Abstract
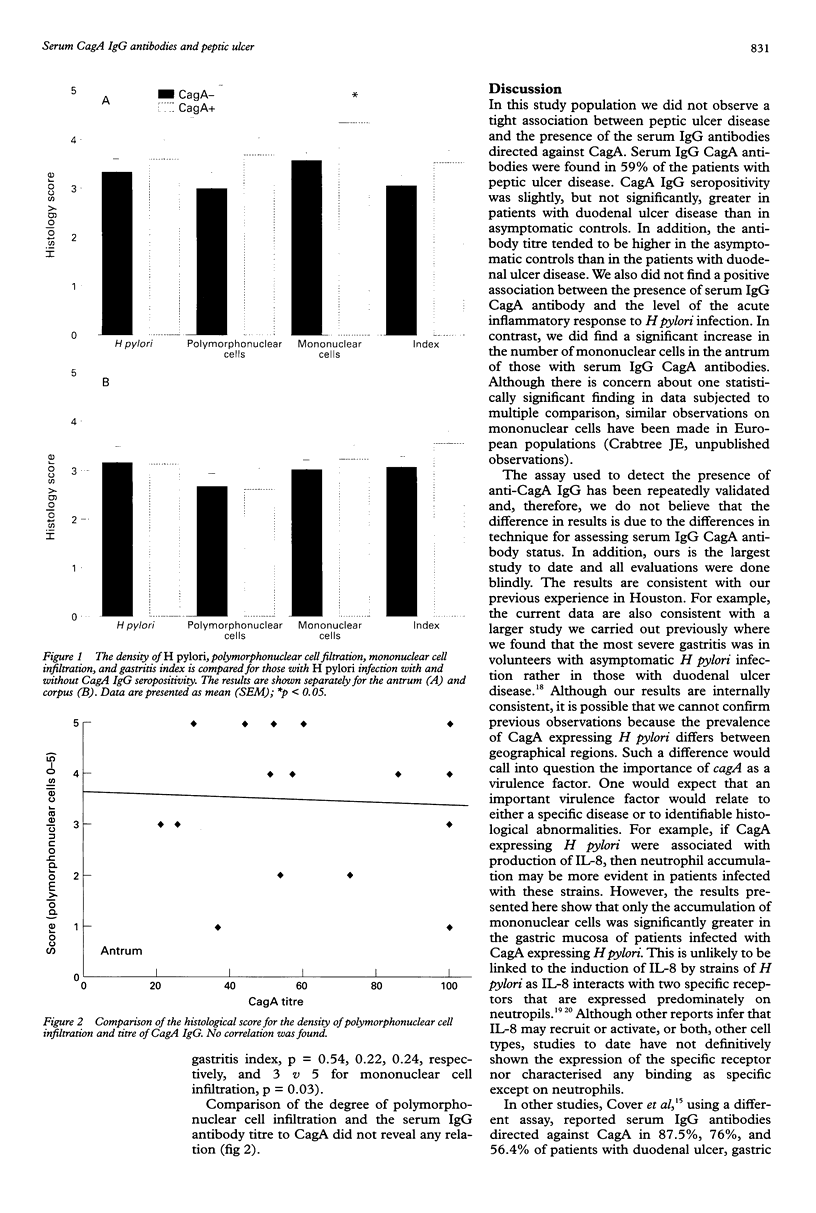
AIM/BACKGROUND: Several studies have suggested that Helicobacter pylori which express CagA may be more virulent than those that do not, but limited populations have been studied to date. The aim of this study was to confirm and extend the association of CagA positive H pylori strains in a different geographical area and to a large, well defined patient population. METHOD: A validated ELISA for serum IgG to CagA was used to investigate the prevalence of CagA seropositivity in 100 patients with peptic ulcer compared with 77 with H pylori infection without ulcer disease in a North American population. The extent of antral and corpus inflammation and H pylori density in relation to CagA seropositivity in 40 subjects with H pylori infection were assessed semiquanitatively. All studies were carried out in a coded and blinded manner. RESULTS: The prevalence of serum IgG CagA antibodies was higher in H pylori infected patients with ulcer (59%) compared with healthy H pylori infected volunteers (44%), but the difference was not significant. In contrast, the titre of serum IgG anti-CagA antibodies was higher among the seropositive subjects without ulcer disease, but again the difference was not significant. Comparison of histological features between asymptomatic individuals with H pylori infection in relation to CagA IgG antibody status revealed no differences in infiltration with acute inflammatory cells, H pylori density, or gastritis index. There was no relation evident between the degree of polymorphonuclear cell infiltration and the serum IgG antibody titre to CagA. Mononuclear cell infiltration in the antrum, but not the corpus, was greater in those with CagA IgG compared with those without (median score 5 v 3). CONCLUSIONS: A right association between the presence or titre of serum IgG to CagA and peptic ulcer disease, greater H pylori density or infiltration of the mucosa with acute inflammatory cells could not confirmed in a North American population. Perhaps geographical differences in the prevalence of circulating H pylori strains are responsible for the discrepant results reported.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Glupczynski Y., Lage A. P., Burette A., Tummuru M. K., Perez-Perez G. I., Blaser M. J. Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol. 1995 Jun;33(6):1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Covacci A., Farmery S. M., Xiang Z., Tompkins D. S., Perry S., Lindley I. J., Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995 Jan;48(1):41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genta R. M., Robason G. O., Graham D. Y. Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol. 1994 Mar;25(3):221–226. doi: 10.1016/0046-8177(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Hayward R. A., Shapiro M. F., Oye R. K. Laboratory testing on cerebrospinal fluid. A reappraisal. Lancet. 1987 Jan 3;1(8523):1–4. doi: 10.1016/s0140-6736(87)90698-2. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Thomas K. M., Kaufmann M. E., Mark R., White M., Taylor L., Gray G., Witt D., Navarro J. Amino terminus of the interleukin-8 receptor is a major determinant of receptor subtype specificity. J Biol Chem. 1992 Dec 15;267(35):25402–25406. [PubMed] [Google Scholar]
- Leunk R. D., Ferguson M. A., Morgan D. R., Low D. E., Simor A. E. Antibody to cytotoxin in infection by Helicobacter pylori. J Clin Microbiol. 1990 Jun;28(6):1181–1184. doi: 10.1128/jcm.28.6.1181-1184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Peek R. M., Jr, Miller G. G., Tham K. T., Pérez-Pérez G. I., Cover T. L., Atherton J. C., Dunn G. D., Blaser M. J. Detection of Helicobacter pylori gene expression in human gastric mucosa. J Clin Microbiol. 1995 Jan;33(1):28–32. doi: 10.1128/jcm.33.1.28-32.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. A., Tummuru M. K., Miller G. G., Blaser M. J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995 May;63(5):1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Bugnoli M., Ponzetto A., Morgando A., Figura N., Covacci A., Petracca R., Pennatini C., Censini S., Armellini D. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128 kilodalton protein (CagA) of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1993 Oct;12(10):739–745. doi: 10.1007/BF02098460. [DOI] [PubMed] [Google Scholar]