Abstract
Background:
Losing the ability to walk safely and independently is a major concern for many older adults. The Lifestyle Interventions and Independence for Elders study recently demonstrated that a physical activity (PA) intervention can delay the onset of major mobility disability. Our objective is to examine the resources required to deliver the PA intervention and calculate the incremental cost-effectiveness compared with a health education intervention.
Methods:
The Lifestyle Interventions and Independence for Elders study enrolled 1,635 older adults at risk for mobility disability. They were recruited at eight field centers and randomly assigned to either PA or health education. The PA program consisted of 50-minute center-based exercise 2× weekly, augmented with home-based activity to achieve a goal of 150min/wk of PA. Health education consisted of weekly workshops for 26 weeks, and monthly sessions thereafter. Analyses were conducted from a health system perspective, with a 2.6-year time horizon.
Results:
The average cost per participant over 2.6 years was US$3,302 and US$1,001 for the PA and health education interventions, respectively. PA participants accrued 0.047 per person more Quality-Adjusted Life-Years (QALYs) than health education participants. PA interventions costs were slightly higher than other recent PA interventions. The incremental cost-effectiveness ratios were US$42,376/major mobility disability prevented and US$49,167/QALY. Sensitivity analyses indicated that results were relatively robust to varied assumptions.
Conclusions:
The PA intervention costs and QALYs gained are comparable to those found in other studies. The ICERS are less than many commonly recommended medical treatments. Implementing the intervention in non-research settings may reduce costs further.
Key Words: Cost-effectiveness, Physical activity, Older adults
As the global population of older adults continues to increase, the quality of life and functional independence of older adults are increasingly important. One aspect of functional independence, the ability to walk safely and independently (1), is often lost during aging (2). Impaired mobility is associated with broader disability including reductions in activities of daily living that are essential for independent living (3,4). Many older adults who are at risk for experiencing disability are sedentary and may benefit from increased physical activity (PA) (2,5).
The goal of the Lifestyle Interventions and Independence for Elders Study (LIFE) was to test whether a comprehensive PA intervention could prevent sedentary older adults from experiencing major mobility disability (MMD), defined as the inability to complete a 400-m walk (6). The PA intervention was compared with a “successful aging” health education (HE) intervention in a large-scale, randomized controlled trial (7). After an average follow-up of 2.6 years, HE participants were more likely to reach the endpoint of MMD than PA participants (8).
PA interventions often vary in their intensity, methodology, and resource requirements (9–11). A description of the intervention costs allows organizations to evaluate, plan, and choose an intervention for implementation. Further, when an intervention has been shown to produce health benefits, it is important to quantify the resources required to produce those benefits, thus informing health policy decisions (12). Our objective was to describe the resources required to deliver the two LIFE interventions and examine the incremental cost-effectiveness of the PA intervention relative to its comparator.
Methods
Detailed descriptions of the study design and methods (7), recruitment and baseline characteristics (13), and the main results (8) of LIFE have been published.
Study Design
LIFE was a large, multisite, randomized controlled trial in which older U.S. adults at risk for developing mobility disability were assigned to either an HE intervention or a PA intervention for the duration of the study. The study was conducted from February 2010 to December 2013 at eight geographically diverse field centers across the United States.
Interventions
PA Intervention
The PA intervention focused primarily on walking, with additional secondary components of strength, flexibility, and balance training. Participants were asked to attend two center-based intervention sessions each week and given home-based activity plans to perform 3–4 times a week. The intervention was designed to be consistent with the U.S. Physical Activity Guidelines that recommend moderate PA be performed at least 150min/wk (14).
PA sessions were led by interventionists, who typically held a Bachelor’s or Master’s degree in exercise science. Interventionists were assisted by one or more exercise facilitators/assistants. All intervention staff received centralized training. Telephone reminders from administrative personnel were used to encourage participation.
Exercise equipment such as ankle weights and pedometers were purchased for participants. Other intervention materials included exercise videotapes, water bottles, and behavioral monitoring folders. Intervention safety supplies included first-aid kits, defibrillators, and glucometer supplies. Refreshments provided at intervention sessions included water, sports drinks, and small snacks. Participant incentives, which differed by site, included t-shirts, pens, gift cards, etc. Intervention-related office supplies included materials for posters, newsletters, and retention efforts.
HE Intervention
The HE program consisted of weekly workshops for the first 26 weeks, followed by monthly workshops until the end of the trial. Health educators provided instructional lectures on successful aging topics such as nutrition, medication use, and preventive medicine. Each HE class included a short, upper extremity stretching program led by the instructor. Contact by phone or mailed reminders was maintained to encourage participation and follow-up after missed sessions. Intervention equipment included media projectors and portable microphones. Intervention materials included educational posters, binders, handouts, and field trips. First-aid kits were purchased at some sites. Refreshments provided at HE sessions included water and small snacks. Incentives such as gift cards or gas cards were raffled off to encourage attendance. Office supplies were purchased for communication and retention efforts.
Participants
Participants included 1,635 sedentary older adults who were recruited over 21 months, primarily via targeted mass mailings and word of mouth. Inclusion criteria were (a) age 70–89 years; (b) sedentary lifestyle (<20min/wk of formal exercise in the past month and <125min/wk of moderate PA); (c) at risk for mobility disability (Short Physical Performance Battery [SPPB] score of <10); (d) ability to complete a 400-m walk within 15 minutes; (e) no major cognitive impairment; and (f) approved to participate by a physician who applied medical exclusion criteria. More detailed inclusion and exclusion criteria (13), a CONSORT diagram (8), and details of the trial design (7) are provided elsewhere.
Measures
The primary measure of intervention effectiveness was time to initial occurrence of MMD, measured at 6-month intervals. The Quality of Well-being Scale Self-Administered (QWB-SA) was a secondary measure of effectiveness for cost-effectiveness analyses. With staggered enrollment, follow-up varied from 24 to 36 months and loss to follow-up for the primary outcome occurred at 4% per year. The mean length of follow-up was 2.6 years.
Major Mobility Disability
A timed 400-m walk was used to measure mobility. Trained assessors, blinded to group assignment, used a stopwatch to time the 400-m walk. Participants were allowed to use a cane, but not a walker during the test, and were instructed that successful completion required that they not sit, lean, or be assisted by another person (15). MMD was considered present when participants could not complete the 400-m walk within 15 minutes.
Quality of Well-being Scale Self-Administered
The QWB-SA measures preference-based health-related quality of life and produces a single, summary score that facilitates cost-effectiveness comparisons across disease states (16). Changes in Quality-Adjusted Life-Years (QALYs) for each group were calculated by multiplying 6-month time intervals by QWB-SA scores. A score of 0 was inserted for deceased participants (12).
Health care utilization
Health care utilization was estimated using self-report via the UCSD Healthcare Utilization Questionnaire (17). The Healthcare Utilization Questionnaire consists of 12 questions that ask participants to list the number of times each type of utilization occurred in the last 6 months. Questions address inpatient hospital days, surgeries, days in nursing home, emergency room visits, ambulance use, physician visits, home-care visits, telephone calls to health care providers, and both prescription and over-the-counter medications used. Self-reported health care utilization has been shown to be both reliable and valid in community-dwelling seniors (18). The mean cost of nursing home days was obtained from the 2012 Genworth Cost of Care survey (19). The cost for other contacts was based on national means from the 2012 Medical Expenditure Panel Survey (20).
Statistical Methods
We compared changes in QWB-SA scores and health care costs between groups using repeated-measures analysis of variance, adjusting for clinic, age, gender, and baseline scores; a compound symmetry structure was used to model the within-person variability (convergence problems occurred with the unstructured covariance matrix).
Cost Analysis
The cost analysis was conducted from the “organization” perspective, which allows health care organizations, employers, or community organizations to gauge the approximate cost of offering this program. The analytic time horizon was 2.6 years. Intervention costs were calculated using the actual cost of materials used in the trial and the mean personnel time required to deliver the intervention in year 2013U.S. dollars. Overhead costs were estimated at 69% of personnel costs, accounting for facilities costs, indirect support personnel, and other typical indirect costs associated with running an outpatient health care program (see Supplementary material) (21). All research-related study costs were excluded.
Cost-effectiveness
To calculate the incremental cost-effectiveness ratio (ICER), we calculated the difference in costs and effectiveness between the PA intervention and the HE intervention. Next, the incremental cost per participant was divided by the incremental effectiveness to get the ICER. Analyses begin with a base-case analysis that includes direct intervention costs and overhead. Because some health care costs may be intervention related, the impact of general health care costs is also evaluated.
Sensitivity Analyses
Because cost-effectiveness analyses usually require the use of cost estimates based on national data or means across study sites, sensitivity analyses were used to examine how the results changed when input data were varied. Personnel wages, fringe benefit rates, and overhead expenses for facilities were estimated based on national or clinical site means or medians, and thus, were varied by 20% in either direction before recalculating the ICERs.
Results
The 1,635 participants were 78.9 years of age on average, 67% women, and 76% White. Detailed characteristics of study participants are shown in Table 1.
Table 1.
Participant Characteristics
| Variable | Physical Activity | Health Education |
|---|---|---|
| Number | 818 | 817 |
| Age | 78.7±5.2 | 79.1±5.2 |
| Gender | ||
| Female | 547 (66.9%) | 551 (67.4%) |
| Ethnicity/race | ||
| Caucasian/White | 604 (73.8%) | 635 (77.7%) |
| African-American/Black | 163 (19.9%) | 125 (15.3%) |
| Latino, Hispanic or Spanish | 31 (3.7%) | 30 (3.8%) |
| Other or mixed | 17 (2.1%) | 25 (3.1%) |
| Refused/missing | 3 (0.4%) | 2 (0.2%) |
| Education | ||
| Elementary school or less | 22 (2.7%) | 23 (2.8%) |
| High School/equivalent | 248 (30.3%) | 236 (28.9%) |
| College | 321 (39.2%) | 320 (39.2%) |
| Post graduate | 194 (23.7%) | 208 (25.5%) |
| Other | 32 (3.9%) | 26 (3.2%) |
| Missing | 1 (0.1%) | 4 (0.5%) |
| Body mass index, mean (SD) | 30.3±6.2 | 30.1±5.9 |
| Short Physical Performance Battery score | ||
| Mean (SD) | 7.4±1.6 | 7.3±1.6 |
| <8 | 353 (43.3) | 378 (46.2) |
Effectiveness
As reported in the main results manuscript, 290/817 or 35.5% of the participants in the HE intervention progressed to MMD compared with 246/818 or 30.1% of participants in the PA intervention (8), yielding an absolute reduction of 5.43% (HR, 0.82 [95% CI, 0.69–0.98]; p = .03).
QWB-SA scores for the two groups over time are shown in Supplementary Table 1, along with calculations of the changes in QALYs over time, and group differences. Controlling for baseline values, QWB scores were significantly higher across follow-up assessments through 36 months for the PA participants relative to HE participants (p = 0.03). The difference in QALYs was 0.0468 over the course of 2.6 years.
Intervention Costs
Direct costs for the HE and the PA interventions are shown in Table 2.
Table 2.
Direct Costs of the Physical Activity and Health Education Interventions
| Item | Provider | Units | Time (h) | Cost/h | Total US$ Cost/Participant |
|---|---|---|---|---|---|
| Physical activity | |||||
| Physical activity sessions | Exercise Interventionist | 18.28 | 1.5 | US$29.09 | 798 |
| Intervention assistant | 18.28 | 1.5 | US$16.49 | 452 | |
| Intervention assistant | 18.28 | 1.5 | US$16.49 | 452 | |
| Phone call reminders | Intervention assistant | 18.28 | 0.5 | US$16.49 | 151 |
| Personnel subtotal | 1,853 | ||||
| Exercise equipment | 8 | ||||
| Intervention materials | 15 | ||||
| Safety supplies | 5 | ||||
| Refreshments | 36 | ||||
| Incentives | 68 | ||||
| Office supplies | 38 | ||||
| Overhead (69% of personnel costs) | 1,279 | ||||
| Total cost/participant | 3,302 | ||||
| Health education | |||||
| Intervention sessions | Health Educator | 5.85 | 1.5 | US$31.11 | 273 |
| Intervention assistant | 5.85 | 1.5 | US$16.49 | 145 | |
| Phone call reminders | Intervention assistant | 5.85 | 0.5 | US$16.49 | 48 |
| Personnel subtotal | 466 | ||||
| Intervention equipment | 7 | ||||
| Intervention materials | 24 | ||||
| Safety supplies | 1 | ||||
| Refreshments | 71 | ||||
| Incentives | 56 | ||||
| Office supplies | 54 | ||||
| Overhead (69% of personnel costs) | 322 | ||||
| Total cost/participant | 1,001 | ||||
Across the 8 clinical research sites, a total of 14,950 center-based PA sessions were held. When divided by the total number of participants randomized to the PA intervention (n = 818), 18.3 sessions were held per participant. The total costs for the PA intervention were estimated to be US$3,302 per participant over 2.6 years, or US$1,270 per year. For the HE intervention, 4,779 intervention sessions were held across the 8 sites, resulting in 5.9 sessions per participant. The total costs for the HE intervention were estimated to be US$1,001 per participant over 2.6 years, or US$385 per year.
As indicated in Table 2, personnel costs accounted for the majority of PA intervention costs (56%), followed by facilities and administrative overhead costs (39%), and other intervention costs (5%). Within PA intervention materials, exercise equipment, intervention materials, and safety supplies accounted for a small portion of these costs. The equipment, materials, and safety costs were such a small proportion of intervention costs because these items were purchased in bulk at the beginning of the study and did not need to be replaced, whereas other intervention costs occurred repeatedly on a twice weekly basis for the duration of the study.
Subtracting US$1,001 from US$3,302, we find the incremental cost per participant was US$2,301/participant. As shown in Table 3, dividing US$2,301 by 0.0543 (5.43%) provides an ICER of US$42,376/MMD prevented. Table 4 shows results of the analysis using QALYs as the measure of effectiveness, producing an ICER of US$49,167/QALY.
Table 3.
Incremental Cost per Disability Prevented
| Total Costs US$/ Participant | Proportion Becoming Disabled | Incremental Cost | Incremental Reduced Disability | Incremental Cost-effectiveness | |
|---|---|---|---|---|---|
| Health education | 1,001 | 290/817 (35.5%) | — | — | |
| Physical activity | 3,302 | 246/818 (30.1%) | 2,301 | 5.43% | US$42,376/ disability prevented |
Table 4.
Incremental Cost per Quality-Adjusted Life-Year (QALY)
| Total Costs US$/ Participant | QALYs | Incremental Cost | Incremental QALYs | Incremental Cost-effectiveness | |
|---|---|---|---|---|---|
| Health education | 1,001 | −0.1302 | — | — | |
| Physical activity | 3,302 | −0.0834 | 2,301 | 0.0468 | US$49,167/QALY |
As reported previously, the intervention effect was the strongest among participants with an SPPB < 8 (8). After a mean of 2.6 years, MMD had occurred in 38.2% of the PA group and 46.8% of the HE group. Assuming the SPPB < 8 subgroup did not consume more resources than other participants, the ICER drops to US$26,756/MMD avoided in this more vulnerable subgroup.
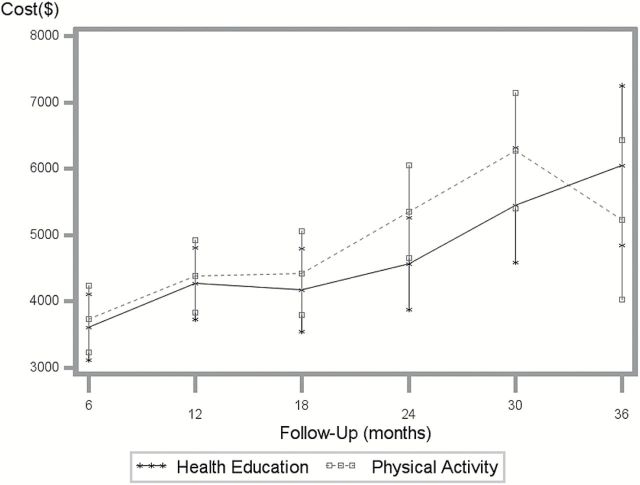
Total health care costs for both groups rose steadily over time but were US$1,583 greater for the PA group than that for the HE group (see Figure 1 and Supplementary Table 2). After adjustment for baseline costs, age, gender, and research site, the difference in total health care costs across these time periods was not statistically significant (p = .573). When health care costs were included in the ICER analysis, the ICER was US$71,529/MMD prevented and US$82,991/QALY.
Figure 1.
Mean 6-month health care costs ($), adjusted for site, gender, age, and baseline costs. Graphed values are least squares means with 95% confidence intervals.
Sensitivity Analyses
Some LIFE field centers spent varying amounts on transportation for participants to the interventions with median transportation costs of US$1345/person and US$330/person for the PA and HE interventions, respectively. When transportation costs were included, the ICERs were US$61,068/MMD avoided and US$70,855/QALY.
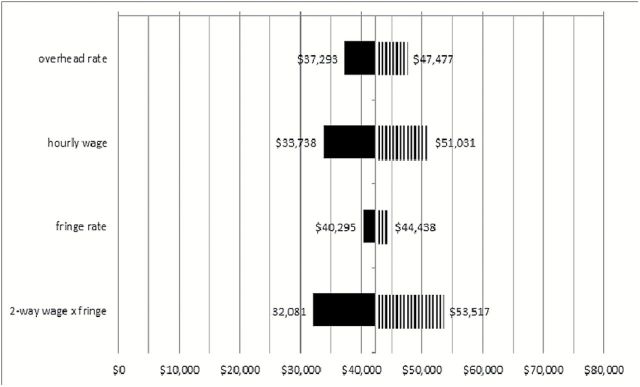
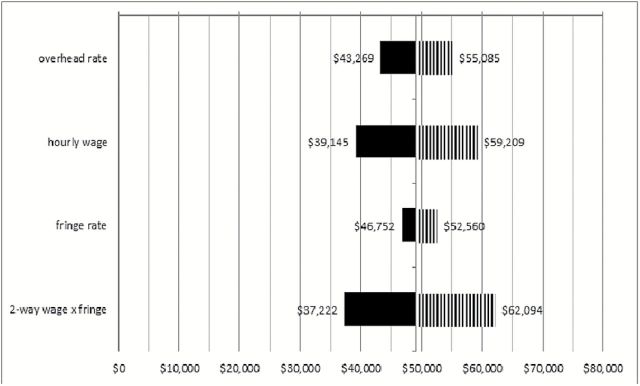
Figures 2 and 3 depict the range of ICERs obtained when overhead and other estimated values were varied 20% in each direction, including a two-way analysis in which wages and fringe benefits were varied together. The figure indicates that organizations that have lower rates of overhead costs, fringe benefits, and/or pay lower hourly wages than the estimates used for analysis can expect a lower cost per MMD or QALY. Higher costs in these areas relative to the estimates can expect slightly higher cost-effectiveness ratios. Paying lower or higher hourly wages had the largest impact on the ration, whereas variation in fringe benefit rates had very little impact on the ICERs.
Figure 2.
Sensitivity analyses for costs ($)/major mobility disability prevented. Solid and striped shading indicate decreased and increased cost/major mobility disability as a result of varying assumptions indicated on the left.
Figure 3.
Sensitivity analyses for costs ($)/QALY. Solid and striped shading indicate decreased and increased cost/major mobility disability as a result of varying assumptions indicated on the left. QALY = Quality-Adjusted Life-Years.
Discussion
Using previously published effectiveness data (8), and additional effectiveness data for QALY, cost-effectiveness analyses produced an ICER of US$42,376 per MMD prevented and US$49,167 per QALY. The QALY data help confirm that the PA intervention provided significant health benefit to older adults and helps validate MMD as a significant source of reduced quality of life.
The total estimated cost of delivering the PA intervention for 2.6 years was US$3,302/person or US$1,270/person annually. These PA intervention costs are quite similar to those found in the LIFE-P study (22) where annual PA intervention costs came to US$1130/person. Beyond the LIFE-P study, few good comparisons exist for gauging whether the estimated intervention costs for the current study are reasonable. Although the interventions and populations studied are different, the Diabetes Prevention Program lifestyle intervention is one of the few behavioral, preventive interventions for which long-term cost data are available. When adjusting for inflation of 35% between 2000 and 2013, the Diabetes Prevention Program lifestyle intervention cost of US$2,780/person over 3 years (9) become very similar to the LIFE PA intervention costs (23).
When considering both cost and health effects, the PA intervention was more costly but produced significant health benefit. PA participants had both an 18% reduction in the incidence of MMD and gained 0.0468 more QALYs per person than HE participants, demonstrating solid quality of life benefits. The 0.0468 QALY benefit is considered clinically important (24) and is comparable to the benefits found in the Diabetes Prevention Program study (25). The HE group showed a consistent decline as might be expected in older adults (26), whereas the PA group remained stable for the first 6–12 months of the study. This finding provides further evidence that prevention of MMD is an important outcome as it converges with a validated measure of preference-based quality of life.
The impact of the ICER of US$42,376 per disability avoided is not easily interpreted because few other studies have measured MMD in the same way. The LIFE-P study with a duration of 12 months (precursor to LIFE) used the same measure of effectiveness and found an ICER of about US$28,000/MMD avoided when unadjusted for inflation. This finding coincides with QWB data showing that a sizable portion of the health benefit from the PA intervention occurs in the first 12 months but keeps accruing over time. Further analyses of intervention adherence over time may assist in identifying ways to create more efficiency and conserve resources by achieving higher attendance per PA session offered through rolling enrollment in an ongoing program.
Although condition-specific measures of effectiveness can limit comparisons, using common units across studies such as QALYs allows for direct comparisons regardless of health condition or primary outcome measures (12). The ICER of US$49,167/QALY is very similar to the inflation adjusted (35%) figure of US$42,541/QALY found in the Diabetes Prevention Program study (25). Beyond Sabor, a recent study of a PA-based weight loss intervention with a low socioeconomic status population estimated ICERs ranging from US$57,000 to US$62,000 per QALY depending on the weight loss goal (27). Thus, the LIFE results are impressive given the fact that both Diabetes Prevention Program and Beyond Sabor involved younger, healthier populations and used placebo or usual care interventions as their comparators.
Inflation should also be considered when comparing current results with previous guidelines for evaluating ICERs (28). The ICER of US$49,167/QALY is in the “reasonable” range of US$20,000–US$100,000 per QALY recommended over 20 years ago (29). The more commonly referenced cutoff of US$50,000/QALY (societal perspective) was proposed in 1982 (30) and becomes US$120,000/QALY when adjusted for inflation. Thus, the LIFE ICER is lower than or similar to the ratios of many commonly recommended medical treatments and procedures (31).
When health care costs were included in the analysis, the ICERs increase to US$69,926/MMD prevented and US$81,132/QALY. However, total health care costs are typically only considered when taking the societal perspective and may be considered revenue, costs, or irrelevant depending on the structure of the health care system when viewed from the organizational perspective.
Additionally, a significant amount of resources were spent on transportation costs at some field centers. Although these costs are important to consider because they could directly facilitate intervention attendance, and in turn, improve health, these costs could be substantially reduced in a variety of ways depending on the intervention location or existing transportation resources at a health care organization.
Sensitivity analyses, used to vary analysis inputs across a range of values, provide a picture of what may occur in different contexts in which the intervention may be implemented (12). Figure 2 indicates that the LIFE ICERs are only mildly sensitive to typical variation in the cost input values if the intervention were held at various sites across the United States. Costs are expected to vary depending on geographical location and other logistics or cost of living factors. For example, large cities that typically pay higher wages or may have higher overhead are expected to have higher costs. Variation in hourly wage costs had the largest impact on the ICERs.
Our analysis was limited to self-reported health care utilization at 6-month intervals. Although self-report has been used in prior studies (17,18,22), national Medicare data are more objective. Significant differences were not found in health care costs, yet the slightly higher health care costs among PA participants are consistent with the study main results (8). The study was also limited to a mean follow-up of 2.6 years. Although this follow-up period is longer than most behavioral randomized controlled trials, it is unknown whether the benefits of PA would continue to accrue or whether health care costs would eventually be offset.
In conclusion, the LIFE PA intervention reduced mobility disability and increased health-related quality of life over time. The intervention costs are comparable to those of other similar PA interventions after adjusting for inflation, despite having older, more impaired participants and using an active intervention for comparison. The ICERs for the LIFE intervention are in a range that warrants implementation on a larger scale. Other behavioral interventions with very similar cost/QALY ratios have been implemented on a wide scale (see Supplementary Table 3).
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement (#UO1 AG22376) and a supplement from the National Heart, Lung and Blood Institute (3U01AG022376–05A2S), and sponsored in part by the Intramural Research Program, National Institute on Aging (No. 1I01CX000927-01A1) and National Institutes of Health. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342); and the National Institutes of Health/National Center for Research Resources Clinical and Translational Science Awards at Stanford University (UL1 RR025744). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688–01A1).
Supplementary Material
Acknowledgments
LIFE investigators are also partially supported by the following: TMG (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging. Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs. Dr. Roger Fielding (Tufts University) is partially supported by the US Department of Agriculture, under agreement 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture. ClinicalTrials.gov Identifiers: NCT01072500.
References
- 1. Patla AE, Shumway-Cook A. Dimensions of mobility: defining the complexity and difficulty associated with community mobility. J Aging Phys Act. 1999;7:7–19. [Google Scholar]
- 2. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:43–52. [DOI] [PubMed] [Google Scholar]
- 4. Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–857. [DOI] [PubMed] [Google Scholar]
- 5. Guralnik JM, Leveille S, Volpato S, Marx MS, Cohen-Mansfield J. Targeting High-Risk Older Adults Into Exercise Programs for Disability Prevention. J Aging Phys Act. 2003;11:219–228. [Google Scholar]
- 6. Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26:141–154. [DOI] [PubMed] [Google Scholar]
- 7. Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi:10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi:10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herman WH, Brandle M, Zhang P, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patrick DL, Ramsey SD, Spencer AC, Kinne S, Belza B, Topolski TD. Economic evaluation of aquatic exercise for persons with osteoarthritis. Med Care. 2001;39:413–424. [DOI] [PubMed] [Google Scholar]
- 11. Sevick MA, Dunn AL, Morrow MS, Marcus BH, Chen GJ, Blair SN. Cost-effectiveness of lifestyle and structured exercise interventions in sedentary adults: results of project ACTIVE. Am J Prev Med. 2000;19:1–8. [DOI] [PubMed] [Google Scholar]
- 12. Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 13. Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi:10.1093/gerona/glt064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 15. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. [DOI] [PubMed] [Google Scholar]
- 16. Andresen EM, Rothenberg BM, Kaplan RM. Performance of a self-administered mailed version of the Quality of Well-Being (QWB-SA) questionnaire among older adults. Med Care. 1998;36:1349–1360. [DOI] [PubMed] [Google Scholar]
- 17. Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med. 2003;167:880–888. [DOI] [PubMed] [Google Scholar]
- 18. Lubeck DP, Hubert HB. Self-report was a viable method for obtaining health care utilization data in community-dwelling seniors. J Clin Epidemiol. 2005;58:286–290. [DOI] [PubMed] [Google Scholar]
- 19. Genworth 2012. Cost of Care Survey: Home Care Providers, Adult Day Health Care Facilities, Assisted Living Facilities and Nursing Homes. Richmond, VA: Genworth Financial; 2012. [Google Scholar]
- 20. Medical Expenditure Panel Survey—Insurance Component: Technical Notes and Survey Documentation. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 21. Latimer EA, Becker ER. Incorporating practice costs into the Resource-Based Relative Value Scale. Med Care. 1992;30(suppl):NS50–NS60. [DOI] [PubMed] [Google Scholar]
- 22. Groessl EJ, Kaplan RM, Blair SN, et al. A cost analysis of a physical activity intervention for older adults. J Phys Act Health. 2009;6:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. US Bureau of Labor Statistics. Consumer Price Index Inflation Calculator 2014. http://www.bls.gov/data/inflation_calculator.htm Accessed January 23, 2015.
- 24. Kaplan RM. The minimally clinically important difference in generic utility-based measures. J Chron Obstruct Pulmon Dis. 2005;2:91–97. [DOI] [PubMed] [Google Scholar]
- 25. Diabetes Prevention Program Research Group. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Groessl EJ, Kaplan RM, Barrett-Connor E, Ganiats TG. Body mass index and quality of well-being in a community of older adults. Am J Prev Med. 2004;26:126–129. [DOI] [PubMed] [Google Scholar]
- 27. Wilson KJ, Brown HS, 3rd, Bastida E. Cost-effectiveness of a community-based weight control intervention targeting a low-socioeconomic-status Mexican-origin population. Health Promot Pract. 2015;16:101–108. doi:10.1177/1524839914537274 [DOI] [PubMed] [Google Scholar]
- 28. King JT, Jr, Tsevat J, Lave JR, Roberts MS. Willingness to pay for a quality-adjusted life year: implications for societal health care resource allocation. Med Decis Making. 2005;25:667–677. [DOI] [PubMed] [Google Scholar]
- 29. Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 30. Kaplan RM, Bush JW. Health-related quality of life measurement for evaluation research and policy analysis. Health Psychol. 1982;1:61–80. [Google Scholar]
- 31. Tufts Medical Center. Center for the Evaluation of Value and Risk in Health. Cost-Effectiveness Analysis Registry. 2007–2013 https://research.tufts-nemc.org/cear4/Resources/LeagueTable.aspx#detailed Accessed March 27, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.