Abstract
Background:
The physical impairments that affect participation in life roles among older adults have not been identified. Using the International Classification of Functioning Disability and Health as a conceptual framework, we aimed to determine the leg and trunk impairments that predict participation over 2 years, both directly and indirectly through mediation by changes in activities.
Methods:
We analyzed 2 years of data from the Boston Rehabilitative Impairment Study of the Elderly, a cohort study of 430 primary care patients with self-reported mobility limitation (mean age 77 years; 68% female; average of four chronic conditions). Frequency of and limitations in participation were examined using the Late-Life Disability Instrument. Baseline physical impairments included: leg strength, leg speed of movement, knee range of motion (ROM), ankle ROM, leg strength asymmetry, kyphosis, and trunk extensor endurance. Structural equation modeling with latent growth curve analysis was used to identify the impairments that predicted participation at year 2, mediated by changes in activities. Models were adjusted for baseline participation, age, and gender.
Results:
Leg speed and ankle ROM directly influenced participation in life roles during follow-up (βdirect = 1.39–4.53 and 4.70, respectively). Additionally, ankle ROM and trunk extensor endurance contributed indirectly to participation score at follow-up via effects on changes in activities (βindirect = −1.06 to −4.24 and 1.01 to 4.18, respectively).
Conclusions:
Leg speed, ankle ROM, and trunk extensor endurance are key physical impairments predicting participation in life roles in older adults. These results have implications for the development of exercise interventions to enhance participation.
Key Words: Disablement, Disability evaluation, Geriatric assessment, Social participation, Rehabilitation
Participation is recognized as a key component of health associated with successful aging and with survival (1,2). Defined as a person’s involvement in life situations, participation is one of the three main components in the World Health Organization’s International Classification of Functioning, Disability and Health (ICF) (3). Up to 52% of adults aged above 50 years have restrictions in participation and this number increases with age (4). Importantly, participation restrictions represent the broad impact of health on people’s lives; a concept more meaningful to individuals than problems in performing basic physical tasks and activities (5)—areas that have traditionally been emphasized in both geriatric care and gerontological research.
Within the ICF framework, participation is described as resulting from the complex interaction between a health condition or disease, body functions, and structures (anatomic and physiologic functioning of organs and body systems), activities (execution of actions by an individual), and personal and environmental factors (3). Examples of participation include involvement in home or community life such as taking care of the home or taking part in active recreation, while the activity domain includes discrete physical tasks such as walking and getting up from a chair. The ICF thus provides a useful framework for studying critical health outcomes and their determinants. Despite its recognized importance, gaps in evidence on the determinants of participation hinder efforts for disability prevention among older adults. While medical, demographic, and socioeconomic factors as well as some aspects of physical function have been shown to be associated with participation in prior work (6–11), less attention has been paid to the physical impairments at the body functions and structures level that may influence participation. This is important for informing evidence-based exercise interventions targeting the impairments most critical for participation in life roles among older adults. Although it is well established that exercise-based interventions improve activity limitations, evidence that exercise can improve participation is mixed (12,13). In part, this may be due to the lack of understanding of the specific impairments that underlie participation and can be targeted as part of interventions. To date, most exercise studies of older adults have emphasized leg strength and aerobic capacity (14,15); it is possible that interventions targeting other physical impairments would have greater effects.
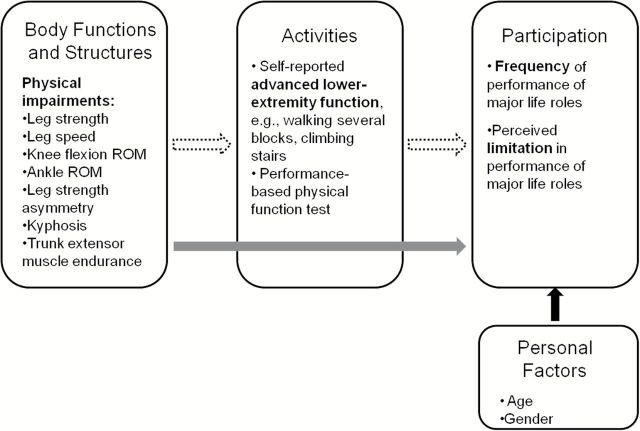
The Boston Rehabilitative Impairment Study of the Elderly (Boston RISE) is a prospective cohort study of older primary care patients with the specific aim of identifying the physical impairments amenable to rehabilitation that are most relevant for activities and participation (16). The primary aim of this analysis of Boston RISE is to investigate the most important leg and trunk impairments that predict an older adult’s subsequent level of participation at 2 years. This information is critical for informing evidence-based exercise interventions to enhance participation in life roles among older adults. In addition, although the ICF is widely accepted as a framework for describing factors that influence the disablement process, little empirical evidence exists confirming the pathways between impairments, activities, and participation. Prior work has shown that both impairments and activities affect participation; however, the limited work evaluating the interplay among these constructs suggests that the main influence of impairments is through an intermediary effect on activities which in turn, has a direct impact on participation (8,17). Therefore, our secondary aim is to determine whether changes in activities mediate the impact of leg and trunk impairments on participation over 2 years. Our structural model of disability tested the following hypotheses: (i) baseline leg and trunk impairments will be identified that predict participation at year 2, both directly and indirectly through changes in activities and (ii) there is a direct relationship between changes in activities over 2 years and participation at year 2. A conceptual model illustrating these relationships is provided in Figure 1.
Figure 1.
Conceptual model for the hypothesized relationships between physical impairments, activities, and participation. The solid arrow represents the direct effect of the impairment on participation and the dotted arrow represents the indirect effect mediated by activities.
Methods
Design and Participants
We analyzed 2 years of follow-up data from Boston RISE, a cohort study of 430 older adults. The study was approved by the relevant Institutional Review Boards and all participants provided written informed consent. Details of the methods have been described previously (16). Briefly, primary care patients in the Greater Boston Area who met the following criteria were recruited: age ≥65 years, ability to communicate in English, self-reported difficulty or task modification walking ½ mile and/or climbing stairs, no planned major surgery, and expectation of remaining in the area. Exclusion criteria comprised: significant visual impairment, uncontrolled hypertension, lower-extremity amputation, use of supplemental oxygen, myocardial infarction or major surgery in the previous 6 months, Mini-Mental State Exam score <18 and Short Physical Performance Battery (SPPB) score <4. Baseline assessments were conducted during an initial screening visit and a subsequent visit within 2 weeks. Follow-up assessments were conducted at 12 and 24 months.
Measures
Physical Impairments
Five categories of physical impairments were evaluated in Boston RISE: strength, speed of movement, range of motion (ROM), asymmetry, and trunk stability. This analysis focused on the following leg and trunk impairments selected from each category based on their association with mobility activities shown previously (18): leg strength, leg speed, knee flexion ROM, ankle ROM, leg strength asymmetry, kyphosis, and trunk extensor endurance.
Participants underwent physical impairment testing during the second baseline visit; all measures were collected by a trained assessor using a standardized protocol. Assessment sessions lasted 2 hours and participants were permitted to rest as needed. Leg strength was the maximum value obtained from either leg using a 1-repetition maximum test on a Keiser A420 pneumatic leg press machine. Leg speed was calculated by dividing peak leg press power by the concurrently recorded force obtained from a single leg press repetition pushing out as quickly as possible (18). Knee and ankle ROM were measured with a goniometer. Ankle ROM was defined as intact or impaired based on dorsiflexion less than 90° or plantarflexion less than 20°. Strength asymmetry was calculated by dividing the higher leg strength value from the two sides by the lower strength value. Trunk extensor endurance was assessed by recording the time a participant was able to maintain a neutral trunk position with their lower body supported on a specialized plinth leaning forward at a 45° angle (19). Kyphosis was measured using a validated flexible ruler technique (20).
Activities
Activities were assessed using the advanced lower-extremity scale of the Late-Life Function Instrument (LLFI) (21). The LLFI assesses a broad range of activity limitations, consistent with the ICF model. Participants are asked to report their current degree of difficulty in performing 32 physical activities on a typical day without the help of someone else and without the use of assistive devices. Response options include: none, a little, some, quite a lot, and cannot do. The advanced lower-extremity function scale includes activities involving a significant degree of physical ability and endurance such as walking several blocks and climbing a flight of stairs without a handrail. The scale is calibrated from 0 to 100, where 0 indicates poor function and 100 indicates good function. Evidence supports the validity, reliability, and responsiveness of the LLFI in community-dwelling older adults (21–23).
The SPPB (24) was used as a secondary measure of activity in order to identify any additional physical impairments that might affect participation through a performance-based test of basic lower-extremity mobility.
Participation
Participation was assessed with the Late-Life Disability Instrument (LLDI) that measures both frequency of participation in 16 major life tasks and limitations in capability to perform each task consistent with the ICF model (25). Frequency dimension questions ask, “How often do you” do a specific task while limitation dimension questions ask “To what extent do you feel limited” in doing the task. Questionnaire items encompass a wide variety of participation tasks including: personal care; mobility and travel; exchange of information; social, community, and civic activities; home life; paid or volunteer work; and involvement in economic activities. Raw scores for each dimension are transformed to scaled scores from 0 to 100 with higher scores indicating a greater level of participation. The LLDI has strong construct validity and reliability, and is predictive of poor self-rated health, hospitalizations, and emergency room visits (23,25,26).
Statistical Analysis
We used structural equation modeling based on latent growth curve analysis to determine the baseline leg and trunk impairments that predicted participation directly at year 2, and indirectly through their effect on changes in activities (Figure 1). Two-step procedures were used to build the models. First, we used the unconditional latent growth model to describe the change in activity across the three time-points (baseline, year 1, and year 2). Second, we used a series of mediation models to examine whether change in activities would mediate the impact of the physical impairment on participation. To facilitate interpretation, the impairments were dichotomized using the median as the cut-point; this approach also yielded the best model fit. Results are presented in terms of performance on the physical impairment test above the median (ie, less impairment). For each physical impairment, the mediation effect was calculated as the product of the impact of that impairment on change in activities and the impact of change in activities on the participation outcome. Based on 5,000 bootstrap samples, the bias-corrected confidence interval was used to determine the statistical significance of the mediation effect. A mediation (indirect) effect was established if the 95% confidence interval did not include 0, indicating that change in activity mediates the impact of the impairment on participation. Models were adjusted for age, gender, and baseline level of participation. An effect size (ES) estimate for the impairments that predicted participation was calculated as ES = β/SD; where β is the direct/indirect coefficient for the impairment and SD is the standard deviation of the participation score at year 2. As a sensitivity analysis to examine the effect of missing data for the entire sample (n = 430), we applied two-stage maximum likelihood estimation (27): in the first stage, we estimated the saturated mean and covariance matrix of the observed variables based on the expectation–maximization algorithm. In the second stage, the mediation model parameters were estimated based on the saturated mean and covariance matrix generated from the first stage; the bias-corrected bootstrap method was used to create the confidence intervals (CIs) (28).
Overall model fit was examined using the chi-square test (nonsignificant p value indicates good model fit), comparative fit index (CFI) and Tucker Lewis index (TLI) (values >0.95 indicate acceptable fit), and root mean square error of approximation (RMSEA; values <0.05 indicate good model fit). Maximum likelihood estimation implemented in MPlus was used in the analysis.
Results
Participants’ baseline characteristics and values for the leg and trunk impairments are provided in Table 1. Demographics were consistent with census data for older adults living in the recruitment area. At baseline, 430 Boston RISE participants had a mean age of 76.6 years, 68% were female and 82.6% were white. At 2 years, 366 participants remained in the study; 15 were deceased, 17 withdrew due to medical reasons and 32 withdrew due to non-health reasons or were lost to follow-up. Over 2 years, the mean change in activities measured by the LLFI was −3.23±10.26 points; the mean change in SPPB score was 0.53±1.77. At 2 years, mean participation scores were 51.12±6.27 for the LLDI frequency scale and 69.11±13.84 for limitation.
Table 1.
Baseline Characteristics
| Variable | n | mean ± SD or % |
|---|---|---|
| Age (years) | 430 | 76.6±7.0 |
| Sex: women (%) | 291 | 67.7 |
| Race: white (%) | 355 | 82.6 |
| Number of chronic medical conditions | 430 | 4.0±1.9 |
| Hypertension (%) | 297 | 69.1 |
| Osteoarthritis (%) | 293 | 68.3 |
| Heart disease (%) | 158 | 36.8 |
| Cancer (%) | 140 | 32.6 |
| Lung disease (%) | 98 | 22.8 |
| Diabetes (%) | 82 | 19.1 |
| Neurologic disease (%) | 80 | 18.6 |
| Mini-Mental State Exam (18–30) | 430 | 27.4±2.4 |
| Habitual gait speed (m/s) | 430 | 0.9±0.2 |
| Physical Activity Scale for the Elderly (0–793) | 429 | 174.9±69.6 |
| Number using assistive device | 118 | 27.4 |
| Leg strength (N/kg) | 387 | 9.45±2.54 |
| Limb velocity (m/s) | 381 | 1.00±0.25 |
| Maximal knee flexion (°) | 425 | 124.6±13.6 |
| Ankle range | 429 | |
| Intact (%) | 307 | 71.6 |
| Impaired (%) | 122 | 28.4 |
| Leg strength asymmetry | 372 | 1.18±0.29 |
| Kyphosis (height/length × 100 of flexicurve instrument) | 430 | 10.52±3.08 |
| Trunk extensor endurance (s per kg) | 405 | 1.30±0.88 |
| Lower-extremity activities (0–100) | 430 | 41.8±14.7 |
| SPPB (4–12) | 430 | 8.7±2.3 |
| Participation frequency (0–100) | 430 | 52.3±5.7 |
| Participation limitation (0–100) | 430 | 68.9±11.8 |
Note: SPPB = Short Physical Performance Battery.
The direct and indirect effect coefficients and 95% CI for the leg and trunk impairments that predicted frequency of and limitations in participation are shown in Tables 2 and 3, respectively. Positive coefficients indicate a positive relationship between the physical impairment and subsequent participation; that is, better scores on the physical impairment tests predict higher participation in life roles. Specifically, the interpretation of β is the expected change in participation score associated with baseline performance above the median on the physical impairment test when holding all other model parameters constant. Both the participation frequency and limitation models met criteria for good model fit. For participation frequency χ2 (df = 13) = 13.98, p = .3753; CFI = 0.999; TLI = 0.997; and RMSEA = 0.015. For participation limitation χ2 (df = 13) = 12.834, p = .4607; CFI = 1; TLI = 1; and RMSEA = 0.
Table 2.
Leg and Trunk Impairments Predicting Frequency of Participation in Life Roles Mediated by Changes in Activities Over 2 Years
| Variable | Beta Coefficient (95% CI) | ||
|---|---|---|---|
| Direct Effect | Indirect Effect | Total | |
| Changes in lower-extremity activities | 0.82 (0.28 to 1.93)* | — | — |
| Ankle ROM | 0.96 (−0.63 to 2.96) | −1.06 (−3.56 to −0.07)* | −0.10 (−1.29 to 1.11) |
| Leg speed | 1.39 (0.05 to 3.28)* | −0.52 (−2.4 to 0.47) | 0.86 (−0.25 to 2.00) |
| Trunk extensor endurance | −0.32 (−2.4 to 1.03) | 1.01 (0.05 to 3.50)* | 0.68 (−0.36 to 1.78) |
Notes: Models adjusted for baseline participation, age, and sex. Coefficients are presented for physical impairment test performance above the median. Participation and activity score range: 0–100; higher is better. ROM = range of motion.
*95% CI does not contain 0
Table 3.
Leg and Trunk Impairments Predicting Limitations in Participation in Life Roles Mediated by Changes in Activities Over 2 Years
| Variable | Beta Coefficient (95% CI) | ||
|---|---|---|---|
| Direct Effect | Indirect Effect | Total | |
| Changes in lower-extremity activities | 2.87 (1.78 to 5.58)* | — | — |
| Ankle ROM | 4.70 (0.15 to 10.70)* | −4.24 (−10.73 to −0.5)* | 0.46 (−2.80 to 3.73) |
| Leg speed | 4.53 (0.58 to 9.65)* | −1.81 (−6.81 to 1.80) | 2.72 (−0.52 to 6.08) |
| Trunk extensor endurance | -0.86 (-6.05 to 3.13) | 4.18 (0.93 to 10.81)* | 3.33 (0.21 to 6.57)* |
Notes: Models adjusted for baseline participation, age, and sex. Coefficients are presented for physical impairment test performance above the median. Participation and activity score range: 0–100; higher is better. ROM = range of motion.
*95% CI does not contain 0.
Participation Frequency Model
Change in activities measured with the LLFI had a significant direct effect on frequency of participation in life roles (standardized β = 2.19). Leg speed was the only physical impairment that had a direct effect on frequency of participation at year 2 (βdirect = 1.39, ES = 0.22) (Table 2). Ankle ROM (βindirect = −1.06, ES = −0.17) and trunk extensor endurance (βindirect = 1.01, ES = 0.16) had indirect effects on frequency of participation acting though changes in activities.
Participation Limitation Model
Change in activities measured with the LLFI had a significant direct effect on limitations in participation in life roles (standardized β = 8.43). Leg speed (βdirect = 4.53, ES = 0.32) and ankle ROM (βdirect 4.70, ES = 0.34) both had a direct effect on limitations in participation at year 2. Ankle ROM (βindirect = −4.24, ES = −0.31) and trunk extensor endurance (βindirect = 4.18, ES = 0.30) had indirect effects on limitations in participation acting though changes in activities.
Models Using SPPB
In the models in which the LLFI was replaced with the SPPB for the activity measure, trunk extensor endurance had a significant indirect effect (βindirect = 6.0, 95% CI 0.91–20.67, ES = 0.43) on limitations in participation mediated by changes in the SPPB. No other physical impairments contributed to participation acting through the SPPB. Change in the SPPB had a direct effect on limitations in participation (β = 0.17, 95% CI 0.06–0.46) but no effect on participation frequency.
Sensitivity Analysis
In the models using maximum likelihood estimation for missing data, the same physical impairments predicted participation frequency and limitations, with the exception of ankle ROM, which did not predict either dimension of participation.
Discussion
To our knowledge, this is the first study to investigate the specific leg and trunk impairments amenable to treatment that are most predictive of an older person’s participation in major life roles. Our results identified leg speed, ankle ROM, and trunk extensor endurance as key physical impairments predicting participation levels both directly and indirectly through changes in activities over 2 years of follow-up. In addition, these results validate a clinically relevant model of disablement with implications for late-life disability prevention.
Further understanding of the factors underlying the disablement process has been highlighted as a key research priority in geriatric rehabilitation (29). Despite widespread acceptance of the ICF as a framework for studying health and disability, there is scarce empirical evidence confirming the associations between impairments, activities and participation. Our results support the hypotheses proposed in our conceptual model (Figure 1) and demonstrate that leg and trunk impairments have both a direct and indirect influence on subsequent participation in life roles acting through changes in activities. These data add to previous work on the disablement process (8,17) and provide a framework through which interventions to enhance participation can be identified. This is particularly important given the limited evidence demonstrating that exercise has a beneficial impact on participation in life roles (12,13,30).
A recent statement from the Australian and New Zealand Society for Geriatric Medicine (31) emphasized the need for research to determine the most effective types of exercise to optimize outcomes in older adults. There are two major trials to date showing that exercise in older adults can prevent or delay the onset of limitations in activities of daily living (15) and walking ability (14), respectively: the Fitness Arthritis and Seniors Trial (15) and the Lifestyle Interventions and Independence for Elders Study (14). Common to both studies is exercise focusing primarily on improving aerobic capacity and muscle strength. The current analysis from Boston RISE makes two important contributions that have the potential to inform future exercise trials: (i) we consider a comprehensive set of leg and trunk impairments linked to mobility status and (ii) we identify their impact on participation in life roles- a broader disability outcome of significance to individuals (5). Our results suggest that if the goal is to have an impact on participation, leg speed, ankle ROM, and trunk endurance are specific physical impairments that could be targeted to maximize the benefits of exercise for older adults.
It is interesting to note that leg speed had a direct effect on both frequency of participation in life roles and perceived limitations in participation. There is increasing literature documenting the importance of leg speed as a predictor of walking ability and other activity outcomes (32–35); however, ours is the first study to demonstrate its prospective association with participation, a global disability outcome. Given that leg speed was directly associated with participation, exercises aimed at increasing the speed generating capacity of the muscle (eg, repeated chair-stands where the patient is asked to stand up as quickly as possible) might have a positive impact on participation in life roles. While there is preliminary evidence demonstrating the potential of task-specific training emphasizing speed of movement for improving activity limitations (36), there is a need to evaluate exercise directly targeting this attribute as part of interventions designed to enhance participation in older adults.
Trunk extensor endurance had a consistent indirect effect on subsequent participation in life roles acting through changes in self-reported activities. It was also the only neuromuscular attribute to have an effect on participation acting through changes in a performance-based measure of activity (the SPPB). A hypothesized mechanism through which trunk endurance may influence participation is through its association with balance (34)—a complex skill required for daily living to avoid falls. Trunk endurance is likely critical for supporting activities requiring a higher degree of balance control which in turn, may have a significant impact on participation in life roles. This theory is supported by a recent meta-analysis which found that exercise interventions designed to reduce falls—many of which included balance exercises challenging trunk stability—had a small effect on participation in life roles (12). Regardless of the specific mechanism, our results suggest that activity-specific functional training with an emphasis on tasks that require trunk endurance might have a positive impact on participation.
Although the direct effect of ankle ROM on participation was positive, its indirect effect mediated by changes in activity was negative. This is a reflection of the negative association between baseline ankle ROM and change in activities; those with impaired ankle ROM at baseline showed greater improvement in activities over 2 years than those with intact ROM. These patients may have had more room to improve and possibly underwent rehabilitation. Further research is necessary to confirm this theory; however, the positive direct effect on participation limitation nevertheless highlights ankle ROM as another understudied target for geriatric research.
A unique strength of our analysis is that we evaluated two key dimensions of participation: frequency of and limitations in participation in major life roles. Although leg speed, ankle ROM, and trunk endurance had modest effects on both participation dimensions, the magnitude of their effect was greatest on limitations in participation. Of note, changes in advanced lower-extremity activities had an almost fourfold greater influence on limitations in participation than on frequency of participation. In addition, the SPPB, reflecting a more basic measure of activity, had no effect on participation frequency. These results extend prior work which showed differences in the responsiveness and predictive value of the LLDI limitation and frequency dimensions in older adults (26), and point to the need for further understanding of the myriad of other potential factors that might underlie the different dimensions of participation (11). This is particularly important for frequency of participation given its association with adverse outcomes such as hospitalizations and emergency room visits (26) and its tendency to show less improvement following interventions (23).
A limitation of this study is that our findings may not be generalizable to primary care patients living outside the Boston area. However, the participation scores in Boston RISE are similar to those of the LLDI development study (25), which included a more general sample of community-dwelling older adults. A small proportion of participants were unable to complete all the baseline impairment tests and, although loss to follow-up was low (16%), there were some missing outcome data at the 2-year assessment. Our sensitivity analysis using two-stage maximum likelihood estimation to account for both missing data and attrition suggested this had little impact on our findings, with the exception of ankle ROM, which was not retained as a predictor of participation. This finding along with our observation of a negative indirect effect of ankle ROM on participation warrant further investigation. Although patients with severe cognitive impairment were excluded from Boston RISE, it is unclear the extent to which error may have been introduced in the self-report measures by those with milder cognitive impairment. Finally, the Boston RISE study did not include a direct measure of aerobic capacity or a comprehensive evaluation of upper extremity impairments; further research is required to evaluate their impact on participation.
In summary, our results demonstrating the relationships between trunk and lower-extremity physical impairments, activities, and participation validate a conceptual model of disability in older adults that highlights opportunities for geriatric research and practice. In particular, we have shown that leg speed, ankle ROM, and trunk extensor endurance have direct and indirect effects on participation—a key component of health. Investigation of interventions for enhancing participation in life roles among older adults should include task-specific exercise emphasizing trunk stability and training directly targeting leg speed and ankle ROM.
Funding
This work was supported by the National Institute on Aging (R01 AG032052-03); the Canadian Institutes of Health Research (to M.K.B.); Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K24HD070966-01 to J.F.B.); and the National Institute on Disability and Rehabilitation Research (H133P120001 to A.M.J.).
References
- 1. Glass TA, de Leon CM, Marottoli RA, Berkman LF. Population based study of social and productive activities as predictors of survival among elderly Americans. BMJ. 1999;319:478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reichstadt J, Sengupta G, Depp CA, Palinkas LA, Jeste DV. Older adults’ perspectives on successful aging: qualitative interviews. Am J Geriatr Psychiatry. 2010;18:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 4. Wilkie R, Peat G, Thomas E, Croft P. The prevalence of person-perceived participation restriction in community-dwelling older adults. Qual Life Res. 2006;15:1471–1479. doi:10.1007/s11136-006-0017-9 [DOI] [PubMed] [Google Scholar]
- 5. Hammel J, Magasi S, Heinemann A, Whiteneck G, Bogner J, Rodriguez E. What does participation mean? An insider perspective from people with disabilities. Disabil Rehabil. 2008;30:1445–1460. doi:10.1080/09638280701625534 [DOI] [PubMed] [Google Scholar]
- 6. Jette AM, Keysor J, Coster W, Ni P, Haley S. Beyond function: predicting participation in a rehabilitation cohort. Arch Phys Med Rehabil. 2005;86:2087–2094. doi:10.1016/j.apmr.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 7. Fairhall N, Sherrington C, Cameron ID, et al. Predicting participation restriction in community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. 2014;43:31–37. doi:10.1093/ageing/aft111 [DOI] [PubMed] [Google Scholar]
- 8. Sullivan KJ, Cen SY. Model of disablement and recovery: knowledge translation in rehabilitation research and practice. Phys Ther. 2011;91:1892–1904. doi:10.2522/ptj.20110003 [DOI] [PubMed] [Google Scholar]
- 9. Maxwell JL, Keysor JJ, Niu J, et al. Participation following knee replacement: the MOST cohort study. Phys Ther. 2013;93:1467–1474. doi:10.2522/ptj.20130109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilkie R, Peat G, Thomas E, Croft P. Factors associated with participation restriction in community-dwelling adults aged 50 years and over. Qual Life Res. 2007;16:1147–1156. doi:10.1007/s11136-007-9221-5 [DOI] [PubMed] [Google Scholar]
- 11. Arnadottir SA, Gunnarsdottir ED, Stenlund H, Lundin-Olsson L. Participation frequency and perceived participation restrictions at older age: applying the International Classification of Functioning, Disability and Health (ICF) framework. Disabil Rehabil. 2011;33:2208–2216. doi:10.3109/09638288.2011.563818 [DOI] [PubMed] [Google Scholar]
- 12. Fairhall N, Sherrington C, Clemson L, Cameron ID. Do exercise interventions designed to prevent falls affect participation in life roles? A systematic review and meta-analysis. Age Ageing. 2011;40:666–674. doi:10.1093/ageing/afr077 [DOI] [PubMed] [Google Scholar]
- 13. Keysor JJ, Jette AM. Have we oversold the benefit of late-life exercise? J Gerontol A Biol Sci Med Sci. 2001;56:M412–M423. [DOI] [PubMed] [Google Scholar]
- 14. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi:10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penninx BW, Messier SP, Rejeski WJ, et al. Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med. 2001;161:2309–2316. [DOI] [PubMed] [Google Scholar]
- 16. Holt NE, Percac-Lima S, Kurlinski LA, et al. The Boston Rehabilitative Impairment Study of the Elderly: a description of methods. Arch Phys Med Rehabil. 2013;94:347–355. doi:10.1016/j.apmr.2012.08.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawrence RH, Jette AM. Disentangling the disablement process. J Gerontol B Psychol Sci Soc Sci. 1996;51:S173–S182. [DOI] [PubMed] [Google Scholar]
- 18. Bean JF, Latham NK, Holt N, et al. Which neuromuscular attributes are most associated with mobility among older primary care patients? Arch Phys Med Rehabil. 2013;94:2381–2388. doi:10.1016/j.apmr.2013.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM R. 2009;1:916–924. doi:10.1016/j.pmrj.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009:CD003288. doi:10.1002/14651858.CD003288.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57:M217–M222. [DOI] [PubMed] [Google Scholar]
- 22. Beauchamp MK, Jette AM, Ward RE, et al. Predictive validity and responsiveness of patient-reported and performance-based measures of function in the Boston RISE Study. J Gerontol A Biol Sci Med Sci. 2014. doi:10.1093/gerona/glu227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beauchamp MK, Schmidt CT, Pedersen MM, Bean JF, Jette AM. Psychometric properties of the Late-Life Function and Disability Instrument: a systematic review. BMC Geriatr. 2014;14:12. doi:10.1186/1471-2318-14-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 25. Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57:M209–M216. [DOI] [PubMed] [Google Scholar]
- 26. Beauchamp MK, Bean JF, Ward RE, Kurlinski LA, Latham NK, Jette AM. How should disability be measured in older adults? An analysis from the Boston Rehabilitative Impairment Study of the Elderly. J Am Geriatr Soc. 2015;63:1187–1191. doi:10.1111/jgs.13453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan KH, Bentler PM. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol Methodol. 2000;30:165–200. doi:10.1111/0081-1750.00078 [Google Scholar]
- 28. Zhang Z, Wang L. Methods for mediation analysis with missing data. Psychometrika. 2013;78:154–184. doi:10.1007/s11336-012-9301-5 [DOI] [PubMed] [Google Scholar]
- 29. Lee L, Siebens H. Geriatric Rehabilitation. In: LoCicero J, Rosenthal RA, Katlic MR, Pompei P, eds. A Supplement to New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2007. [Google Scholar]
- 30. Keysor JJ, Brembs A. Exercise: necessary but not sufficient for improving function and preventing disability? Curr Opin Rheumatol. 2011;23:211–218. doi:10.1097/BOR.0b013e3283432c41 [DOI] [PubMed] [Google Scholar]
- 31. Australian and New Zealand Society for Geriatric Medicine. Position statement - exercise guidelines for older adults. Australas J Ageing. 2014;33:287–294. doi:10.1111/ajag.12194 [DOI] [PubMed] [Google Scholar]
- 32. Marsh AP, Miller ME, Saikin AM, et al. Lower extremity strength and power are associated with 400-meter walk time in older adults: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bean J, Kiely D, LaRose S, Leveille S. Which impairments are most associated with high mobility performance in older adults? Implications for a rehabilitation prescription. Arch Phys Med Rehabil. 2008;89:2278–2284. [DOI] [PubMed] [Google Scholar]
- 34. Thomas JC, Odonkor C, Griffith L, et al. Reconceptualizing balance: attributes associated with balance performance. Exp Gerontol. 2014;57:218–223. doi:10.1016/j.exger.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beauchamp MK, Leveille SG, Patel KV, et al. What physical attributes underlie self-reported vs. observed ability to walk 400 m in later life? An analysis from the InCHIANTI Study. Am J Phys Med Rehabil. 2014;93:396–404. doi:10.1097/PHM.0000000000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bean JF, Kiely DK, LaRose S, O’Neill E, Goldstein R, Frontera WR. Increased velocity exercise specific to task training versus the National Institute on Aging’s strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci. 2009;64:983–991. doi:10.1093/gerona/glp056 [DOI] [PMC free article] [PubMed] [Google Scholar]