Abstract
Background:
Disability in older African American adults is common, but its basis is unclear. We tested the hypothesis that the level of motor function is associated with incident disability in older African Americans after adjusting for cognition.
Methods:
A prospective observational cohort study of 605 older community-dwelling African American adults without dementia was carried out. Baseline global motor score summarized 11 motor performances, cognition was based on 19 cognitive tests, and self-reported disability was obtained annually. We examined the association of motor function with incident disability (instrumental activities of daily living [IADL], activities of daily living [ADL], and mobility disability) with a series of Cox proportional hazards models which controlled for age, sex, and education.
Results:
Average follow-up was about 5 years. In proportional hazards models, a 1-SD increase in baseline level of global motor score was associated with about a 50% decrease in the risk of subsequent IADL, ADL, and mobility disability (all p values < .001). These associations were unchanged in analyses controlling for cognition and other covariates. Further, the association of global motor score and incident ADL disability varied with the level of cognition (estimate −5.541, SE 1.634, p < .001), such that higher motor function was more protective at higher levels of cognition. Mobility and dexterity components of global motor score were more strongly associated with incident disability than strength (all p values < .001).
Conclusions:
Better motor function in older African Americans is associated with a decreased risk of developing disability. Moreover, the association of motor function and disability is stronger in individuals with better cognitive function.
Key Words: Aging, African Americans, Disability, Motor function, Cognition
Late-life motor impairments are common and associated with a wide range of adverse health outcomes including mortality and disability (1–5). Although disability is common in older adults, many studies suggest that disability is more prevalent in older African Americans than in whites, and the basis of this racial difference is unclear (6–9). Prior work in both African Americans and whites suggests that level differences in cognitive function may contribute to the increased disability observed in older African Americans (10). The daily activities assessed by scales employed to identify disabilities leverage a combination of both cognitive and motor resources for successful completion of their diverse tasks. However, there are few studies which have examined the degree to which objective measures of motor performances are associated with incident disability in older African Americans when controlling for concurrently measured cognitive function. Furthermore, because cognitive resources may be crucial for planning and monitoring movement, it is possible that like education, having more cognitive resources may provide reserve which might modify the association between motor function and incident disability (11).
In this study, we tested the hypothesis that motor function in older blacks is associated with incident disability. We used clinical data collected from more than 600 nondemented older adults participating in the Minority Aging Research Study (MARS), a community-based cohort study of chronic conditions of aging in African Americans (12,13). Subjects underwent structured testing at baseline and assessment of self-report disability at baseline and at annual follow-up. A global motor score was employed to summarize 11 motor performances as previously described, and we examined its relationship to the subsequent development of disability (14). Next we examined whether cognitive function based on 19 cognitive tests and chronic health conditions attenuated the association of motor function with incident disability (13). Finally, we examined whether the association of motor function and incident disability varied with the level of cognitive function.
Methods
Participants
Participants included self-identified African Americans from an epidemiologic cohort study of risk factors for cognitive impairment called the Minority Aging Research Study (MARS) (12). The cohort consists of noninstitutionalized seniors older than 65 years without known dementia who agreed to annual clinical evaluations and cognitive testing. The cohort was recruited from various community-based organizations, churches, and senior subsidized housing facilities in and around the Chicago metropolitan area. The study was approved by the Institutional Review Board of Rush University Medical Center.
Since its inception in August, 2004, 926 subjects have been recruited into MARS, including 283 recruited form from the Clinical Core of the Rush Alzheimer’s Disease Center using identical recruitment procedures by same staff (15). Due to participant burden, motor testing was only recently introduced in the MARS subjects from the Clinical Core. At the time of these analyses, 643 participants had enrolled and completed their baseline clinical assessment including a valid motor assessment. There were 15 who were excluded because of baseline dementia and 23 with incomplete motor assessment leaving 605 participants for these analyses. Average follow-up was 5.0 years (SD = 3.27 years); interquartile range 6.57 years and maximum of 10 years. On average, there were five annual assessments (5.5 exams, SD = 2.89 exams)
Assessment of Cognitive Function and Clinical Diagnoses
A uniform structured clinical evaluation is performed each year that includes medical history, physical function, and neuropsychological performance tests. A composite measure of cognitive function based on 19 tests was used in these analyses. Psychometric information on the construction of this composite measure is contained in previous publications (13,16).
Cognitive status was diagnosed in a three-step process. Nineteen cognitive tests were scored by a computer and reviewed by a neuropsychologist to diagnose cognitive impairment. Then participants were evaluated by a physician who used all cognitive and clinical data to diagnose cognitive status. Medications were inspected and coded using the Medi-Span system (Medi-Span, Inc.) (17,18)
Assessment of Disability
Disability was assessed annually via three self-report instruments. Instrumental activities of daily living (IADLs) were assessed using items adapted from the Duke Older Americans Resources and Services project, which assess eight activities: telephone use, meal preparation, money management, medication management, light and heavy housekeeping, shopping, and local travel (19). Basic activities of daily living (ADLs) were assessed using a modified version of the ADL scale which assesses six activities: feeding, bathing, dressing, toileting, transferring, and walking across a small room (20). Mobility disability was assessed using the Rosow-Breslau scale, which assesses three activities: walking up and down a flight of stairs, walking a half mile, and doing heavy housework such as washing windows, walls, or floors (21).
Participants were given the following response choices with regard to their ability to perform each of the above activities: no help, help, and unable to do. For these analyses, the total disability was employed as well as whether participants were classified as being disabled based on a reported needing help with or an inability to perform one or more tasks on each of the three scales.
Assessment of Motor Function
Eleven motor performances employed by other investigators were assessed. (i) Grip and (ii) pinch strength were measured bilaterally using the Jamar hydraulic hand and pinch dynamometers (Lafayette Instruments, Lafayette) to assess manual strength. Upper extremity dexterity was based on (iii) the number of pegs that could be placed (Purdue Pegboard) in 30 seconds. Two trials were recorded for each hand. The four trials were averaged to provide a Purdue Pegboard score. In addition, (iv) participants tapped an electronic tapper (Western Psychological Services, Los Angeles, CA) with their index finger as quickly as possible for 10 seconds. Two trials were performed for each hand. The four trials were averaged together to yield a tapping score. To evaluate gait, we asked people to walk eight feet and turn 360° and measured the (v, vi) time and (vii, viii) number of steps taken on each task. (ix) To assess balance, we asked people to stand on each leg for 10 seconds. (x) Persons were asked to stand on their toes for 10 seconds. (xi) We also asked people to walk an eight foot line heel to toe and counted the number of steps off line (14,22). The intercorrelation of these 11 motor performances are shown in Supplementary Table 1.
These measures were scaled and averaged to obtain a summary global motor score which has previously been reported to be associated with risk of mortality, incident disability, and dementia (14,23). Summary measures of manual strength (two tests), manual dexterity (two tests), and gait (four tests) were formed in a similar manner. We did not form a balance measure because the balance tests, unlike the other motor tests, were sometimes not attempted (22,23).
Assessment of Other Covariates
Demographic information including date of birth, sex, and years of education were collected via participant interview. Body mass index (BMI) was determined by dividing measured weight represented in kilograms with the square of measured height represented in meters. The frequency of physical activity was assessed using questions adapted from the 1985 National Health Interview Survey (16). We summarized the number of three vascular risk factors: hypertension, diabetes mellitus, and smoking and the number of four vascular diseases: stroke, myocardial infarction, congestive heart failure, and claudication, as previously described (16).
Statistical Analysis
Pearson correlations were used to examine the bivariate associations between the global motor scores, disability measures, and other continuous covariates at baseline. We employed a Cox proportional hazards model to examine whether global motor score at baseline predicted incident disability. All models controlled for age, sex, and education. We then added terms for a number of potential confounders including global cognition which might affect the association of motor function and disability. We employed both linear and quadratic terms for BMI, because both high and low BMI may be associated with adverse health outcomes. In subsequent analyses, we added interaction terms to examine whether the associations of motor function and risk of disability varied with cognition. A priori level of statistical significance was .05. All models were validated graphically and analytically. Analyses were programmed in SAS, Version 9.3 for LINUX (SAS Institute Inc., Cary, NC) (24).
Results
Motor Function and Disability at Baseline
The baseline characteristics of individuals in this study including each of 11 motor performances used to construct global motor score are shown in Table 1. Global motor score ranged from 0.53 to 1.33 with higher levels representing better function and a mean of 0.94 (SD = 0.17) On average, participants with a higher global motor score were younger, had more education, higher cognition, and lower BMI, were more physically active, and had fewer vascular risk factors and diseases (Supplementary Table 2). A higher global motor score was associated with a lower disability score (IADL disability score [r = −.25, p < .001]; ADL disability score [r = −.45, p < .001] and mobility disability score [r = −.49, p < .001]).
Table 1.
Clinical Characteristics of Cohort at Baseline (N = 605)
| Variable | Mean (SD) or n (%) |
|---|---|
| Age (y) | 73.6 (6.25) |
| Sex (% female) | 459 (75.9%) |
| Education (y) | 14.8 (3.56) |
| Minimental status testing (0–30) | 27.7 (2.36) |
| Global cognition (z-score) | 0.05 (0.55) |
| BMI | 30.3 (6.59) |
| Motor performances | |
| Grip strength (lbs) | 57.8 (21.02) |
| Pinch strength (lbs) | 14.6 (5.34) |
| Finger taps in 10 s | 55.0 (8.90) |
| Purdue pegboard | 11.1 (2.25) |
| Time to walk 8 feet (s) | 4.4 (2.03) |
| Steps 8 foot walk | 6.6 (1.60) |
| Time to turn 360° | 5.3 (2.24) |
| Steps 360° turn | 8,4 (2.55) |
| Foot stand duration (s) | 6.5 (3.25) |
| Toe stand duration (s) | 8.5 (2.81) |
| Errors on 8 foot tandem walk | 2.2 (2.53) |
| Self-reported disability | |
| IADL disability | 173 (28.6%) |
| ADL disability | 90 (14.9%) |
| Mobility disability | 166 (27.4%) |
| Vascular risk factors (0–4) | 1.5 (0.86) |
| Hypertension | 465 (76.9%) |
| Diabetes | 163 (26.9%) |
| Smoking | 345 (57.0%) |
| Vascular diseases (0–3) | 0.24 (0.54) |
| Stroke | 21 (3.5%) |
| Myocardial infarction | 50 (8.3%) |
| Congestive heart failure | 31 (5.1%) |
| Claudication | 35 (9.1%) |
| Physical activities (0–3) | 1.2 (0.94) |
Note: ADL = activities of daily living; BMI = body mass index; IADL = instrumental activities of daily living.
Motor Function and Incident Disability
To test the hypothesis that global motor score is associated with the risk of developing disability in IADLs, we restricted the analysis to the individuals who did not report IADL disability at baseline. Over a mean of 4.9 (SD =3.26) years of follow-up, 172 of 355 persons (48.5%) reported impairment in IADLs. In a proportional hazards model which controlled for age, sex, and education, a 1-SD increase in global motor score was associated with a 50% decrease in risk of developing IADL disability. Global motor scores were also associated with incident ADL disability in 90 of 491 persons (18.3%) and 166 of 331 (50.2%) who developed mobility disability (Table 2). These results were unchanged in sensitivity analyses in which we excluded cases with Parkinson’s disease (n = 6) and clinical stroke (n = 21; results not shown).
Table 2.
Motor Function and Incident Disability in Older Black Adults
| Predictor | IADL Disability | ADL Disability | Mobility Disability |
|---|---|---|---|
| Global motor | 0.50 (0.42, 0.61)*** | 0.43 (0.33, 0.56)*** | 0.52 (0.43, 0.65)*** |
| Mobility | 0.63 (0.53, 0.76)*** | 0.49 (0.37, 0.65)*** | 0.67 (0.55, 0.81)*** |
| Dexterity | 0.60 (0.51, 0.72)*** | 0.54 (0.42, 0.69)*** | 0.69 (0.58, 0.82)*** |
| Strength | 0.72 (0.61, 0.85)*** | 0.76 (0.59, 0.97)* | 0.83 (0.70, 0.98)* |
Notes: ADL = activities of daily living; IADL = instrumental activities of daily living.
Each cell shows the results for a separate Cox proportional hazard model which included terms for age, sex and education (not shown) and a term for a motor predictor for each of the three disabilities. Each cell shows the hazard’s ratio (95% confidence interval). Hazard’s ratio is with respect to 1-SD increase of the motor predictor.
*p < .05. **p < .01. ***p < .001.
Global motor score is constructed from different motor performances which might be differentially associated with disability. All three components were associated with reduced risk of subsequent disability, but gait and upper extremity dexterity were more strongly associated with disability than hand strength (Table 2).
Motor and Cognitive Function and Incident Disability
Cognition may contribute to the development of disability or impaired motor function and could therefore attenuate the association of motor function with disability. Global motor scores remained strongly associated with incident disability in models which included a term for global cognition (Supplementary Table 3). Next we added terms for several other covariates including body composition, several vascular diseases and risk factors, and physical activity to the previous models. Global motor score remained strongly associated with the development of disability when adjusting for all of these covariates simultaneously (Table 3).
Table 3.
Motor Function, Cognition, Other Covariates, and Incident Disability in Older Black Adults
| Predictors | Incident IADL Disability (n = 172, 48.5%) |
Incident ADL Disability (n = 90, 18.3%) |
Incident Mobility Disability (n = 166, 50.2%) |
|---|---|---|---|
| Global motor score | 0.56 (0.46, 0.69)*** | 0.48 (0.36, 0.64)*** | 0.59 (0.47, 0.75)*** |
| Global cognition | 0.69 (0.48, 1.01) | 0.57 (0.35, 0.94)* | 0.88 (0.61, 1.27) |
| BMI | 1.02 (0.99, 1.06) | 1.00 (0.96, 1.05) | 1.02 (0.99, 1.06) |
| BMI × BMI | 1.001 (1.000, 1.004) | 1.003 (1.000, 1.006) | 1.002 (0.999, 1.005) |
| Vascular diseases | 1.38 (0.98, 1.93) | 1.38 (0.85, 2.24) | 1.64 (1.14, 2.36)** |
| Vascular risk factors | 1.13 (0.93, 1.39) | 1.08 (0.81, 1.45) | 1.20 (0.98, 1.47) |
| Physical activity | 0.98 (0.84, 1.16) | 1.06 (0.82, 1.36) | 0.79 (0.66, 0.94)** |
Notes: ADL = activities of daily living; BMI = body mass index; IADL = instrumental activities of daily living.
Each column shows the results for a single Cox proportional hazard model for each of the disabilities which were examined. The model included terms for age, sex, and education (not shown) and terms for each of the seven predictors. Each cell shows the hazard’s ratio (95% confidence interval). Hazard’s ratio is shown for 1-SD of global motor score.
*p < .05. **p < .01. ***p < .001.
Both global cognition and global motor scores were both associated with incident ADL disability when included in a single model (Supplementary Table 4, Model A). Nonetheless, the association of motor function and ADL disability might vary with the level of cognitive function. To test whether cognitive function modified the association of motor function and incident disability, we added an interaction term to the previous model (Supplementary Table 4, Model B). The interaction term was significant, showing that the association of motor function with incident ADL disability varied with the level of cognition (Global motor score × Global cognition, estimate, ×5.541, SE 1.634, p < .001).
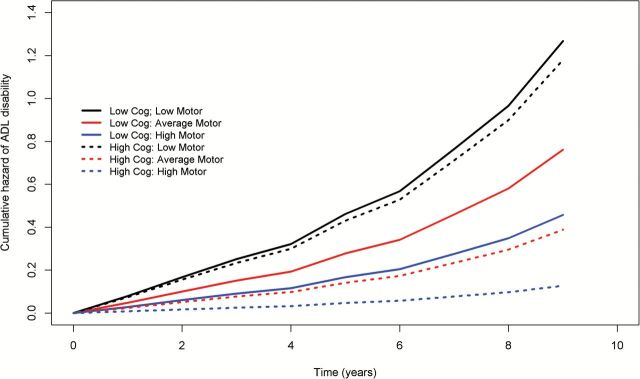
Figure 1 is based on the complete model described earlier that included all participants and illustrates this interaction between motor and cognitive function by contrasting the risk of developing ADL disability for three pairs of average individuals with high (blue), average (red), and low (black) motor function with either high (solid line) or low (dashed line) cognition. The risk of ADL disability in this figure is lowest for the individual with high motor function and high cognitive function. One also observes that the distance between the solid (low cognition) and dashed lines (high cognition) is much greater for the blue (high motor function) as compared with the black lines (low motor function). Thus, at low levels of motor function, cognition has a negligible effect on the development of disability, that is, the risk of developing disability is primarily determined by the level of motor function and the effect of cognition in reducing the risk of incident ADL disability appears to vary and increase with better motor function.
Figure 1.
Cognition modifies the association of global motor scores and subsequent risk of developing disability in activities of daily living (ADL). To illustrate how the association of global motor scores and incident ADL disability varies with cognition, three pairs of hypothetical average participants with their estimated risk of developing ADL disability during the course of the study are shown. The illustration is based on a model including an interaction term between global cognition and global motor scores, with all the cases analyzed in this study. Model derived risk of developing ADL disability are illustrated for average participants with low (solid line, 25th percentile) and high (dashed line, 75th percentile) cognitive function with three levels of motor function: low (−1 SD below the mean global motor score; black), median (mean global motor score: Red), and high (+1 SD above the mean global motor score; blue). At both levels of cognitive function, the blue line (high motor function) is below the red line (median motor function) and both are below the black line (low motor function); the risk of developing ADL disability is lower in individuals with high motor function versus low motor function. Furthermore, it is evident that the distance between the black and blue lines (low and high motor function) is much wider for the dashed lines (high cognitive function) as compared with the solid lines (low cognitive function); thus cognitive function strongly modifies risk of developing ADL disability in individuals with high motor function (blue) with only a negligible effect on low motor function (black).
In secondary analyses, we quantified features of the interaction illustrated in Figure 1 by examining how the association of motor function with incident disability varied between three groups of subjects with low, moderate, and high cognitive function (Supplementary Table 5). In all the three groups, higher motor function is associated with a lower risk for incident ADL disability. However, the risk reduction of ADL disability by cognition was strongest for individuals with high motor function similar to the individuals depicted by the blue lines in Figure 1. Specifically, in the group with low cognitive function, with every 1-SD increase in motor function, the hazard ratio of incident ADL disability was 0.534 (95% confidence interval 0.352–0.809). By contrast, in the group with high cognitive function, the hazard ratio was reduced to 0.182 (95% confidence interval 0.105–0.317). This group difference was highly significant (p = .002). In practical terms, for the group with higher cognitive function, the association of motor function with incident ADL disability was almost threefold stronger as compared with the group with lower cognitive function.
There was no interaction between motor and cognitive function and incident IADL or mobility disability (results not shown).
Discussion
In a cohort of more than 600 community-dwelling older African Americans, better motor function was associated with a reduced risk of developing disability during follow-up. Motor function remained a robust predictor of the subsequent development of all three disabilities when controlling for baseline cognition and other chronic health conditions and physical activity. Furthermore, there was evidence that the association of motor function with incident ADL disability varied with the level of cognition. This significant interaction demonstrates that the inter-relationship between cognitive and motor function with incident disability is complex, involving more than just additive effects. Together these findings suggest that better motor function may contribute to a decreased risk of disability in older African Americans and is most strongly associated with incident disability in individuals with better cognitive function.
Longer life spans and aging baby boomers will lead to a projected doubling of adults older than 65 years by 2030, to more than 70 million persons who will account for 20% of the U.S. population (25). At the same time, our population is becoming more racially and ethnically diverse. By 2030, the percentage of non-Hispanic white older Americans is projected to fall from 80% to 70%, and African Americans will comprise about 10% of older adults. Currently, about two thirds of older Americans have several chronic conditions whose treatment accounts for about two thirds of the U.S. health care budget (25). Prior work suggests that older African Americans develop more disability as compared with white older adults, but the basis for increased disability in African Americans is unclear (6,9). Given the growing magnitude of this public health challenge, there is an urgent need to identify modifiable risk factors and the biology which underlies the development of disability in older African Americans.
Some have suggested that lower performance on cognitive function tests in older African Americans may contribute to their risk of greater disability (10). Prior work has linked lower extremity physical function and mobility disability (26) and several motor performances with ADL disability (27) in African Americans. A recent work modeling both cognitive and lower extremity physical function together reported that both were associated with the development of ADL disability but did not examine other disabilities (28). Prior studies have suggested that cognitive abilities may more strongly impact IADL disability and motor abilities may more strongly affect ADL disability (29,30). The current study extends these prior studies by showing that objective motor performance of both arms and legs is a strong predictor of all IADL, ADL, and mobility disability in older African Americans, when simultaneously adjusting for cognition.
A high burden of poor health may also contribute to disability in older African Americans. Nonetheless, we found that motor function remained a robust predictor of disability even after controlling for factors that are often influenced by race and contribute to health disparities in old age, including common health conditions, years of education, and frequency of physical activity. Our results suggest that these and other determinants of health and health disparities in African Americans, many that begin in early- or mid-life, may lead to lower levels of motor function and ultimately contribute to increased disability in old age (26). Thus, strategies aimed at improving motor function, a modifiable risk factor, may reduce or prevent disability in older African Americans (8,31). Moreover, efforts to improve motor function to reduce disability represent concrete actions and are not contingent on public policy efforts to decrease social inequities that lead to health disparities, such as health service utilization and economic inequalities. Finally, because disease prevention provides the optimal long-term strategy for reducing the burden of disability in our aging population, public health efforts to improve motor function could focus on the entire life span as a means of decreasing the burden of disability in old age.
A novel finding in the current study was that the association of motor function and risk of developing ADL disability varied with an individual’s level of cognition and was stronger in individuals with better cognitive function. Cognitive resources, especially executive function, are essential for the initiation and monitoring of movement. It is possible that higher levels of cognition may optimize movements and decrease falls, thereby leading to more judicious movement strategies that prevent other adverse consequences including disability. Additionally, cognitive function may protect or provide reserve for motor impairment in older African Americans. The concept of reserve applies to many bodily functions including pulmonary, renal, hepatic, and cardiac. Redundancy in these systems provides protection which minimizes functional impairment despite mild damage. Similar evidence has emerged to suggest that brain reserve can protect against the development of impaired cognition in the face of old age and brain damage (32). Thus, for a given amount of Alzheimer’s disease (AD) pathology, some individuals will show impaired cognition, whereas others with more brain reserve may maintain normal cognition. However, the concept of reserve is likely broader and may also include other potential compensatory and neuroprotective mechanisms (32). This has led to the search and identification of various lifestyle factors which may mitigate the deleterious effects of accumulating age-related pathologies and disease on cognitive function. There are few reports which have identified factors which provide motor reserve (11,33). Thus, the current findings, showing that higher cognitive function may provide “motor reserve” to further reduce the risk of developing disability in older adults, may have important translational consequence for the development of non-motor interventions which may be employed to decrease the risks of disability in older African Americans.
In the current study, better cognitive function provided reserve against the development of disability in individuals with high but not with low global motor performance scores. Prior reports about cognitive reserve suggest that there are limits to the ability of education to buffer and provide reserve against the untoward effects of AD pathology (34). Thus, while having more years of education can mitigate the deleterious effects of some AD pathology, as AD pathology accumulates eventually even well-educated individuals manifest AD dementia when the reserve capacity for education is exceeded. Motor function has an essential role in the disablement process, and performance on the global motor scale reflects a range of function on a continuum from that observed in healthy young adults to mild or severe impairment in older adults (35). Hence, in the presence of severe motor impairment, reserve factors (in this case cognitive resources) may be unable to prevent disability. So in the current study, individuals with low global motor performance scores may have a degree of motor impairment which exceeds the reserve capacity provided by cognitive resources. Consequently, high cognition is insufficient to provide reserve in individuals with low global motor scores and does not prevent disability. High and low motor functions, in this study, are relative labels which identify the upper and lower percentiles of participants’ when considering the entire range of performance measured in this study. These labels are not linked to other reports about normal function. Thus, we would suggest that even older individuals with high global motor scores, in the current study, have some degree of motor impairment. However, in contrast to those with low motor function, participants with high motor function have a milder degree of motor impairment which is amenable to the reserve provided by cognitive resources and which delays the development of ADL disability (35).
Although motor function was associated with incident disability for all three scales assessed in this study, an unexpected finding was that cognition only modified the association of motor function with incident ADL disability but not with IADL or mobility disability. First, it is important to recognize that the diverse tasks assessed by these three scales are likely to vary with respect to the relative contributions of cognitive and motor resources needed for their successful completion. Moreover, the constructs employed to assess cognitive and motor functions in this study may not capture the specific cognitive and motor or other resources underlying the different tasks assessed by each of these disability scales. Thus, whereas our results suggest that cognition may provide some reserve in the context of ADL disability, the factors that interact to support IADL and mobility disability need further exploration. Filling these crucial gaps in our knowledge is essential to facilitate targeted interventions which may provide reserve for a wider range of disabilities in older adults.
Our study has some limitations. Most importantly, inferences regarding causality must be drawn with great caution from observational studies. Given the selected nature of the cohort, including their relatively high education and good health, our findings will need replication in a more representative population, which can also assess the extent to which cohort and period may affect results (36–39). Many of the covariates for health conditions employed were based on self-reported data, further studies employing objective measures of vascular risk factors and diseases are needed. Furthermore, objective measures of physical activity are imperative given its importance for health and motor function in particular. Because some work suggests that modifiable risk factors may vary with race, studies are needed which directly compare African Americans directly to non-Hispanic whites (8). Finally, although we employed traditional objective measures of physical performance, many aspects of motor function were not measured. Further studies using objective measures to capture a wider range of motor abilities may help optimize interventions to improve those aspects of movement most salient for the development of disability in older adults (40).
However, several factors increase confidence in our findings. Perhaps most importantly, the study enjoys high follow-up participation reducing bias due to attrition. In addition, motor function was evaluated as part of a uniform clinical evaluation which incorporated many widely accepted and reliable strength and motor performance measures. Furthermore, strength and motor performance testing was done in arms and legs. In addition, a relatively large number of older persons were studied, so that there was adequate statistical power to identify the associations of interest while controlling for several potentially confounding demographic variables.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institute of Health, grants R01AG22018 (L.L.B), P30AG10161 (D.A.B), and R01NS78009 (A.S.B), and the Illinois Department of Public Health.
Conflict of Interest
The authors report no disclosures for this manuscript.
Supplementary Material
Acknowledgments
We thank all the participants in the MARS and the Rush Clinical Core. We also thank staff employed at the Rush Alzheimer’s Disease Center.
References
- 1. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379–1386. doi:10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. [DOI] [PubMed] [Google Scholar]
- 4. Rantanen T, Guralnik JM, Ferrucci L, Leveille S, Fried LP. Coimpairments: strength and balance as predictors of severe walking disability. J Gerontol A Biol Sci Med Sci. 1999;54:M172–M176. [DOI] [PubMed] [Google Scholar]
- 5. Rantanen T, Guralnik JM, Sakari-Rantala R, et al. Disability, physical activity, and muscle strength in older women: the Women’s Health and Aging Study. Arch Phys Med Rehabil. 1999;80:130–135. [DOI] [PubMed] [Google Scholar]
- 6. Arbeev KG, Butov AA, Manton KG, Sannikov IA, Yashin AI. Disability trends in gender and race groups of early retirement ages in the USA. Sozial- und Praventivmedizin. 2004;49(2):142–151. [DOI] [PubMed] [Google Scholar]
- 7. Whitson HE, Hastings SN, Landerman LR, Fillenbaum GG, Cohen HJ, Johnson KS. Black–White disparity in disability: the role of medical conditions. J Am Geriatr Soc. 2011;59(5):844–850. doi:10.1111/j.1532-5415.2011.03401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Latham K. Racial and educational disparities in mobility limitation among older women: what is the role of modifiable risk factors? J Gerontol B Psychol Sci Soc Sci. 2014;69:772–783. doi:10.1093/geronb/60.5.S263 [DOI] [PubMed] [Google Scholar]
- 9. Mendes de Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA. Racial disparities in disability: recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. J Gerontol B Psychol Sci Social Sci. 2005;60(5):S263–S271. doi:10.1093/geronb/gbu028 [DOI] [PubMed] [Google Scholar]
- 10. Zsembik BA, Peek MK, Peek CW. Race and ethnic variation in the disablement process. J Aging Health. 2000;12:229–249. doi:10.1177/089826430001200205 [DOI] [PubMed] [Google Scholar]
- 11. Elbaz A, Vicente-Vytopilova P, Tavernier B, et al. Motor function in the elderly: evidence for the reserve hypothesis. Neurology. 2013;81:417–426. doi:10.1212/WNL.0b013e31829d8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, Wilson RS. Perceived discrimination and cognition in older African Americans. J Int Neuropsychol Soc. 2012;18:856–865. doi:10.1017/s1355617712000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the rush memory and aging project, a community-based cohort study. BMC Med. 2011;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi:10.1159/000096129 [DOI] [PubMed] [Google Scholar]
- 16. Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. [DOI] [PubMed] [Google Scholar]
- 17. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 20. Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. [DOI] [PubMed] [Google Scholar]
- 21. Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. [DOI] [PubMed] [Google Scholar]
- 22. Buchman AS, Yu L, Boyle PA, et al. Microvascular brain pathology and late-life motor impairment. Neurology. 2013;80:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson RS, Segawa E, Buchman AS, Boyle PA, Hizel LP, Bennett DA. Terminal decline in motor function. Psych Aging. 2012;4:988–1007. doi:10.1037/a0028182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. SAS/STAT® Software for Unix, Version (9.18) [computer program]. Cary, NC: SAS Institute; 2002–2003. [Google Scholar]
- 25. CDC. The State of Aging and Health in America 2013. Atlanta, GA: U.S. Department of Health and Human Services; 2013. [Google Scholar]
- 26. Thorpe RJ, Koster A, Kritchevsky SB, et al. Race, socioeconomic resources, and late-life mobility and decline: findings from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2011;66A(10):1114–1123. doi:10.1093/gerona/glr102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirsch CH, Buzková P, Robbins JA, Patel KV, Newman AB. Predicting late-life disability and death by the rate of decline in physical performance measures. Age Ageing. 2012;41:155–161. doi:10.1093/ageing/afr151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajan KB, Hebert LE, Scherr P, et al. Cognitive and physical functions as determinants of delayed age at onset and progression of disability. J Gerontol A Biol Sci Med Sci. 2012;67:1419–1426. doi:10.1093/gerona/gls098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyle PA, Cohen RA, Paul R, Moser D, Gordon N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. Int J Geriatr Psychiatry. 2002;17:164–169. [DOI] [PubMed] [Google Scholar]
- 30. Cahn DA, Sullivan EV, Shear PK, Pfefferbaum A, Heit G, Silverberg G. Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson’s disease. Arch Clin Neuropsychol. 1998;13(7):575–583. [PubMed] [Google Scholar]
- 31. Satariano WA, Guralnik JM, Jackson RJ, Marottoli RA, Phelan EA, Prohaska TR. Mobility and aging: new directions for public health action. Am J Public Health. 2012;102:1508–1515. doi:10.2105/ajph.2011.300631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17:502–509. doi:10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleischman DA, Yang J, Arfanakis K, et al. Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology. 2015;84:1294–1300. doi:10.1212/wnl.0000000000001417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu L, Boyle P, Schneider JA, et al. APOE epsilon4, Alzheimer’s disease pathology, cerebrovascular disease, and cognitive change over the years prior to death. Psychol Aging. 2013;28(4):1015–1023. doi:10.1037/a0031642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langlois JA, Keyl PM, Guralnik JM, Foley DJ, Marottoli RA, Wallace RB. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87:393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin S-F, Beck AN, Finch BK. Black–White disparity in disability among U.S. older adults: age, period, and cohort trends. J Gerontol B Psychol Sci Social Sci. 2014;69(5):784–797. doi:10.1093/geronb/gbu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin SF, Beck AN, Finch BK, Hummer RA, Masters RK. Trends in US older adult disability: exploring age, period, and cohort effects. Am J Public Health. 2012;102(11):2157–2163. doi:10.2105/ajph.2011.300602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hung WW, Ross JS, Boockvar KS, Siu AL. Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr. 2011;11:47. doi:10.1186/1471-2318-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66:75–86. doi:10.1093/geronb/gbq088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiss A, Mirelman A, Buchman AS, Bennett DA, Hausdorff JM. Using a body-fixed sensor to identify subclinical gait difficulties in older adults with IADL disability: maximizing the output of the timed up and go. PLoS One. 2013;8:e68885. doi:10.1371/journal.pone.0068885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.