different types of tissues including heart, liver, cornea, bladder, esophagus and bone. CSE is a promising technique to help with in vivo vascularization from the cell sheets able to directly attach to the host tissue and incorporate its ECM and growth factors to expand the vasculature network. In bone tissue regeneration, vascularization is important to suffice the growth and development of tissues being grown. In particular, the vascularization of bone tissue was discussed in great detail on improvements with the technique of CSE and how to better improve those techniques in the future. Discussed previously was how to prevascularize the cell sheet before implementing the tissue into the host and that different cells on the cell sheet can help in the promotion of a vascular network.
In CSE there are a few variable techniques to improve the vascularization but the number of cell sheets is limited by the thickness [7]. This happens due to the complications when the cell sheet has too many layers upon each other showing the cells going through hypoxia and ischemia. The remaining problem with CSE is that it is newly explored for bone TE. It still needs more studies to improve the vascularization of cell sheets for bone tissue regeneration. In implantation, the cell sheet may not repair large bone defects due to weak mechanical support. Future studies may need to focus on how to improve the mechanical properties through combining scaffolds or other synthetic grafts, thus further widening the application of CSE in tissue regeneration.
Future perspective
Developing reproducible and cost-effective cell sheets can provide favorable long-term vascularization for tissue regeneration. Undoubtedly, cell sheet technology has a promising potential to make more functional vascularized cell sheet that can provide transplantable tissues with a high metabolic-rate like in the kidney and heart. The cell sheet method can be used for many different kinds of tissue and organ structures. Additionally, 2- and 3D structures of CSE will create great progress in the next-generation of regenerative medicine and TE. The combination of cell sheet technology with other techniques such as scaffolding, microfluidics or nanomedicine will bring promising potential to regenerative medicine.
Executive summary.
Principle of cell sheet engineering
One of the key challenges in tissue engineering is vascularization.
Cell sheet engineering (CSE) is based on a temperature-responsive culture dish which is covalently grafted with thermosensitive polymer poly(N-isopropylacrylamide). A dense cell sheet formed on a thermosensitive surface can be detached when the temperature decreases.
The application of cell sheets in tissue vascularization
Cell sheets can be easy to shape into different structures for different applications of regenerating new tissues, such as heart, liver, cornea, bladder, esophagus and bone.
Cell sheets for the cardiac tissue are layered with multiple sheets in a 3D fashion.
The liver cell sheet approach is developed as a multilayer and formed in a 3D structure.
The cornea is the visual part of the eye and if damaged, it can impair the vision of a person. CSE shows the potential to regenerate the cornea.
Urothelial cells from a bladder can be used to grow urothelial cell sheet for tissue regeneration.
Cell sheet technology integrated with endoscopic technology has created a new therapy for treating patients with esophageal disorders, which has brought tremendous potential for clinical application.
Bone is highly vascularized tissue. Prevascularization is a key process in CSE to promote vascularization of the synthetic bone tissue.
Clinical potential of CSE in tissue regeneration
CSE technique provides excellent microenvironment for vascularization since the technique can maintain the intact cell matrix that is crucial for angiogenesis.
CSE is a promising technique to promote in vivo vascularization from the cell sheets able to directly attach to the host tissue and incorporate its extracellular matrix and growth factors to expand the vasculature network for tissue regeneration.
Footnotes
Financial & competing interests disclosure
We acknowledge the financial supported from the following agencies: NIH (NCI, R03CA201960) and Florida Atlantic University Internal Awards (OURI and SURF). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Yang J, Yamato M, Kohno C, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]; • Presents several significant advantages of cell sheet engineering.
- 2.Vacanti JP. Beyond transplantation. Third annual Samuel Jason Mixter lecture. Arch Surg. 1988;123:545–549. doi: 10.1001/archsurg.1988.01400290027003. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Vacanti CA, Vacanti JP. The science of tissue engineering. Orthop. Clin. North Am. 2000;31:351–356. doi: 10.1016/s0030-5898(05)70155-3. [DOI] [PubMed] [Google Scholar]
- 5.Ikada Y. Challenges in tissue engineering. J. Royal Soc. Interface. 2006;3:589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Adv. Drug Deliv. Rev. 2007;60:277–283. doi: 10.1016/j.addr.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Ren L, Ma D, Liu B, et al. Preparation of three-dimensional vascularized MSC cell sheet. BioMed Res. Int. 2014;2014:1–10. doi: 10.1155/2014/301279. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A new variant of cell sheet engineering for a 3D tissue construct.
- 8.Natoli RM. Doctoral, Rice University: 2009. Impact loading and functional tissue engineering of articular cartilage.http://hdl.handle.net/1911/88472 [Google Scholar]
- 9.Chen GN, Qi YY, Niu L, et al. Application of the cell sheet technique in tissue engineering. Biomed. Rep. 2015;3:749–757. doi: 10.3892/br.2015.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phelps EA, García AJ. Engineering more than a cell: vascularization strategies in tissue engineering. Curr. Opin. Biotechnol. 2010;21:704–709. doi: 10.1016/j.copbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novosel E, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenge. Crit. Rev. Biomed. Eng. 2013;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamato M, Okano T. Cell sheet engineering. Mater. Today. 2004;7:42–47. [Google Scholar]
- 14.Sakaguchi K, Shimizu T, Okano T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J. Control. Release. 2014;205:1–6. doi: 10.1016/j.jconrel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Yan W, Chen G, Qi Y, et al. Application of the cell sheet technique in tissue engineering. Biomed. Rep. 2015;3:749–757. doi: 10.3892/br.2015.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada N, Okano T, Sakai H, et al. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 1990;11:571–576. [Google Scholar]
- 17.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J. Biomed. Mater. Res. Part A. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 18.Heskins M, Guilent JE. Solution properties of poly(N-isopropylacrylamide) J. Macromol. Sci. Chemical: Part A. 1968;2:1441–1455. [Google Scholar]
- 19.Matsuura K, Utoh R, Nagase K, Okano T. Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release. 2014;190:228–236. doi: 10.1016/j.jconrel.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Kushida A, Yamato M, Konno C, et al. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999;45:355–362. doi: 10.1002/(sici)1097-4636(19990615)45:4<355::aid-jbm10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura K, Shimizu T, Okano T. Toward the development of bioengineered human three-dimensional vascularized cardiac tissue using cell sheet technology. Int. Heart J. Assoc. 2013;55:1–7. doi: 10.1536/ihj.13-337. [DOI] [PubMed] [Google Scholar]
- 22.Colton CK. Implantable biohybrid artificial organs. Cell Transplant. 1995;4:415–436. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 23.Hossler FE, Douglas JE. Vascular corrosion casting: review of advantages and limitations in the application of some simple quantitative methods. Microsc. Microanal. 2001;7:253–264. doi: 10.1017.S1431927601010261. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Yamato M, Nishida K, et al. Cell delivery in regenerative medicine: the cell sheet engineering approach. J. Control. Release. 2006;116:193–203. doi: 10.1016/j.jconrel.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Porrello ER, Mahmond AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Memon IA, Sawa Y, Fukushima N, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J. Thorac. Cardiovasc. Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Sekiya N, Matsumiya G, Miyagawa S, et al. Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart. J. Thorac. Cardiovasc. Surg. 2009;138:985–993. doi: 10.1016/j.jtcvs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Kondoh H, Sawa Y, Miyagawa S, et al. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc. Res. 2006;69:466–475. doi: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Hoashi T, Matsumiya G, Miyagawa S, et al. Skeletal myoblast sheet transplantation improves the diastolic function of a pressure-overloaded right heart. J. Thorac. Cardiovasc. Surg. 2009;138:460–467. doi: 10.1016/j.jtcvs.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Hata H, Matsumiya G, Miyagawa S, et al. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J. Thorac. Cardiovasc. Surg. 2006;132:918–924. doi: 10.1016/j.jtcvs.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Miyagawa S, Saito A, Sakaguchi T, et al. Impaired myocardium regeneration with skeletal cell sheets – a preclinical trial for tissue-engineered regeneration therapy. Transplantation. 2010;90:364–372. doi: 10.1097/TP.0b013e3181e6f201. [DOI] [PubMed] [Google Scholar]
- 32.Hamdi H, Planat-Benard V, Bel A, et al. Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc. Res. 2011;91:483–491. doi: 10.1093/cvr/cvr099. [DOI] [PubMed] [Google Scholar]
- 33.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 34.Stoker ME, Gerdes AM, May JF. Regional differences in capillary density and myocyte size in the normal human heart. Anat. Rec. 1982;202:187–191. doi: 10.1002/ar.1092020203. [DOI] [PubMed] [Google Scholar]
- 35.Sekiya S, Shimizu T, Yamato M, et al. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem. Biophys. Res. Commun. 2006;341:573–582. doi: 10.1016/j.bbrc.2005.12.217. [DOI] [PubMed] [Google Scholar]
- 36.Brown MF, Gratton TP, Stuart JA. Metabolic rate does not scale with body mass in cultured mammalian cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R2115–R2121. doi: 10.1152/ajpregu.00568.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kidambi S, Yarmush RS, Novik E, et al. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc. Natl Acad. Sci. USA. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utheim TP, Utheim ØA, Khan QE, Sehic A. Culture of oral mucosal epithelial cells for the purpose of treating limbal stem cell deficiency. J. Funct. Biomater. 2016;7:1–27. doi: 10.3390/jfb7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umemoto T, Yamato M, Nishida K, Okano T. Regenerative medicine of cornea by cell sheet engineering using temperature-responsive culture surfaces. Chin. Sci. Bull. 2013;58:4349–4356. [Google Scholar]
- 40.Nishida K, Yamato M, Hayashida Y, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 41.Asakawa N, Shimizu T, Tsuda Y, et al. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. 2010;31:3903–3909. doi: 10.1016/j.biomaterials.2010.01.105. [DOI] [PubMed] [Google Scholar]
- 42.Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]; • The clinical application of epithelial cell sheet in corneal reconstruction.
- 43.Staack A, Hayward SW, Baskin LS, Cunha GR. Molecular, cellular and developmental biology of urothelium as a basis of bladder regeneration. Differentiation. 2005;73:121–133. doi: 10.1111/j.1432-0436.2005.00014.x. [DOI] [PubMed] [Google Scholar]
- 44.Ohki T, Yamamoto M, Ota M, Okano T, Yamamoto M. Application of cell sheet technology for esophageal endoscopic submucosal dissection. Tech. Gastrointest. Endosc. 2011;13:105–109. [Google Scholar]
- 45.Ohki T, Yamato M, Ota M, et al. Application of regenerative medical technology using tissue-engineered cell sheets for endoscopic submucosal dissection of esophageal neoplasms. Dig. Endosc. 2015;27:182–188. doi: 10.1111/den.12354. [DOI] [PubMed] [Google Scholar]; •• New clinical application of cell sheets in the treatment of submucosa after the resection of esophageal tumor.
- 46.Takagi R, Yamato M, Kanai N, et al. Cell sheet technology for regeneration of esophageal Mucosa. World J. Gastroenterol. 2012;18:5145–5150. doi: 10.3748/wjg.v18.i37.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Yamato M, Shimizu T, et al. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28:5033–5043. doi: 10.1016/j.biomaterials.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 49.Shen KR, Austen WG, Jr, Mathisen DJ. Use of a prefabricated pectoralis major muscle flap and pedicled jejunal interposition graft for salvage esophageal reconstruction after failed gastric pull-up and colon interposition. J. Thorac. Cardiovasc. Surg. 2008;135:1186e7. doi: 10.1016/j.jtcvs.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 50.Pierie JP, de Graaf PW, van Vroonhoven TJ, Obertop H. The vascularization of a gastric tube as a substitute for the esophagus is affected by its diameter. Dis. Esophagus. 1998;11:231–235. doi: 10.1093/dote/11.4.231. [DOI] [PubMed] [Google Scholar]
- 51.Hayari L, Hershko DD, Shoshani H, Maor R, Mordecovich D, Shoshani G. Omentopexy improves vascularization and decreases stricture formation of esophageal anastomoses in a dog model. J. Pediatr. Surg. 2004;39:540–544. doi: 10.1016/j.jpedsurg.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Ye Z, Zhou Y, Tan WS. Towards engineering complex tissue/organs: a co culture perspective. Austin J. Biomed. Eng. 2015;2:1–2. [Google Scholar]
- 53.Battiston KG, Cheung JW, Jain D, Santerre JP. Biomaterials in co-culture systems: towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials. 2014;35:4465–4476. doi: 10.1016/j.biomaterials.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Kang Y, Yin G, Luo L, et al. Effects of mechanical stress on the in vitro degradation of porous composite scaffold for bone tissue engineering. Key Eng. Mater. 2007;342–343:273–276. [Google Scholar]
- 55.Knothe Tate ML. “Whither flows the fluid in bone?” An osteocyte's perspective. J. Biomech. 2003;36:1409–1424. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 56.Jain RK, Au P, Tam J, et al. Engineering vascularized tissue. Nat. Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 57.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Roux BM, Cheng MH, Brey EM. Engineering clinically relevant volumes of vascularized bone. J. Cell. Mol. Med. 2015;19:903–914. doi: 10.1111/jcmm.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang Y, Mochizuki N, Khademhosseini A, et al. Engineering a vascularized collagen-β-tricalcium phosphate graft using an electrochemical approach. Acta Biomater. 2015;11:449–458. doi: 10.1016/j.actbio.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl Acad. Sci. USA. 2006;103:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiu LL, Montgomery M, Liang Y, Liu H, Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc. Natl Acad. Sci. USA. 2012;109:E3414–E3423. doi: 10.1073/pnas.1210580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nillesen ST, Geutjes PJ, Wismans R, et al. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–1131. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 63.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab. Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 64.Ling Y, Rubin J, Deng Y, et al. A cell-laden microfluidic hydrogel. Lab. Chip. 2007;7:756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 65.Choi NW, Cabodi M, Held B, et al. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 66.Cuchiara MP, Allen AC, Chen TM, et al. Multilayer microfluidic PEGDA hydrogels. Biomaterials. 2010;31:5491–5497. doi: 10.1016/j.biomaterials.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 67.Therriault D, White SR, Lewis JA. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2003;2:265–271. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 68.Wu W, Hansen CJ, Aragon AM, et al. Direct-write assembly of biomimetic microvascular networks for efficient fluid transport. Soft Matter. 2010;6:739–742. [Google Scholar]
- 69.Miller JS, Stevens KR, Yang MT, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visconti RP, Kasyanov V, Gentile C, et al. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin. Biol. Ther. 2010;10:409–420. doi: 10.1517/14712590903563352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zorlutuna P, Annabi N, Camci-Unal G, et al. Microfabricated biomaterials for engineering 3D tissues. Adv. Mater. 2012;24:1782–1804. doi: 10.1002/adma.201104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nikkhah M, Eshak N, Zorlutuna P, et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials. 2012;33:9009–9018. doi: 10.1016/j.biomaterials.2012.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren L, Kang Y, Browne C, et al. Fabrication, vascularization and osteogenic properties of a novel synthetic biomimetic induced membrane for the treatment of large bone defects. Bone. 2014;64:173–182. doi: 10.1016/j.bone.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendes LF, Pirraco RP, Szymczyk W, et al. Perivascular-like cells contribute to the stability of the vascular network of osteogenic tissue formed from cell sheet-based constructs. PLoS ONE. 2012;7(7):e41051. doi: 10.1371/journal.pone.0041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ouhtit A, Gaur RL, Abd Elmageed ZY, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochimica Biophys. Acta. 2009;1795:130–136. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Zhang R, Gao Z, Geng W, Yan X, Chen F, Liu Y. engineering vascularized bone graft with osteogenic and angiogenic lineage differentiated bone marrow mesenchymal stem cells. Artif. Organs. 2012;36:1036–1046. doi: 10.1111/j.1525-1594.2012.01529.x. [DOI] [PubMed] [Google Scholar]
- 77.Kang Y, Ren L, Yang Y. Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold. ACS Appl. Mat. Interfaces. 2014;6:9622–9633. doi: 10.1021/am502056q. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A new application of cell sheets with scaffolds for bone tissue regeneration.
- 78.Akita S, Tamai N, Myoui A, et al. Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng. 2004;10(5–6):789–795. doi: 10.1089/1076327041348338. [DOI] [PubMed] [Google Scholar]
- 79.Elcin YM, Dixit V, Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif. Organs. 2001;25:558–565. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 80.Royce PM, Kato T, Ohsaki K, Miura A. The enhancement of cellular infiltration and vascularization of a collagenous dermal implant in the rat by platelet-derived growth factor BB. J. Dermatol. Sci. 1995;10:42–52. doi: 10.1016/0923-1811(95)93713-b. [DOI] [PubMed] [Google Scholar]
- 81.Ueha T, Akahane M, Shimizu T, et al. Utility of tricalcium phosphate and osteogenic matrix cell sheet constructs for bone defect reconstruction. World J. Stem Cells. 2015;7:873–882. doi: 10.4252/wjsc.v7.i5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao RR, Stegemann JP. Cell-based approaches to the engineering of vascularized bone tissue. Cytotherapy. 2013;15:1309–1322. doi: 10.1016/j.jcyt.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sakaguchi K, Shimizu T, Horaguchi S, et al. In vitro engineering of vascularized tissue surrogates. Sci. Rep. 2013;3:1316. doi: 10.1038/srep01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sekine H, Shimizu T, Sakaguchi K, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masuda S, Shimizu T. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv. Drug Deliv. Rev. 2016;96:103–109. doi: 10.1016/j.addr.2015.05.002. [DOI] [PubMed] [Google Scholar]