ABSTRACT
The genetic heterogeneity of the close relatives Vibrio anguillarum and Vibrio ordalii, both serious pathogens of fish causing extensive losses in aquaculture, was studied. Eight housekeeping genes, i.e., atpA, ftsZ, gapA, gyrB, mreB, rpoA, topA, and pyrH, were partially sequenced in 116 isolates from diverse fish species and geographical areas. The eight genes appear to be under purifying selection, and the genetic diversity in the total data set was estimated to be 0.767 ± 0.026. Our multilocus sequence analysis (MLSA) scheme identified several widespread clonal complexes and resolved the isolates, for the most part, according to serotype. Serotype O2b isolates from diseased cod in Norway, Ireland, and Scotland were found to be extremely homogeneous. Horizontal gene transfer appears to be fairly common within and between clonal complexes. Taken together, MLSA and in silico DNA-DNA hybridization (DDH) calculations suggest that some isolates previously characterized as V. ordalii, i.e., 12B09, FF93, FS144, and FS238, are in fact V. anguillarum isolates. The precise taxonomic situation for two isolates from Atlantic cod that display several traits consistent with V. ordalii, i.e., NVI 5286 and NVI 5918, and a single environmental strain that was previously considered to represent V. ordalii, i.e., FF167, is less clear.
IMPORTANCE It is still being debated whether V. anguillarum and V. ordalii represent separate bacterial species. Our study addresses this issue and elucidates the degree of genetic variability within this group of closely related bacteria, based on a substantial number of isolates. Our results clearly illustrate the existence of different populations among putative V. ordalii isolates. On the basis of additional full-length genomic analysis, we conclude that most environmental isolates previously identified as V. ordalii lie firmly within the species V. anguillarum. While bona fide fish-pathogenic V. ordalii isolates display a very close genetic relationship with V. anguillarum, they combine a clearly divergent evolutionary pattern with clear phenotypic differences. The study also highlights the need for further characterization of fish-pathogenic isolates from the northern Atlantic region that share phenotypic characteristics with V. ordalii but are genetically closer to V. anguillarum. The retention of taxonomic distinctions between the phenotypically different groups of bacteria is of practical advantage to microbial ecologists and veterinarians.
INTRODUCTION
Vibriosis, i.e., systemic infection with either Vibrio anguillarum or Vibrio ordalii (1), probably constitutes the most significant bacterial disease of wild and farmed fish in temperate marine environments worldwide. While 23 different serotypes of V. anguillarum have been identified (2), by far the majority of fish-pathogenic isolates belong to serotypes O1 and O2. In addition, strains not corresponding to known serotypes are commonly identified, mainly from environmental sources (3). Despite considerable phenotypic differences, V. ordalii and V. anguillarum are genetically very closely related (4); V. ordalii was initially characterized as V. anguillarum biotype 2, prior to its description as an independent species (1).
Vibriosis was previously a significant problem in salmonid farming in the North Atlantic area, but its impact in this industry has been drastically reduced due to the development of effective vaccination procedures (3). Despite vaccination, vibriosis caused by V. anguillarum and, to a lesser degree, V. ordalii remains a major problem in the relatively novel marine fish (e.g., cod and cleaner fish) farming industries in Norway (5), Ireland (6), and Scotland (7). The disease also remains a significant problem in farmed fish in Europe and Asia (8). In order to facilitate vaccine development and better understanding of the epidemiology of vibriosis, more information on the population structure is needed. Despite a considerable number of studies focusing on phenotypic (9, 10), serotypic (11–14), and genetic (15) variations among strains of fish-pathogenic V. anguillarum, few studies have performed detailed taxonomic analyses of both V. ordalii and V. anguillarum. None of the previous genus-wide studies addressing the phylogeny of vibrios (16–20) included significant numbers of V. anguillarum/V. ordalii isolates.
Multilocus sequence analysis (MLSA), relying on essentially unequivocal sequence information from several genetic loci, addresses most of the shortfalls associated with PCR-based DNA fingerprinting or single-gene phylogenetic studies. MLSA has the advantage of being portable, reproducible, and taxonomically sound, allowing the precise identification and classification of Vibrio isolates. Therefore, the main aim of the current study was to perform MLSA with a large number of V. anguillarum and V. ordalii isolates representing different geographical locations and fish species, to improve our knowledge of the population structure within and between these closely related fish-pathogenic species.
MATERIALS AND METHODS
Strains, serotyping, and culture conditions.
A total of 103 V. anguillarum isolates of diverse serotypes and 13 isolates previously identified as V. ordalii, from different geographical regions, environments, and fish hosts (Tables 1 and 2), were analyzed by MLSA. Isolates available for testing were identified to the species level using standard phenotypic and biochemical tests (21). Essentially, both species were identified as Gram-negative, highly motile, oxidase- and catalase-positive, short, curved rods, which produced acid from glucose both aerobically and anaerobically and were sensitive to vibriostatic agent 0/129. V. anguillarum and V. ordalii were differentiated primarily by the former's ability to produce arginine dihydrolase and the latter's markedly slower growth and lack of hemolysis in blood agar cultures. All V. anguillarum isolates of unknown serotype were tested with the slide agglutination method, as described by Sørensen and Larsen (11), using antisera raised against V. anguillarum serotype O1 (isolated from Atlantic salmon), serotype O2a (isolated from Atlantic salmon), and serotype O2b (isolated from turbot). V. ordalii isolates were not serotyped. Isolates donated to the present study were not reserotyped. All strains were cultured on blood agar at 22°C for 48 h prior to DNA extraction and were maintained at −80°C.
TABLE 1.
Geographic origins, host species, and haplotypes of 116 Vibrio anguillarum-related isolates examined in the present study
| Designation and referencea | Species and serotype | Origin | Haplotype(s) |
|---|---|---|---|
| IU-01, IU-02, IU-03, IU-5 | V. anguillarum, non-O1/O2 | Dicentrarchus labrax; Turkey | HT15, HT16, HT3, HT4 |
| IU-07, IU-08, IU-10, IU-12 | HT17, HT4, HT18, HT19 | ||
| IU-11 | V. anguillarum, non-O1/O2 | Thunnus thynnus; Turkey | HT4 |
| NVI 4317, 5180, 6258 | V. anguillarum, O1 | Oncorhynchus mykiss; Norway | HT26, HT4, HT33 |
| NCIMB 2131 | V. anguillarum, O1 | Oncorhynchus mykiss; Norway | HT4 |
| NCIMB 2132 | V. anguillarum, O1 | Oncorhynchus mykiss; Norway | HT4 |
| IU-4 | V. anguillarum, O1 | Mugil cephalus; Turkey | HT4 |
| ATCC 43305 | V. anguillarum, O1 | Oncorhynchus mykiss; Denmark | HT4 |
| VIB 253b | V. anguillarum, O1 | Oncorhynchus mykiss; Australia (Tasmania) | HT37 |
| VIB 266Ab | V. anguillarum, non-O1/O2 | Rotifers; Australia (Tasmania) | HT34 |
| VIB 605b | V. anguillarum, O1 | Plecoglossus altivelis; Japan | HT4 |
| NVI 5589 | V. anguillarum, O1 | Salmo salar; Norway | HT4 |
| VIB 266Bb | V. anguillarum, O1 | Rotifers; Australia (Tasmania) | HT4 |
| ATCC 43314 | V. anguillarum, O10 | Gadus morhua; Denmark | HT12 |
| VIB77b | V. anguillarum, O2a | Plecoglossus altivelis; Japan | HT38 |
| LMG 13224 | V. anguillarum, non-O1/O2 | Rotifers; Greece | HT20 |
| LMG 13225 | V. anguillarum, non-O1/O2 | Rotifers; Greece | HT20 |
| LMG 13227 | V. anguillarum, non-O1/O2 | Sparus aurata; Greece | HT21 |
| NVI 4591, 4601, 4631 | V. anguillarum, O2a | Salmo salar; Norway | HT3, HT3, HT28 |
| NVI 5070 | V. anguillarum, O2a | Oncorhynchus mykiss; Norway | HT3 |
| NVI 4792, 5065, 6404 | V. anguillarum, O2a | Gadus morhua; Norway | HT3, HT3, HT3 |
| NVI 5031, 5034, 6401 | V. anguillarum, O2a biotype II | Gadus morhua; Norway | HT3, HT3, HT3 |
| NCIMB 6T | V. anguillarum, O2a | Gadus morhua; Norway | HT1 |
| ATCC 43306 | V. anguillarum, O2a | Gadus morhua; Denmark | HT1 |
| ATCC 14181 | V. anguillarum, O2a | Salmo trutta; UK | HT3 |
| N3A3-2c (41) | V. anguillarum, non-O1/O2 | Gadus morhua; Scotland | HT22 |
| NVI 3379 | V. anguillarum, O2a | Scophthalmus maximus; Norway | HT3 |
| NVI 5064 | V. anguillarum, O2a | Pollachius virens; Norway | HT3 |
| NVI 4351, 4845, 5022, 5039, 5056, 5121, 6036, 6037, 6409 | V. anguillarum, O2a biotype II | Gadus morhua; Norway | HT27, HT29, HT28, HT29, HT29, HT29, HT29, HT29, HT29 |
| NVI 4299, 4353, 4590, 4750, 5042, 5043, 5067, 5106, 5543, 5546, 6100, 6396 | V. anguillarum, O2b | Gadus morhua; Norway | HT2, HT2, HT2, HT2, HT2, HT2, HT2, HT2, HT2, HT2, HT2, HT2 |
| NCIMB 2133 | V. anguillarum, O2b | Pollachius virens; Norway | HT2 |
| 114-02c | V. anguillarum, O2b | Gadus morhua; Scotland | HT2 |
| NVI 6331 | V. anguillarum, O2b | Gadus morhua; Ireland | HT2 |
| NVI 5356 | V. anguillarum, O2b | Gadus morhua; Ireland | HT2 |
| NVI 4614 | V. anguillarum, O2b | Pollachius virens; Norway | HT2 |
| 820723 (42) | V. anguillarum, O2b | Unknown; Denmark | HT1 |
| NVI 6078, 6099, 6243, 6273, 6398, 6419 | V. anguillarum, O2b | Gadus morhua; Norway | HT2, HT32, HT2, HT2, HT2, HT2 |
| NCIMB 2130 | V. anguillarum, O2b | Pollachius virens; Norway | HT23 |
| NVI 6168 | V. anguillarum, O2b | Gadus morhua; Norway | HT2 |
| 860708-6/1 (43) | V. anguillarum, O2c | Oncorhynchus mykiss; Denmark | HT3 |
| 860813-17/1b (43) | V. anguillarum, O2c | Oncorhynchus mykiss; Denmark | HT3 |
| 910614-1/1 (43) | V. anguillarum, O2c | Gadus morhua; Denmark | HT3 |
| Se-1/1 (43) | V. anguillarum, O2c | Sediment; Denmark | HT3 |
| ATCC 43307 | V. anguillarum, O3 | Oncorhynchus mykiss; Denmark | HT5 |
| ATCC 43308 | V. anguillarum, O4 | Gadus morhua; Denmark | HT6 |
| ATCC 43309 | V. anguillarum, O5 | Gadus morhua; Denmark | HT7 |
| ATCC 43310 | V. anguillarum, O6 | Gadus morhua; Denmark | HT8 |
| ATCC 43311 | V. anguillarum, O7 | Anguilla anguilla; Denmark | HT9 |
| ATCC 43312 | V. anguillarum, O8 | Gadus morhua; Denmark | HT10 |
| ATCC 43313 | V. anguillarum, O9 | Gadus morhua; Denmark | HT11 |
| HI 21412, 13, 14, 29 (40) | V. anguillarum, untyped | Gadus morhua; Norway | HT13, HT14, HT14, HT14 |
| VIB 149b | V. anguillarum, untyped | Water; Denmark | HT36 |
| N8D10-8c (41) | V. anguillarum, untyped | Gadus morhua; Scotland | HT22 |
| NCIMB 2129 | V. anguillarum, O1 | Oncorhynchus mykiss; Norway | HT4 |
| VIB 64b | V. anguillarum, O1 | Scophthalmus maximus; Spain | HT4 |
| NCIMB 1873 | V. anguillarum, O1 | Oncorhynchus tshawytscha; USA | HT4 |
| VIB 127b | V. anguillarum, non-O1/O2 | Oncorhynchus mykiss; Italy | HT35 |
| NVI 5286, 5918 | V. ordalii | Gadus morhua; Norway | HT31, HT31 |
| NCIMB 2168 | V. ordalii | Oncorhynchus kisutch; USA | HT25 |
| NVI 5190, 5191 | V. ordalii | Salmo salar; Chile | HT30, HT30 |
| NVI 5084 | V. ordalii | Salmo salar; Chile | HT30 |
| NVI 5087 | V. ordalii | Salmo salar; Chile | HT30 |
IU, Istanbul University; NVI, Norwegian Veterinary Institute; NCIMB, National Collection of Industrial, Food, and Marine Bacteria; ATCC, American Type Culture Collection; VIB, Heriot-Watt University (Edinburgh, Scotland); LMG, Belgian Coordinated Collections of Microorganisms/LMG Bacteria Collection; HI, Institute for Marine Research (Bergen, Norway).
Kindly donated by Dawn Austin (Heriot-Watt University).
Kindly donated by Harry Birkbeck (University of Glasgow).
TABLE 2.
Publicly available draft or complete full-genome sequences
| Designation and reference | Previous species designation and serotype | Proposed species designation | Origin | Haplotype |
|---|---|---|---|---|
| V. ordalii NCIMB 2167T | V. ordalii | Oncorhynchus kisutch; USA | HT24 | |
| V. anguillarum 775 (ATCC 68554) | V. anguillarum, O1 | Oncorhynchus mykiss; USA | HT39 | |
| V. anguillarum NB10 (44) | V. anguillarum, O1 | Oncorhynchus mykiss; Sweden | HT4 | |
| V. anguillarum (45) | V. anguillarum, O1 | Paralichthys olivaceus; China | HT4 | |
| V. anguillarum 96F | V. anguillarum, O1 | Morone saxatilis; USA | HT40 | |
| V. anguillarum RV22 | V. anguillarum, O2b | Scophthalmus maximus; Spain | HT3 | |
| V. ordalii 12B09 (38) | V. ordalii | V. anguillarum | Filtered seawater; USA | HT41 |
| V. ordalii FF93 (38) | V. ordalii | V. anguillarum | Filtered seawater; USA | HT42 |
| V. ordalii FF167 (38) | V. ordalii | V. ordalii? | Filtered seawater; USA | HT43 |
| V. ordalii FS144 (38) | V. ordalii | V. anguillarum | Filtered seawater; USA | HT41 |
| V. ordalii FS238 (38) | V. ordalii | V. anguillarum | Filtered seawater; USA | HT44 |
Selection of housekeeping gene loci and primer design.
Housekeeping genes were selected based on previous reports of orthologous genes used for Vibrio MLSA (17, 18). Eight gene loci were investigated, and primers were either retrieved from previous studies or designed using Vector NTI 11 (Invitrogen), as part of this study. Specific primer sets for atpA, ftsZ, gapA, mreB, rpoA, and topA were designed based on publicly available sequences for V. anguillarum LMG4437 retrieved from GenBank (GenBank accession numbers EF601227, DQ907334, DQ907275, DQ907406, AJ842561, and DQ907471, respectively). For gyrB, previously published primers were used (5). For pyrH, new specific primers were designed from a sequence obtained from V. anguillarum ATCC 43312, following sequencing of amplicons generated using the degenerate Vibrio primers from a previous study (17). A summary of all gene loci and corresponding primer sets is presented in Table 3.
TABLE 3.
Overview of the eight Vibrio anguillarum-related gene loci studied and primer sequences
| Locus and size (bp) | Description | Z-scorea | P | Primer name | Primer sequence (5′ to 3′) | Amplicon size (bp) | Source |
|---|---|---|---|---|---|---|---|
| atpA (1,038) | ATP synthase A subunit | −4.039 | 0 | Vib-atpA-01-F | GACGTGATGCAAGGTGAAAT | 1,167 | This study (LMG 4437) |
| Vib-atpA-02-R | TTTCTGCTTCATCAGCTCTG | ||||||
| Vib-atpA-03-R | ACAATCAGTGCATCTTCACC | ||||||
| Vib-atpA-04-F | AGGTTGTGCAATGGGTGAAT | ||||||
| Vib-atpA-05-F | TCATTCGCATTCACGGCCTA | 1,247 | |||||
| Vib-atpA-06-R | CGCTCTGCCGCAAAGATAA | ||||||
| ftsZ (459) | Cell division protein | −5.095 | 0 | Vib-ftsZ-F | CACTAAAGGTTTGGGTGCTGG | 536 | This study (LMG 4437) |
| Vib-ftsZ-R | CCATCTCTGCCGCTTCTTCT | ||||||
| Vib-ftsZ-03-F | TTGGTGGTGGTGGCGGTAA | 678 | |||||
| Vib-ftsZ-04-R | TTGCCATCTCTGCCGCTT | ||||||
| gapA (483) | Glyceraldehyde-3-phosphate dehydrogenase | −2.972 | 0.004 | Vib-gapA-F | AACAGGTCTATTCCTAGACGA | 570 | This study (LMG 4437) |
| Vib-gapA-R | CCGATGAAGTCTTGAGAAAC | ||||||
| gyrB (936) | DNA gyrase subunit B | −4.861 | 0 | Vib-gyrB-F | GTCTGCATGGTGTCGGTGT | 1,092 | Colquhoun et al. 2007 (5) |
| Vib-gyrB-R | CACAGCCTAATGGCGGTATA | ||||||
| Vib-gyrB-1 | CATTTACTGCTTTACCAA | ||||||
| Vib gyrB-4 | CTAGAGAACTTAGGATCC | ||||||
| mreB (543) | Rod shape-determining protein | −4.221 | 0 | Vib-mreB-F | AAGCGTTGCTGCTGTCGGTC | 772 | This study (LMG 4437) |
| Vib-mreB-R | GCAACCATCACCGCAGAAAC | ||||||
| pyrH (513) | Uridylate kinase | −3.249 | 0.002 | pyrH-02-R | GTRAABGCNGMYARRTCCA | Thompson et al. (17) | |
| pyrH-04-F | ATGASNACBAAYCCWAAACC | ||||||
| Vib-pyrH-05-F | AGCCTATCAACGTATCCTGT | 587 | This study (ATCC 43312) | ||||
| Vib-PyrH-06-R | CAGGTCCATCACTTTTAGCT | ||||||
| rpoA (801) | RNA polymerase alpha subunit | −2.251 | 0.03 | Vib-rpoA-07-F | TCTTAAGCCACGTCTTGTTG | 919 | This study (LMG 4437) |
| Vib-rpoA-08-R | AGATAGACCACGTGATGCAA | ||||||
| Vib-rpoA-09-R | TGGGCTGTAAGTAGCATCTA | ||||||
| Vib-rpoA-10-F | GATCGCTATGCGTATCAAAG | ||||||
| topA (438) | Topoisomerase I | −2.941 | 0.004 | Vib-topA-F | GTTTCATGGACCGTGTGGTG | 532 | This study (LMG 4437) |
| Vib-topA-R | GCTTGTCCAAACTCACTCCC | ||||||
| Vib-topA-03-F | CAATCCAACAAGCCTTCCAA | 621 | |||||
| Vib-topA-04-R | CGGATTCTCAGGTAAGTAGGCTT |
Results of a codon-based Z-test of selection for each gene locus.
DNA isolation, PCR amplification, and sequencing.
Bacterial genomic DNA was extracted using NucliSENS easyMAG (bioMérieux), according to the manufacturer's instructions. Standard 50-μl PCRs were performed with 1.25 U Taq DNA polymerase, ThermoPol buffer (New England BioLabs), and gene-specific primer sets for the eight housekeeping genes (Table 3). The thermal program consisted of an initial 5-min step at 94°C, 30 cycles of 30 s at 94°C, 30 s at 52°C, and 0.5 to 1 min at 72°C (depending on amplicon length), and a final 7-min step at 72°C. PCR products were purified using a Nucleospin Extract II kit (Macherey-Nagel, Germany), following the manufacturer's instructions. Standard 10-μl sequencing PCRs were performed according to the manual for the DYEnamic dye terminator cycle sequencing kit (Amersham Biosciences). The resulting PCR products for sequencing were purified using the DYE terminator removal kit (ABgene) and sequenced on a MegaBACE 1000 sequencing instrument (Amersham Biosciences).
MLSA and eBURST population snapshot construction.
Raw sequence data were assembled and edited primarily using Vector NTI 11 (Invitrogen). The resulting consensus sequences were aligned in Bioedit (version 7.0.5), utilizing the ClustalX algorithm (22, 23). The concatenated sequences were constructed by joining individual gene sequences, trimmed to the correct reading frame, head to tail alphabetically using Sequence Matrix 1.7.8 software (24). Concatenated sequences were 5,208 bp and consisted of atpA (1,038 bp), ftsZ (456 bp), gapA (483 bp), gyrB (936 bp), mreB (543 bp), pyrH (513 bp), rpoA (801 bp), and topA (438 bp). Allele types (ATs) and haplotypes (HTs) were then assigned with an Excel (Microsoft)-based AT and HT generator developed in house (see Table S1 in the supplemental material). After the assignment of HTs, clonal complexes (CCs) were identified using eBURST 3.7 (25). The predicted ancestral genotype (founder) for each CC was defined as the HT with the greatest number of single-locus variants (SLVs). Major CCs, with three or more HTs, were named according to the HT of the predicted founder, while minor CCs were named after the most represented HT or the HT with the lowest numbering. HTs that did not belong to any CC are referred to as singletons. Clonal relationships were examined using stringent settings (i.e., minimum of 7 shared alleles), and bootstrapping (n = 100,000) was performed to evaluate model robustness. A maximum-parsimony network, reconstructing the phylogenetic relationships among HTs, was generated with Network 4.6 software (Fluxus Engineering). The median joining algorithm was used (26), with transversions and transitions being equally weighted. A codon-based Z-test of selection and all pairwise distance computations were performed with MEGA6 software (27).
Population genetics, phylogenetic analysis, and DDH calculations.
Phylogenetic analysis of concatenated gene sequences was conducted using PhyML (version 3.0) (28), implementing maximum likelihood (ML) analysis using the general time reversible (GTR) base substitution model. This substitution model was identified as the best fit using jModeltest 2.1.7 software (29, 30). Branch support was calculated with the approximate likelihood ratio test (aLRT) Shimodaira-Hasegawa (SH)-like fast likelihood method. Genetic diversity (H) and the level of linkage disequilibrium (nonrandom association) between ATs at the eight MLSA loci were investigated with the standardized index of association (ISA), using LIAN 3.7 (31). Three main data sets were tested, i.e., the whole population, single HT representatives (44 HTs), and finally CC founder sequence types (STs) together with singleton HTs (30 HTs), as well as minor data sets, based on the origin of the isolates. A network representation of the evolutionary relationships between HTs was constructed with concatenated sequences using the SplitsTree4 neighbor-net algorithm (32), applying the uncorrected P method (default settings). Recombination within each HT was estimated by the pairwise homoplasy index (PHI) test implemented in SplitsTree4. Intergenomic distances and DNA-DNA hybridization (DDH) estimates between homologous regions of the well-characterized V. anguillarum 775 genome (4, 33) and 10 other full-genome-sequenced V. anguillarum and putative V. ordalii strains (Table 2) were calculated with GGDC 2.0 software (34).
Accession number(s).
All partial gene sequences obtained during the present study were submitted to GenBank, with the following accession numbers: atpA, KU754602 to KU754707; ftsZ, KU754708 to KU754813; gapA, KU754814 to KU754919; gyrB, KU754920 to KU755025; mreB, KU755026 to KU755131; pyrH, KU755132 to KU755237; rpoA, KU755238 to KU755343; topA, KU755344 to KU755449.
RESULTS
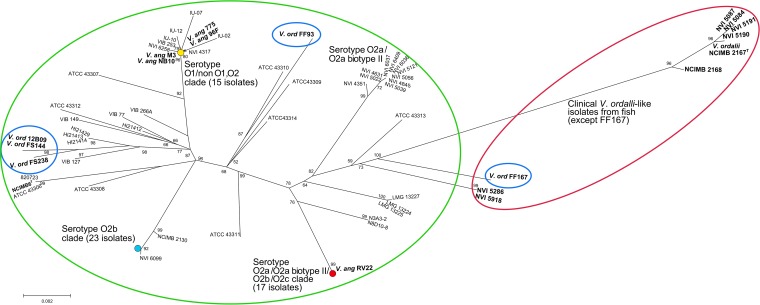
Partial sequences were obtained for eight protein-encoding genes (atpA, ftsZ, gapA, gyrB, mreB, topA, rpoA, and pyrH) from 103 V. anguillarum isolates and 13 isolates previously identified as V. ordalii. A codon-based Z-test of selection indicated that all genes were under purifying selection and thereby suitable for MLSA (Table 3). The Pacific (American and Chilean) V. ordalii isolates, all isolated from fish, clustered separately and apart from the main V. anguillarum clade in the maximum likelihood (ML) phylogenetic tree reconstruction (Fig. 1, far right cluster in red oval). While the Norwegian fish-pathogenic isolates putatively identified as Vibrio ordalii appeared to represent an earlier branch of the same lineage (Fig. 1, left cluster in red oval), the Pacific environmental (putative) V. ordalii isolates were spread around the main V. anguillarum cluster (Fig. 1, green oval), with the exception of FF167, which was closer to V. anguillarum but was placed basally within the fish-pathogenic V. ordalii lineage (Fig. 1, rightmost blue oval). Obvious associations between the host species and the major V. anguillarum serotype clusters could be seen. The serotype O1 clade (Fig. 1, yellow dot) was dominated by Norwegian, Danish, and American salmon and trout isolates but also included isolates from Spain (turbot), Japan (ayu), and Australia (rainbow trout), as well as the well-characterized V. anguillarum 775 (4, 33). The serotype O2b clade (Fig. 1, blue dot) was the most uniform; all 25 isolates originated from gadoid fish from Norway, Scotland, or Ireland, with 23 of them appearing clonal. The O2a/O2aII/O2b/O2c clade (Fig. 1, red dot) consisted of isolates from Norway, Denmark, and the United Kingdom that were identical in all eight sequenced loci. This clade also contained the full-genome-sequenced isolate RV22 (described as serotype O2b) from turbot (Scophthalmus maximus) (from Spain), a single non-O1/O2a/O2b Turkish isolate, and a single O2a biotype II isolate (defined as such due to the ability to produce lysine decarboxylase). The last of the four major clusters, i.e., the O2/O2a biotype II clade, included 10 somewhat diverse isolates, including a single serotype O2a isolate from Norwegian salmon.
FIG 1.
Phylogenetic reconstruction of Vibrio anguillarum intraspecies relationships, based on concatenated partial gene sequences (atpA, ftsZ, gapA, gyrB, mreB, pyrH, ropA, and topA), by ML analysis using the GTR base substitution model. Red oval, V. ordalii-like isolates from fish; green oval, V. anguillarum isolates from fish; blue ovals, isolates from the environment previously identified as V. ordalii; yellow dot, HT4 isolates; blue dot, HT2 isolates; red dot, HT3 isolates. Branch numbers represent aLRT support values.
The MLSA of the 116 isolates identified 44 HTs. eBURST clustering of the 44 HTs (with stringent group definition, i.e., 7 of 8 shared allele types) divided them into 5 CCs and 27 singletons (see Fig. S1 in the supplemental material). Seven singletons (HT1, HT3, HT14, HT22, HT30, HT31, and HT41) were represented by more than one isolate, with the largest (HT3) containing 18 isolates. The majority of the isolates (11 isolates) within HT3 originated from Norwegian turbot, salmonids, and gadoids, while some of the remaining 7 isolates originated from European seabass, brown trout, and a sediment sample (Turkey, United Kingdom, and Denmark). Three of the five identified CCs contained more than 10 isolates. The predominant clonal complex, CC-HT4, included 7 HTs, consisting of 22 isolates, with the predicted founder being HT4. Eight of the 16 isolates in HT4 were isolated from salmonids (Norway, Denmark, Sweden, and the United States), while the remainder were isolated from other fish species (not including Gadidae) in other countries (Turkey, Spain, Japan, Australia, and China). The second major clonal complex, CC-HT2, included 3 HTs and 25 isolates, with HT2 being its predicted founder. In comparison with CC-HT4, CC-HT2 was a much more homogeneous group, with all isolates originating from Norwegian, Irish, and Scottish Gadidae. The third clonal complex, CC-HT28, was made up of 3 HTs containing 10 isolates, with HT29 as the predicted founder. This group was also homogeneous, with 9 of 10 isolates originating from Norwegian gadoids and a single isolate originating from Norwegian Atlantic salmon. The two minor groups, CC-HT20 and CC-HT24, included only 2 HTs each, with 2 or 3 isolates, respectively, and neither had a predicted founder.
The genetic diversity (H) in the total data set (5,208 bp from each of 116 isolates) was estimated to be 0.767 ± 0.026 (Table 4). The standardized index of association (ISA) for the whole population was estimated to be 0.506, which differed significantly from zero (P < 0.05); therefore, the null hypothesis of linkage equilibrium was rejected. When overrepresentation of particular haplotypes was avoided by restricting the analysis to single representatives of the 44 HTs, the ISA value decreased to 0.281 (P < 0.05). When the analysis was further limited to include only CC founders plus all singletons (27 isolates), the ISA value was 0.097, closer to linkage equilibrium, but it remained significantly different from zero (P < 0.05).
TABLE 4.
Calculated mean genetic diversity values and levels of linkage disequilibrium (nonrandom association) between allelic types of eight Vibrio anguillarum-related MLSA locia
| Population | VD | Ve | ISA | Var(VD) | P | L | H |
|---|---|---|---|---|---|---|---|
| All isolates | 6.33 | 1.39 | 0.506 | 0.003 | <1.0 × 10−3 | 1.49 | 0.767 ± 0.026 |
| All HTs | 2.29 | 0.77 | 0.281 | 0.003 | <1.0 × 10−3 | 0.86 | 0.885 ± 0.026 |
| Founder HTs + singletons | 1.06 | 0.63 | 0.097 | 0.003 | <1.0 × 10−3 | 0.72 | 0.906 ± 0.031 |
| Norway/Sweden | 8.20 | 1.81 | 0.503 | 0.020 | <1.0 × 10−3 | 2.06 | 0.603 ± 0.043 |
| Europe | 5.10 | 1.20 | 0.466 | 0.007 | <1.0 × 10−3 | 1.34 | 0.800 ± 0.038 |
| Pacific | 6.79 | 1.18 | 0.680 | 0.015 | <1.0 × 10−3 | 1.40 | 0.815 ± 0.022 |
| Salmonid | 9.36 | 1.59 | 0.700 | 0.020 | <1.0 × 10−3 | 1.83 | 0.714 ± 0.029 |
| Gadiformes | 8.12 | 1.82 | 0.493 | 0.033 | <1.0 × 10−3 | 2.13 | 0.599 ± 0.042 |
| Other fish | 9.00 | 1.72 | 0.602 | 0.120 | <1.0 × 10−3 | 2.36 | 0.551 ± 0.067 |
| Environmentb | 2.50 | 0.61 | 0.441 | 0.012 | <1.0 × 10−3 | 0.79 | 0.909 ± 0.030 |
Calculations were performed using LIAN 3.7 software. VD, observed variance; Ve, expected variance at linkage equilibrium; ISA, standardized index of association; Var(VD), variance of VD; L, critical value; H, genetic diversity.
Seawater, rotifers, and sediment.
When results were sorted by geographical origin, the greatest variation in haplotypes per number of isolates, i.e., 12 HTs/20 isolates (H = 0.815 ± 0.022), was found in the Pacific group (United States, Chile, Japan, and China) (Table 4). When results were sorted by isolation source, the greatest variations in haplotypes per number of isolates, i.e., 9 HTs/11 isolates (H = 0.909 ± 0.030), were found among the environmental isolates (including seawater, sediment, and rotifers) and in the nongadoid nonsalmonid group, with 11 HTs/19 isolates (H = 0.551 ± 0.067).
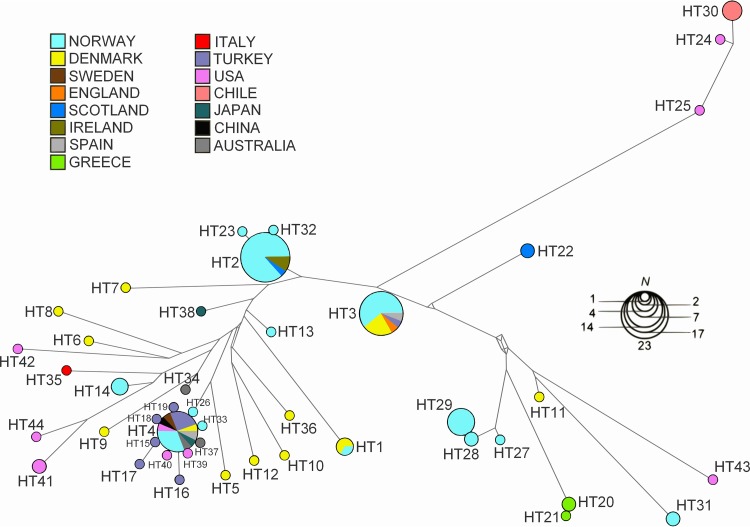
The haplotype network construction revealed that fish disease-related isolates of Vibrio ordalii from Chile and the United States and the isolates from Norway that shared several phenotypic traits with V. ordalii represented distinct populations (HT24, HT25, HT30, and HT31) (Fig. 2). Furthermore, the five environmental isolates from the United States previously considered to represent V. ordalii (HT41, HT42, HT43, and HT44) represented disparate isolates. V. anguillarum isolates from the United States (HT4, HT39, and HT40) grouped with isolates from other countries, including five closely related isolates from Turkey (HT15, HT16, HT17, HT18, and HT19). Three isolates from Greece also constituted a distinct group (HT20 and HT21).
FIG 2.
Eight-locus haplotype (H) network based on allelic profiles of the 116 Vibrio anguillarum-related isolates investigated in the present study. Circle size is proportional to haplotype frequency, and the colors represent the different geographical origins of the haplotypes. The distances between haplotypes are proportional to the number of mutational steps. More details on the haplotypes can be found in Table S1 in the supplemental material.
The PHI test in SplitsTree4 identified statistically significant evidence of recombination among isolates included in the present study. The reticulate structure (see Fig. S2 in the supplemental material) is consistent with such recombination events in the evolutionary history of all studied isolates.
The intergenomic distances and DNA-DNA hybridization (DDH) estimates between V. anguillarum 775 and other full-genome-sequenced V. anguillarum strains (four strains) and putative V. ordalii strains, as well as the V. ordalii type strain (six strains), were calculated (Table 5). Of the V. anguillarum strains studied, RV2 displayed the greatest intergenomic distance to V. anguillarum 775 (distance, 0.0171). V. ordalii NCIMB 2167T (clinical isolate) showed the greatest intergenomic distance to V. anguillarum 775 (distance, 0.0428). The intergenomic distances to the five environmental putative V. ordalii isolates were less (distances, 0.0136 to 0.0234) and similar to the intergenomic distances to bona fide V. anguillarum strains (distances, 0.0012 to 0.0171). The calculated DDH value for V. anguillarum 775 versus V. ordalii NCIMB 2167T was 65.4 ± 2.9% (species delineation norms are defined as DDH values of >70%) (34). It is noteworthy that the calculated DDH values for the only environmental putative V. ordalii isolate, FF167, which groups with clinical isolates from fish, versus V. ordalii NCIMB 2167T and V. anguillarum 775 were 69.3 ± 3.0% and 80.0 ± 2.8%, respectively.
TABLE 5.
Intergenomic distances between Vibrio anguillarum 775 and 10 full-genome-sequenced strains of V. anguillarum and isolates previously identified as Vibrio ordaliia
| Distance measured | Distance | DDH estimate (%) | Probability (%)b |
|
|---|---|---|---|---|
| DDH value of >70% | DDH value of >79% | |||
| From V. anguillarum 775 to: | ||||
| V. anguillarum M3 | 0.0012 | 99.4 ± 0.3 | 98.1 | 79.1 |
| V. anguillarum NB10 | 0.0007 | 100.0 ± 0.2 | 98.2 | 79.7 |
| V. anguillarum 96F | 0.0030 | 98.1 ± 0.7 | 97.9 | 77.2 |
| V. anguillarum RV22 | 0.0171 | 85.5 ± 2.5 | 94.0 | 57.8 |
| V. ordalii NCIMB 2167T | 0.0428 | 65.4 ± 2.9 | 68.4 | 20.8 |
| V. ordalii 12B09 | 0.0144 | 87.9 ± 2.3 | 95.0 | 61.9 |
| V. ordalii FF93 | 0.0136 | 88.7 ± 2.3 | 95.3 | 63.1 |
| V. ordalii FF167 | 0.0234 | 80.0 ± 2.8 | 90.6 | 47.7 |
| V. ordalii FS144 | 0.0143 | 88.0 ± 2.3 | 95.1 | 62.1 |
| V. ordalii FS238 | 0.0136 | 88.7 ± 2.3 | 95.3 | 63.1 |
| From V. ordalii NCIMB 2167T to V. ordalii FF167 | 0.0371 | 69.3 ± 3.0 | 80.0 | 27.4 |
Calculations were performed using GGDC 2.0 software.
Probability that the differences represent species and/or subspecies delineation.
DISCUSSION
The present study represents a multigene analysis of a substantial number of V. anguillarum and V. ordalii isolates. Examination of phylogenetic trees and networks (Fig. 1 and 2; also see Fig. S2 in the supplemental material) based on concatenated sequences revealed a high degree of sequence conservation among pathogenic V. anguillarum serotype O1 isolates from around the world. Norwegian V. anguillarum isolates showed relatively great diversity (13 haplotypes), but Danish isolates showed the greatest diversity of the study, compared to the number of isolates (12 haplotypes) (Fig. 2). Interestingly, most of the Turkish (non-O1/O2a/O2b) isolates were either identical or highly similar in sequence to serotype O1 isolates. Only one distinct serotype/haplotype-host relationship was identified, i.e., serotype O2b was isolated exclusively from gadoid fish from diverse geographical origins in the North Atlantic area. Twenty-three of the 25 serotype O2b isolates that were isolated from gadoids between 1979 and 2008 possessed identical sequences for all eight loci. Our results showed that many V. anguillarum isolates collected internationally grouped together in three main clonal complexes (see Fig. S1 in the supplemental material). The distribution of haplotypes versus serotypes (see Fig. S2) is consistent with relatively frequent horizontal gene transfer within this group of bacteria. For example, HT3 isolates occurred in four different O2 serotypes (O2a, O2aII, O2b, and O2c), while the O2a serotype occurred in three more distantly related haplotypes (HT1, HT26, and HT38), in addition to HT3. According to Jedani et al. (35), V. anguillarum serotype O2 isolates have several intact insertion sequences that may have the potential to move or shuffle genes, thus creating new O-antigen/capsule specificities within Vibrionaceae.
MLSA-based DNA similarities for all eight genes in all isolates were between 97.5 and 99.0%, with the exception of ftsZ (≥95%), and were well above the 95% threshold for Vibrio species differentiation proposed by Sawabe et al. (36). However, the calculated DDH value for V. ordalii NCIMB 2167T versus V. anguillarum 775 of 65.4%, which agrees well with the original DDH estimates of 53% to 67% reported by Schiewe et al. and Thompson et al. (1, 37), clearly suggests that these two strains represent separate bacterial species. The GGDC 2.0 software calculations are independent of genome size, provide results directly comparable to wet-laboratory DDH results, and have been shown to correlate better with DDH results than the more commonly used average nucleotide identity (ANI) method (34).
Naka et al. (4) identified a significantly smaller genome in V. ordalii NCIMB 2167T, compared to V. anguillarum 775, which suggests that physical and ecological differences exist between these two species/strains and that fish-pathogenic V. ordalii may be evolving toward an endosymbiotic lifestyle. The sequences retrieved from public databases for five putative V. ordalii isolates originating from seawater revealed intermediate-size genomes, genetically considerably closer (by MLSA and DDH analysis) to V. anguillarum 775 than the fish-pathogenic V. ordalii isolates. It is not clear from the available literature whether these environmental isolates are phenotypically consistent with V. ordalii (38, 39). With the exception of isolate FF167, the isolates show no relationship to the V. ordalii reference strain but are spread inside the V. anguillarum clade (Fig. 1), which clearly indicates that these isolates represent V. anguillarum. The precise taxonomic situation for the environmental isolate FF167 and the Atlantic cod pathogens NVI 5286 and NVI 5918 is unclear, as the latter two isolates display several phenotypic traits consistent with V. ordalii and appear very closely related to V. anguillarum by ML analysis but are situated at the base of the V. ordalii lineage. Intergenomic distances and in silico DDH values of 80.0% and 69.3% for FF167 versus V. anguillarum 775 and V. ordalii NCIMB 2167T, respectively, indicate that putative V. ordalii strain FF167 is closer to V. anguillarum 775 than V. ordalii NCIMB 2167T. It should be noted, however, that the distance to V. anguillarum type strain NCIMB 6 could not be calculated, as there is no full-genome sequence available for that isolate. Interestingly, FF167 has the smallest genome of the five environmental isolates and is also the only one to possess a full complement of biofilm-associated syp genes (data not shown), which were previously suggested to be species specific and present in V. ordalii NCIMB 2167T but not in V. anguillarum 775 (4). These genes may represent genetic markers for phenotypic and epidemiological separation of V. ordalii linage members from V. anguillarum isolates.
In conclusion, the described MLSA largely resolved V. anguillarum isolates according to serotype. V. anguillarum serotype O1 and O2b isolates associated with disease in fish displayed a high degree of sequence conservation, as expected. Taken together, the phylogenetic relationships revealed by MLSA, DNA similarity values, and DDH estimates do not justify incorporation of V. ordalii within the species V. anguillarum. Further study of larger numbers of North Atlantic fish-pathogenic isolates that share several phenotypic traits with V. ordalii are warranted.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dawn Austin (Heriot-Watt University, Edinburgh, Scotland) and Harry Birkbeck (University of Glasgow, Scotland) for the donation of isolates.
The research was financially supported by the Norwegian Research Council (grant 184634). We are grateful for the support of Istanbul University, which financed a research period in Norway for S.K. P.M.M. and F.L.T. thank CNPq, CAPES, and FAPERJ for support.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00620-16.
REFERENCES
- 1.Schiewe MH, Trust TJ, Crosa JH. 1981. Vibrio ordalii sp. nov.: a causative agent of vibriosis in fish. Curr Microbiol 6:343–348. doi: 10.1007/BF01567009. [DOI] [Google Scholar]
- 2.Pedersen K, Grisez L, van Houdt R, Tiainen T, Ollevier F, Larsen JL. 1999. Extended serotyping scheme for Vibrio anguillarum with the definition and characterization of seven provisional O-serogroups. Curr Microbiol 38:183–189. doi: 10.1007/PL00006784. [DOI] [PubMed] [Google Scholar]
- 3.Colquhoun DJ, Lillehaug A. 2014. Vaccination against vibriosis, p 172–184. In Gudding R, Lillehaug A, Evensen Ø (ed), Fish vaccination. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 4.Naka H, Dias GM, Thompson CC, Dubay C, Thompson FL, Crosa JH. 2011. Complete genome sequence of the marine fish pathogen Vibrio anguillarum harboring the pJM1 virulence plasmid and genomic comparison with other virulent strains of V. anguillarum and V. ordalii. Infect Immun 79:2889–2900. doi: 10.1128/IAI.05138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colquhoun DJ, Aarflot L, Melvold CF. 2007. gyrA and parC mutations and associated quinolone resistance in Vibrio anguillarum serotype O2b isolated from Norwegian farmed Atlantic cod (Gadus morhua). Antimicrob Agents Chemother 51:2597–2599. doi: 10.1128/AAC.00315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodger HD, Colquhoun DJ. 2008. Clinical vibriosis in farmed Atlantic cod (Gadus morhua) in Ireland. Vet Rec 162:94–95. doi: 10.1136/vr.162.3.94. [DOI] [PubMed] [Google Scholar]
- 7.Jones M, Cockerill DJ, Birbeck TH, Cox DI. 2000. Clinical infection of cod (Gadus morhua L.) in Scotland by Vibrio anguillarum: a case history. Bull Eur Assoc Fish Pathol 20:125–128. [Google Scholar]
- 8.Kusuda R, Kawai K. 1998. Bacterial diseases of cultured marine fish in Japan. Fish Pathol 33:221–227. doi: 10.3147/jsfp.33.221. [DOI] [Google Scholar]
- 9.Austin B, Alsina M, Austin DA, Blanch AR, Grimont F, Grimont PAD, Jofre J, Koblavi S, Larsen JL, Pedersen K, Tiainen T, Verdonck L, Swings J. 1995. Identification and typing of Vibrio anguillarum: a comparison of different methods. Syst Appl Microbiol 18:285–302. doi: 10.1016/S0723-2020(11)80400-5. [DOI] [Google Scholar]
- 10.Pedersen K, Larsen JL. 1998. Characterization and typing methods for the fish pathogen Vibrio anguillarum. Recent Res Dev Microbiol 2:17–93. [Google Scholar]
- 11.Sørensen UBS, Larsen JL. 1986. Serotyping of Vibrio anguillarum. Appl Environ Microbiol 51:593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen HB. 1987. Subgrouping of lipopolysaccharide O antigens from Vibrio anguillarum serogroup O2 by immunoelectrophoretic analysis. Curr Microbiol 16:39–42. doi: 10.1007/BF01568167. [DOI] [Google Scholar]
- 13.Bolinches J, Lernos ML, Fouz B, Cambra M, Larsen JL, Toranzo AE. 1990. Serological relationship among Vibrio anguillarum strains. J Aquat Anim Health 2:21–29. doi:. [DOI] [Google Scholar]
- 14.Tiainen T, Pedersen K, Larsen JL. 1997. Immunological reactivity of Vibrio anguillarum sero-subgroups O2a and O2b, and comparison of their lipopolysaccharide profiles. Curr Microbiol 34:38–42. doi: 10.1007/s002849900141. [DOI] [PubMed] [Google Scholar]
- 15.Skov MN, Pedersen K, Larsen JL. 1995. Comparison of pulsed-field gel electrophoresis, ribotyping, and plasmid profiling for typing of Vibrio anguillarum serovar O1. Appl Environ Microbiol 61:1540–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorsch M, Lane D, Stackebrandt E. 1992. Towards a phylogeny of the genus Vibrio based on 16S rRNA sequences. Int J Syst Bacteriol 42:58–63. doi: 10.1099/00207713-42-1-58. [DOI] [PubMed] [Google Scholar]
- 17.Thompson FL, Gevers D, Thompson CC, Dawyndt P, Naser S, Hoste B, Munn CB, Swings J. 2005. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Appl Environ Microbiol 71:5107–5115. doi: 10.1128/AEM.71.9.5107-5115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson FL, Gomez-Gil B, Vasconcelos AT, Sawabe T. 2007. Multilocus sequence analysis reveals that Vibrio harveyi and V. campbellii are distinct species. Appl Environ Microbiol 73:4279–4285. doi: 10.1128/AEM.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson CC, Vicente AC, Souza RC, Vasconcelos AT, Vesth T, Alves N Jr, Ussery DW, Iida T, Thompson FL. 2009. Genomic taxonomy of vibrios. BMC Evol Biol 9:258. doi: 10.1186/1471-2148-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikow RB. 2011. Systematic relationships within the Vibrionaceae (Bacteria: Gammaproteobacteria): step towards a phylogenetic taxonomy. Cladistics 27:9–28. doi: 10.1111/j.1096-0031.2010.00312.x. [DOI] [PubMed] [Google Scholar]
- 21.Austin B, Austin DA. 2007. Bacterial fish pathogens: diseases of farmed and wild fish, 4th ed Springer, Dordrecht, Netherlands. [Google Scholar]
- 22.Hall T. 1999. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp Ser 41:95–98. [Google Scholar]
- 23.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 24.Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–180. doi: 10.1111/j.1096-0031.2010.00329.x. [DOI] [PubMed] [Google Scholar]
- 25.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandelt HJ, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 29.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guindon S, Gascuel O. 2003. A simple, fast and accurate method to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 31.Haubold H, Hudson RR. 2000. LIAN 3.0: detecting linkage disequilibrium in multilocus data. Bioinformatics 16:847–848. doi: 10.1093/bioinformatics/16.9.847. [DOI] [PubMed] [Google Scholar]
- 32.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. [DOI] [PubMed] [Google Scholar]
- 33.di Lorenzo M, Stork M, Tolmasky ME, Actis LA, Farrell D, Welch TJ, Crosa LM, Wertheimer AM, Chen Q, Salinas P, Waldbeser L, Crosa JH. 2003. Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J Bacteriol 185:5822–5830. doi: 10.1128/JB.185.19.5822-5830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jedani KE, Stroeher UH, Manning PA. 2000. Distribution of IS1358 and linkage to rfb-related genes in Vibrio anguillarum. Microbiology 146:323–331. doi: 10.1099/00221287-146-2-323. [DOI] [PubMed] [Google Scholar]
- 36.Sawabe T, Kita-Tsukamoto K, Thompson FL. 2007. Inferring the evolutionary history of vibrios by means of multilocus sequence analysis. J Bacteriol 189:7932–7936. doi: 10.1128/JB.00693-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson FL, Thompson CC, Dias GM, Naka H, Dubay C, Crosa JH. 2011. The genus Listonella MacDonell and Colwell 1986 is a later heterotypic synonym of the genus Vibrio Pacini 1854 (Approved Lists 1980): a taxonomic opinion. Int J Syst Evol Microbiol 61:3023–3027. doi: 10.1099/ijs.0.030015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordero OX, Wildschutte H, Kirkup B, Proehl S, Ngo L, Hussain F, Le Roux F, Mincer T, Polz MF. 2012. Ecological populations of bacteria act as socially cohesive units of antibiotic production and resistance. Science 337:1228–1231. doi: 10.1126/science.1219385. [DOI] [PubMed] [Google Scholar]
- 39.Hunt DE, David LA, Gevers D, Preheim SP, Alm EJ, Polz MF. 2008. Resource partitioning and sympatric differentiation among closely related bacterioplankton. Science 320:1081–1085. doi: 10.1126/science.1157890. [DOI] [PubMed] [Google Scholar]
- 40.Sandlund N, Bergh Ø. 2008. Screening and characterization of potentially pathogenic bacteria associated with Atlantic cod Gadus morhua larvae: bath challenge trials using a multidish system. Dis Aquat Organ 81:203–217. doi: 10.3354/dao01934. [DOI] [PubMed] [Google Scholar]
- 41.Reid H, Treasurer WJ, Adam B, Birkbeck TH. 2009. Analysis of bacterial populations in the gut of developing cod larvae and identification of Vibrio logei, Vibrio anguillarum and Vibrio splendidus as pathogens of cod larvae. Aquaculture 288:36–43. doi: 10.1016/j.aquaculture.2008.11.022. [DOI] [Google Scholar]
- 42.Larsen JL, Olsen JE. 1991. Occurrence of plasmids in Danish isolates of Vibrio anguillarum serovars O1 and O2 and association of plasmids with phenotypic characteristics. Appl Environ Microbiol 57:2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiainen T, Pedersen K, Larsen JL. 1995. Ribotyping and plasmid profiling of Vibrio anguillarum serovar O2 and Vibrio ordalii. J Appl Bacteriol 79:384–392. doi: 10.1111/j.1365-2672.1995.tb03152.x. [DOI] [PubMed] [Google Scholar]
- 44.Holm KO, Nilsson K, Hjerde E, Willassen NP, Milton DL. 2015. Complete genome sequence of Vibrio anguillarum strain NB10, a virulent isolate from the Gulf of Bothnia. Stand Genomic Sci 10:60. doi: 10.1186/s40793-015-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, Mo Z, Li J, Xiao P, Hao B. 2013. Complete genome sequence of Vibrio anguillarum M3, a serotype O1 strain isolated from Japanese flounder in China. Genome Announc 1(5):e00769-13. doi: 10.1128/genomeA.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.