Abstract
We modeled the etiology of postmenopausal biology on ovarian cancer risk using germ cell-deficient white-spotting variant (Wv) mice, incorporating oncogenic mutations. Ovarian cancer incidence is highest in peri- and postmenopausal women, and epidemiological studies have established the impact of reproductive factors on ovarian cancer risk. Menopause as a result of ovarian follicle depletion is thought to contribute to higher cancer risk. As a consequence of follicle depletion, female Wv mice develop ovarian tubular adenomas, a benign epithelial tumor corresponding to surface epithelial invaginations and papillomatosis frequently found in postmenopausal human ovaries. Lineage tracing using MISR2-Cre indicated that the tubular adenomas that developed in Wv mice were largely derived from the MISR2 lineage, which marked only a fraction of ovarian surface and oviduct epithelial cells in wild-type tissues. Deletion of p27, either heterozygous or homozygous, was able to convert the benign tubular adenomas into more proliferative tumors. Restricted deletion of p53 in Wv/Wv mice by either intrabursal injection of adenoviral Cre or inclusion of the MISR2-Cre transgene also resulted in augmented tumor growth. This finding suggests that follicle depletion provides a permissive ovarian environment for oncogenic transformation of epithelial cells, presenting a mechanism for the increased ovarian cancer risk in postmenopausal women.
INTRODUCTION
Epidemiological evidence suggests that the risk of ovarian cancer is associated with the number of ovulatory events (1–3). Two major theories, namely, incessant ovulation (4, 5) and gonadotropin stimulation (6–8), have been postulated to explain the cancer risk association. The incessant ovulation hypothesis (4, 5) postulates that the repeated wounding and proliferative repairing of the ovarian surface epithelium results in mutations accumulating in the epithelial cells and ultimately in tumor formation. Supported by the same epidemiological evidence, the gonadotropin stimulation hypothesis (6–8) suggests that the surges of pituitary gonadotropins (FSH and LH) that initiate each ovulation also stimulate the ovarian surface epithelium and induce cell transformation. The role speculated for gonadotropins is also consistent with the fact that ovarian cancer occurs most frequently in postmenopausal women, when ovulation ceases yet plasma gonadotropins are elevated (1, 3, 9, 10). However, since these hormones have unremarkable effects on the growth of ovarian surface epithelial cells in culture and in mice, a direct impact of the hormones on ovarian epithelial cells is considered unlikely to be a critical causal factor (11, 12).
Presumably, successful modeling of ovarian cancer in mice will provide useful tools to investigate the mechanisms of these reproductive factors in ovarian cancer risk. In the past 2 decades, efforts have been made to develop genetic models of ovarian cancer in mice. First, a mouse model demonstrated that a combination of defined genetic changes, such as k-Ras, v-Akt, v-myc, or loss of p53, could transform mouse ovarian surface epithelial cells, which produced malignant tumors when reintroduced into the ovarian bursa of the mice (13). Expression of T antigen using the Mullerian-inhibiting substance receptor type 2 (MIS R II [MISR2], or anti-Mullerian hormone receptor type II [Amhr2]) promoter led to a transgenic line that developed malignant bilateral ovarian tumors (14). Intrabursa delivery of adenovirus Cre (Adv-Cre) to mediate deletion of both p53 and Rb in ovaries also resulted in the development of malignant ovarian epithelial tumors (15). Other mouse ovarian tumor models are endometriosis and endometrioid carcinomas following conditional expression of K-Ras and deletion of Pten in ovarian surface epithelial cells (16, 17) or by combining beta-catenin activation and Pten loss (18). As the recent findings that the origin of high-grade serous ovarian cancer may be derived from fallopian tube fimbria (19–22), simian virus 40T (SV40T) expression driven by the promoter of the mouse oviductal glycoprotein (OGP) was shown to produce tumors from the fallopian tube and other epithelial cells of the reproductive tract (23). Carcinomas derived from fallopian tube fimbria with morphology resembling high-grade serous ovarian cancer in humans developed in mice following Amhr2-Cre-mediated deletion of Pten and Dicer (24). Transformation of fallopian tube epithelia by deletion of BRCA1, Tp53, and Pten was demonstrated to model high-grade serous cancer in mice, adding to the support of a fallopian tube origin of serous ovarian cancer (25). Additional ovarian cancer animal models that are not mentioned here have also been produced and studied (26–28).
Despite these accomplishments, modeling of human serous ovarian cancer in mice has not successfully replicated the genotype and phenotype link, and p53 mutation alone in mice is not sufficient to produce ovarian serous tumors. Although p53 mutation is the only common genetic mutation in high-grade serous ovarian cancer (29), p53-null mice do not commonly develop ovarian cancer. Even when p53-null ovaries were transplanted into wild-type recipients to allow prolonged aging, the ovarian tumors that developed were of granulosa rather than epithelial origin (30). In several recent studies, concomitant inactivation of BRCA1 and Tp53 or Rb produced leiomyosarcomas, which likely originated from the ovarian bursa (31–33). Further investigation of these animal models will potentially lead to greater understanding of ovarian cancer development. Nevertheless, all of these models lack components related to the reproductive etiology of ovarian cancer.
A unique germ cell-deficient mouse line, the white spotting variant (Wv), recapitulates several aspects of human menopause and has been suggested for use in investigating the link between reproductive factors and ovarian cancer risk (34, 35). Wv female mice have a greatly decreased number of ovarian follicles due to a mutation in c-Kit that greatly reduces tyrosine kinase activity (36–39). At around 8 weeks of age, when wild-type littermates reach reproductive maturity, the Wv/Wv ovaries are depleted of follicles, and subsequently, ovarian tubular adenomas initiate (40, 41). These epithelial tumor cells are derived from the ovarian surface, and the majority of the lesions either exhibit inclusion cyst-like structures or resemble the surface deep invaginations/papillomatosis that is commonly observed in postmenopausal human ovaries (41, 42–44). Although dysplastic morphology is evident in some epithelial compartments of the tubular adenomas, the ovarian epithelial tubular structures in Wv mice are considered benign tumors, and the lesions are confined to ovarian tissues and do not become metastatic.
Previously, in an attempt to convert these benign lesions into more malignant tumors, we added a homozygous p53 deletion in Wv mice (45). However, global deletion of p53 did not enhance, but rather prevented, the development of tubular adenomas in the Wv/Wv; p53−/− mice. Investigation of the mechanism revealed that p53 deletion rescued and prolonged the survival of the ovarian follicles, which in turn suppressed the development of ovarian tubular adenomas (45). This surprising finding indicates that the presence of ovarian follicles suppresses the growth and remodeling of epithelial cells in the ovaries.
In the current study, we further tested the hypothesis that the synergy between oncogenic mutations and the postmenopausal ovarian environment can be achieved by combining the germ cell-deficient phenotype of the Wv/Wv mice and genetic alterations. Here, we investigated ovarian tumor phenotypes in Wv/Wv mice with additional p27 ablation or restricted p53 inactivation, which are commonly found in human ovarian cancer.
MATERIALS AND METHODS
Sources, breeding, and genotyping of compound-mutant mice.
All the mouse strains were kept in the C57BL/6 background. The Wv mouse (C57BL/6J-KitW-v) breeding pairs were originally obtained from Jackson Laboratory in 2002 and were maintained by inbreeding, as well as occasionally backcrossed with C57BL/6J mice in our mouse facility for the last 10 years. Several studies using the mice have been reported (34, 41, 46). The compound-mutant inbreeding colonies were established by crossing Wv/+ mice with p27+/−, p53+/−, or p53fl/fl (p53 allele/gene is flanked by loxP sites) mice. The founder pair of p27+/− mice was a kind gift from Andrew Koff via A. Di Cristofano (47). The following primer sets were used for PCR genotyping to amplify the wild-type and mutant p27 alleles: 5′-AGGTG AGAGT GTCTA ACGG-3′, 5′-AGTGC TTCTC CAAGT CCC-3′, and 5′-GCGAG GATCT CGTCG TGAC-3′. All three primers were used simultaneously in the PCR, yielding a 130-bp wild-type and/or 450-bp p27 mutant band. The p53+/− mice were purchased from Taconic (Hudson, NY) (48). The following primer sets that simultaneously amplify the wild-type and mutant p53 alleles were used for PCR genotyping: 5′-TGGTG CTTGG ACAAT GTGTT-3′, 5′-CTCCG TCATG TGCTG TGACT-3′, and 5′-GGATG ATCTG GACGA AGAGC-3′. A 450-bp PCR product indicated a wild-type allele, and a 650-bp product indicated a mutant p53 allele. The p53fl/fl pair (FVB; 129-Trp53tm1Brn, from the Mouse Models of Human Cancer Consortium [MMHCC] mouse repository, National Cancer Institute, Frederick, MD [http://mouse.ncifcrf.gov/]) (49, 50) was a gift from Denise Connolly (32). The following primer sets were used in PCR genotyping to amplify the wild-type and loxP-flanked intron 1 of the p53 allele: forward primer, 5′-CACAA AAACA GGTTA AACCA G-3′, and reverse primer, 5′-AGCAC ATAGG AGGCA GAGAC-3′, yielding a 288-bp band for the wild-type and a 370-bp band for the loxP-flanked intron 1 of p53, respectively. Another primer set was also used to amplify the wild-type and loxP-flanked intron 10 of the p53 allele: forward primer, 5′ AAGGG GTATG AGGGA CAAGG-3′, and reverse primer, 5′-GAAGA CAGAA AAGGG GAGGG-3′, yielding a 431-bp PCR product for the wild-type and a 584-bp product for the loxP-flanked intron 10. To confirm Cre-mediated excision of exons 2 to 10 of the p53 allele, the forward primer of intron 1 and the reverse primer of intron 10 were used in the PCR, yielding a 612-bp product, indicating the deletion of exons 2 to 10 of the p53 allele (df).
The founder pair of MISR2/Amhr2-Cre mice [strain B6; 129S7-Amhr2tm3(cre)Bhr (http://www.mmrrc.org/strains/14245/014245.html)] was obtained from the Mutant Mouse Regional Resource Centers (MMRRC) (51). The line contains an anti-Mullerian hormone type 2 receptor-targeted mutation by knock-in using the internal ribosome entry site (IRES)-Cre-pA FLP recombination target (FRT)-flanked Pgk-neo-bpA cassette introduced into exon 5 of the Amhr2 locus and was originally generated in the laboratory of Richard Behringer. The strain had a 129/SvEvBrd × C57BL/6J mixed genetic background with subsequent backcrossing to C57BL/6J mice. Generic Cre and LacZ primer sets were used to determine the genotype of these mice according to previously described protocols (51, 52). The ROSA26 Cre reporter mice for lineage-tracing experiments were obtained from Jackson Laboratory [strain B6.129S4-Gt(ROSA)26Sortm1Sor/J] (53). The ROSA26 mice contain a loxP-flanked sequence of a stop codon that can be removed by the presence of Cre expression to activate LacZ. All procedures in animal experimentation described were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Miami Miller School of Medicine.
Adenoviral delivery of Cre to ovarian surface epithelial cells.
At 7 to 8 weeks of age, female Wv/+; p53fl/fl mice were injected with Adv-Cre into the ovarian bursa to delete p53 in the ovarian surface epithelial cells; Adv-LacZ or Adv-green fluorescent protein (GFP) was used as a control. The protocol for adenovirus injection into the ovarian bursa is well established, and Adv-Cre injection has been successfully used in several mouse models of ovarian tumors recently (31, 54). The ovarian injection was performed following a mini-surgical procedure to gain access to the ovaries. In control experiments, we used Evans blue dye (Sigma) to monitor and demonstrate the successful delivery of the injected volume into ovarian bursa without leaking into the peritoneal cavity (see Fig. S1A and B in the supplemental material). Adv-Cre, Adv-LacZ, and Adv-GFP were purchased commercially (Vector Laboratory, Marion, IA). Using Adv-LacZ as a reporter for transfection following adenovirus injection, expression of beta-galactosidase was observed in cells of both the ovarian surface and the bursa inner layer of cells (see Fig. S1C to E in the supplemental material).
Preparation and culturing of primary ovarian epithelial cells from mutant mice.
Primary cultures of mouse ovarian surface epithelial (MOSE) cells were generated using a slightly modified protocol, as described previously (15, 31). Ovaries from adult wild-type and mutant mice were collected, washed with phosphate-buffered saline (PBS), and incubated in 0.25% trypsin solutions (Invitrogen) at 37°C for 1 h. Disassociated MOSE cells were then collected and cultured on 6-well plates previously coated with 0.1% gelatin in high-glucose Dulbecco's modified Eagle's medium (DMEM)–F-12 medium supplemented with 10% fetal bovine serum, 1 mM glutamine, 10 ng/ml epidermal growth factor, 500 ng/ml hydrocortisone, 5 μg/ml insulin, and 5 μg/ml transferrin. The growth of MOSE cells was analyzed using a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)-based WST-1 kit from Roche.
Western blotting, immunohistochemistry, and antibodies.
To prepare cell lysate, MOSE cells in monolayer culture were washed twice with cold PBS and immediately collected in radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris, pH 7.5, 50 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate). Immunoblotting was performed according to standard procedures. Primary antibodies used in the Western blotting included polyclonal antibodies to p27, p16, p19, cyclin-dependent kinase 2 (CDK2), CDK4, and cyclin A, and monoclonal antibodies to PCNA and cyclin E2 were from Santa Cruz; monoclonal antibodies for p21, E-cadherin, and N-cadherin were from BD Bioscience; monoclonal antibodies to β-actin were from Sigma; polyclonal antibodies to claudin 3 were from Invitrogen.
An immunohistochemistry procedure was performed to analyze paraffin-embedded tissues according to the standard procedure (41, 45). Antigen retrieval was performed by boiling the slides submerged in antigen retrieval buffer (Dako) for 20 min. The endogenous peroxidase activity was quenched by incubation with 3% H2O2 for 15 min. Primary antibodies used included monoclonal cytokeratin 8 (CK8) antibodies at 1:600, mouse monoclonal anti-inhibin α (Serotec) at 1:200, and anti-Ki67 antibodies (Dako) at 1:100. For CK8 and Ki67 staining, biotinylated polyclonal rabbit anti-rat bridging antibodies (Dako) at 1:500 dilution were used before horseradish peroxidase (HRP)-conjugated antibodies.
Analysis and quantitation of tumor phenotypes.
The areas of CK8-positive regions, as well as the sizes of whole ovarian tumors, were estimated on cross sections that reflected the maximal diameter of the ovaries from the mutant mice. Briefly, sections from various ovarian tumors were first stained with CK8 and counterstained with hematoxylin. Lower-magnification (×4) images at consistent resolutions were then taken to cover as much of the tumor regions as possible. Areas of the colorimetric development of diaminobenzidine (DAB) substrate were selected using ImageJ. The pixel numbers of the selected regions were automatically measured and converted to units of square micrometers based on the scale bars at the same magnification. The whole tumor area was also selected and quantified using the same methodology. CK8 staining in the adjacent oviduct epithelial cells was excluded from this measurement.
Statistical and analytical considerations.
Basic and standard analytical procedures were applied to examine the statistical significance of the data. Differences in proportions were evaluated by the χ2 or Fisher exact test, as appropriate. Student's t test was used to compare the differences in means between two groups. Statistical significance was considered a P value of <0.05.
RESULTS
Ovarian and oviduct epithelia of mixed MISR2 and non-MISR2 lineages.
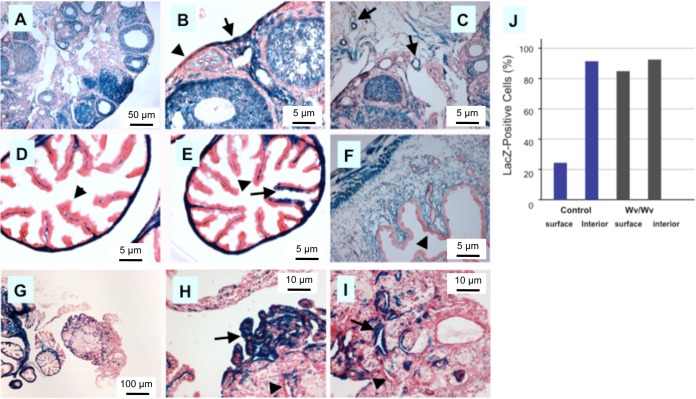
Previously, we found that the tubular adenomas in Wv mice were derived from ovarian surface epithelia (41), as well as the remains of the Mullerian duct, such as rete ovarii in the interior regions of the mouse ovary (35, 46). Here, we determined if these tumor cells were derived from a Mullerian lineage that could be traced by the temporal expression of MISR2, also known as Amhr2. First, using MISR2-Cre (51) in a ROSA26 Cre reporter background, we tracked the LacZ-positive cells in the reproductive tissues of females (Fig. 1). In the ovary, MISR2-Cre marked the ovarian follicles and stroma (Fig. 1A). The ovarian surface epithelial cells were largely negative, although some were mosaic for positive LacZ staining (Fig. 1B; the arrow indicates positive, and the arrowhead indicates negative), and epithelial components (rete ovarii) within the interior of the ovary were generally positive for LacZ staining (Fig. 1C, arrow). The stroma of both the oviduct (Fig. 1D and E) and uterus (Fig. 1F) were positive, though the epithelial linings generally lacked LacZ staining (Fig. 1D to F; the arrowheads indicate negative LacZ staining). Upon examination of multiple cuttings, however, some LacZ-positive epithelial cells lining the oviduct were detected (Fig. 1E; the arrow indicates epithelial cells with positive LacZ staining). Thus, consistent with previous findings (51), MISR2 expression is induced in stroma but mostly absent in epithelial cells. Nevertheless, we did find mosaic patterning of a smaller number of LacZ-positive epithelial cells within the epithelia, and we conclude that a fraction of both surface and oviduct epithelial cells are derived from the MISR2 lineage (Fig. 1B and E; see Fig. S2 in the supplemental material).
FIG 1.
MISR2 lineage tracing in ovarian and oviduct epithelia in wild-type and tubular adenomas of Wv/Wv mice. ROSA26 mice were crossed with Wv mutant and MISR2-Cre transgenic mice. At 3 to 4 months of age, the obtained progeny, including Wv/Wv; ROSA26; MISR2-Cre and ROSA26; MISR2-Cre or Wv/+; ROSA26; MISR2-Cre (as a control) genotypes, were analyzed for LacZ expression as a reporter of MISR2 lineage. More than 5 mice from each genotype were analyzed, and representative results are shown. (A) Typical example of LacZ staining of an ovary from a ROSA26; MISR2-Cre mouse. (B) Higher magnification of the ovary in panel A with both LacZ-positive (arrow) and -negative (arrowhead) ovarian surface epithelial cells. (C) Higher magnification of the ovary in panel A. Epithelial cysts located in the interior of the ovary were often LacZ positive, as shown in the examples (arrow). (D) Typical example showing oviduct fimbria of a ROSA26; MISR2-Cre mouse, where the stromal cells were LacZ positive and the epithelial cells were largely negative (arrowhead). (E) Rare LacZ-positive fallopian tube fimbrial epithelial cells (arrow) among largely LacZ-negative cells (arrowhead) in a ROSA26; MISR2-Cre mouse. (F) Typical example showing LacZ staining of uterine horns of a ROSA26; MISR2-Cre mouse, where the stroma was LacZ positive and the epithelial cells were largely negative (arrowhead). (G) Typical example of LacZ staining of an ovary and associated uterine horn and oviduct from a 3-month-old Wv/Wv; ROSA26; MISR2-Cre mouse. (H) Higher magnification of the ovary in panel G. Tubular adenomas located near the ovarian surface were largely LacZ positive (arrow) and mixed with some LacZ-negative cells (arrowhead). (I) Higher magnification of the ovary in panel G. Epithelial cysts and tubular adenomas located in the interior of the ovary were often LacZ positive (arrow). An example showing LacZ-negative cells is indicated by the arrowhead. (J) Based on the analysis of at the least 5 cases in each category, the percentages of LacZ-positive epithelial cells located on the surface or in the interior were estimated for control (ROSA26; MISR2-Cre or Wv/+; ROSA26; MISR2-Cre) and Wv/Wv (Wv/Wv; ROSA26; MISR2-Cre) mice.
Based on histology, the ovarian tubular adenomas formed in Wv/Wv mice are thought to derive from both surface epithelia and interior epithelial components, including rete ovarii (35, 41). We used MISR2-Cre to trace the origin of the tubular adenomas in Wv mice. Upon analysis of multiple 3- to 6-month-old Wv/Wv; MISR2-Cre; ROSA26 mice, we found that the majority of surface and interior components of the tubular adenoma were LacZ positive (Fig. 1G to I). Based on results from examining three wild-type ovaries and six Wv/Wv tumors, we estimated that 23% of ovarian surface epithelial cells and 92% of interior epithelia were LacZ positive in normal ovaries (heterozygous), while 85% and 93% of surface and interior epithelial cells, respectively, were traced to the MISR2 lineage in the tubular adenomas of follicle-depleted Wv/Wv ovaries (Fig. 1J). These results suggest that the MISR2-labeled Mullerian epithelial cells have a greater capacity for proliferation and expansion when follicles are depleted in the ovaries and the MISR2-derived cells constitute the majority of the tubular adenomas developed.
p27kip1 deficiency enhances growth of Wv/Wv ovarian tumors.
Cyclin-dependent kinase inhibitor 1B (p27kip1, or p27) is a well-established cell cycle inhibitor and tumor suppressor. Reduced p27kip1 expression is commonly found in human malignancies, and its expression predicts the prognosis in human ovarian cancer (55–60). Thus, p27 loss is likely a driving force for ovarian cancer development. Both p27 heterozygous and homozygous mutant mice are predisposed to the development of several tumor types, suggesting p27 is a haploinsufficient tumor suppressor (47, 61, 62). Indeed, in the colonies maintained in our facility, we occasionally observed ovarian epithelial tumors in mice with a p27 mutation alone (see Fig. S3 in the supplemental material). For compound mutant mice, we first tested the effect of the addition of p27 deficiency to the benign ovarian epithelial tumor phenotypes of Wv/Wv mice (35, 41). Because both p27−/− and Wv/Wv mice are infertile, we crossed Wv/+ mice with p27+/− mice and subsequently mated Wv/+; p27+/− mice to produce Wv/Wv; p27+/− and Wv/Wv; p27−/− mice for analysis. Over a period of 2 years, approximately 50 Wv/Wv; p27+/− and 20 Wv/Wv; p27−/− female mice were collected, aged, and analyzed for ovarian tumor phenotype.
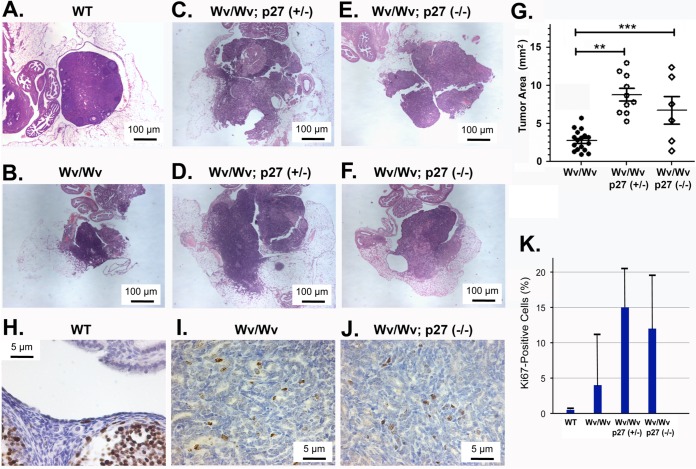
Deletion of either one or both copies of the p27 gene led to enlarged ovarian tumors compared to those of Wv/Wv mice, as shown in the histology of several representative tumors (Fig. 2). Generally, the ovarian tubular adenomas were bilateral, with comparable sizes within an individual compound-mutant mouse. Overall, the sizes of the tubular adenomas were statistically larger than those of Wv/Wv mice (Fig. 2A to G). Somewhat unexpectedly, the Wv/Wv; p27+/− ovaries appeared to have a tumor phenotype similar to, if not more prominent than the tubular adenomas of the Wv/Wv; p27−/− ovaries. The loss of one p27 allele significantly increased the size of CK8-positive epithelial tumors, from an average of 2.73 × 105 ± 0.32 × 105 μm2 in Wv/Wv ovaries to 8.78 × 105 ± 0.82 × 105 μm2 (P < 0.0001), measured using the cross section that contained the maximal diameter of the ovarian tumors. Homozygous knockout of p27 in Wv/Wv ovaries led to a similar increase in tumor area, to 6.77 × 105 ± 1.81 × 105 μm2 (Fig. 2J). In wild-type ovaries, proliferative marker Ki67-positive cells were mostly follicular granulosa but rarely epithelial cells (Fig. 2H). A small fraction (<5%) of tubular adenoma cells became Ki67 positive in Wv/Wv ovaries (Fig. 2I), and reduction or loss of p27 greatly increased the fraction of Ki67-positive tumor cells (Fig. 2J), as quantitated (Fig. 2K). The observed oncogenic effects of the p27 heterozygous deletion were consistent with the findings that p27 acts as a haploinsufficient tumor suppressor (62).
FIG 2.
Enhanced growth of tubular adenomas in Wv/Wv; p27+/− and Wv/Wv; p27−/− mice. Hematoxylin and eosin (H&E)-stained slides showing representative ovarian and tumor morphology from 4- to 6-month-old mice with Wv and p27 mutant genotypes. (A) Typical example of an ovary enclosed in a bursa and associated oviduct from a wild-type mouse. (B) Typical ovarian tubular adenoma from a 4-month-old Wv/Wv mouse. (C and D) Examples of ovarian tumors from Wv/Wv; p27+/− mice. (E and F) Examples of ovarian tumors from Wv/Wv; p27−/− mice. (G) Tumor sizes (areas) were estimated and are shown as means with standard deviations, using Zeiss Axiovision software, from slides near the middle section (with the greatest width) of ovarian tumors. P values were calculated by a nonpaired Student t test. **, P < 0.0001; ***, P < 0.001. (H) Example of Ki67 staining of a wild-type ovary. (I) Example of Ki67 staining of a Wv/Wv ovarian tubular adenoma. (J) Example of Ki67 staining of a Wv/Wv; p27−/− ovarian tubular adenoma. (K) Based on the analysis of at the least 5 cases in each category, the percentages of Ki67-positive epithelial cells among either ovarian surface or tubular adenoma epithelial cells were estimated for wild-type (WT), Wv/Wv, Wv/Wv; p27+/−, and Wv/Wv; p27−/− mice and are shown as averages and ranges. The increases in Ki67 cells were statistically significant (P < 0.005) in comparisons between wild-type and Wv/Wv, Wv/Wv; p27+/−, and Wv/Wv; p27−/− mice. The difference between Wv/Wv and Wv/Wv; p27+/− or Wv/Wv; p27−/− mice was also significant, but no significant difference was found between Wv/Wv; p27+/− and Wv/Wv; p27−/− mice.
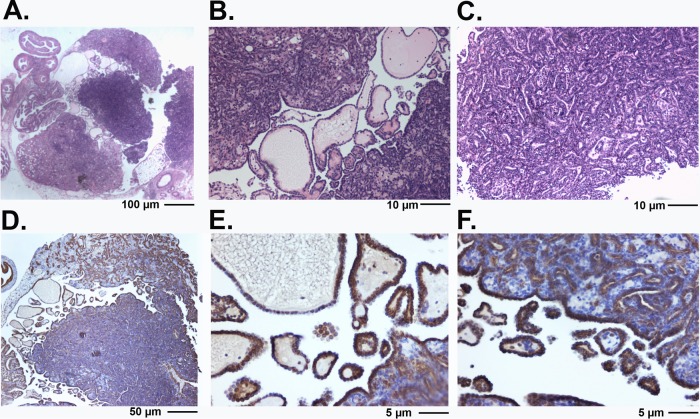
The morphology of tumor lesions in both Wv/Wv; p27−/− and Wv/Wv; p27+/− ovaries became more complex. A representative papillary ovarian tubular adenoma from an 8-month-old Wv/Wv; p27+/− mouse is shown in detail in Fig. 3. CK8-positive epithelial cells infiltrated the entire ovary, as well as the bursa and surrounding areas (Fig. 3D to F). These tumor lesions resembled borderline ovarian cancer or severe ovarian surface papillomatosis. Nevertheless, the epithelial lesions of the Wv/Wv; p27+/− and Wv/Wv; p27−/− ovarian tumors were benign in appearance, resembling the original tubular adenomas of the Wv/Wv ovarian tumors, and significant mitotic figures were absent. In aged Wv/Wv; p27+/− mice, large ovarian tumors developed, infiltrated, and occupied the entire para- and meso-ovarian regions (Fig. 4). Mortality (sudden death) was frequently observed in the tumor-bearing Wv/Wv; p27+/− mice but not in Wv/Wv or p27+/− mice. However, we examined all the mice following a planned time course up to 12 months of age and did not use death as an endpoint for the analysis of these ovarian-tumor-bearing mice.
FIG 3.
Morphology of ovarian tubular adenomas in Wv/Wv; p27+/− mice. (A) H&E staining of a large ovarian tubular adenoma from an 8-month-old Wv/Wv; p27+/− mouse. The tumor is papillary and resembles a serous borderline ovarian tumor. (B) Higher magnification of the tumor in panel A showing the presence of papillary adenoma morphology. (C) Higher magnification of the tumor in panel A showing the crowding of epithelial tubular gland structures. (D) The same tumor stained with cytokeratin 8. (E and F) Cytokeratin 8 staining of the tumor shown at higher magnification.
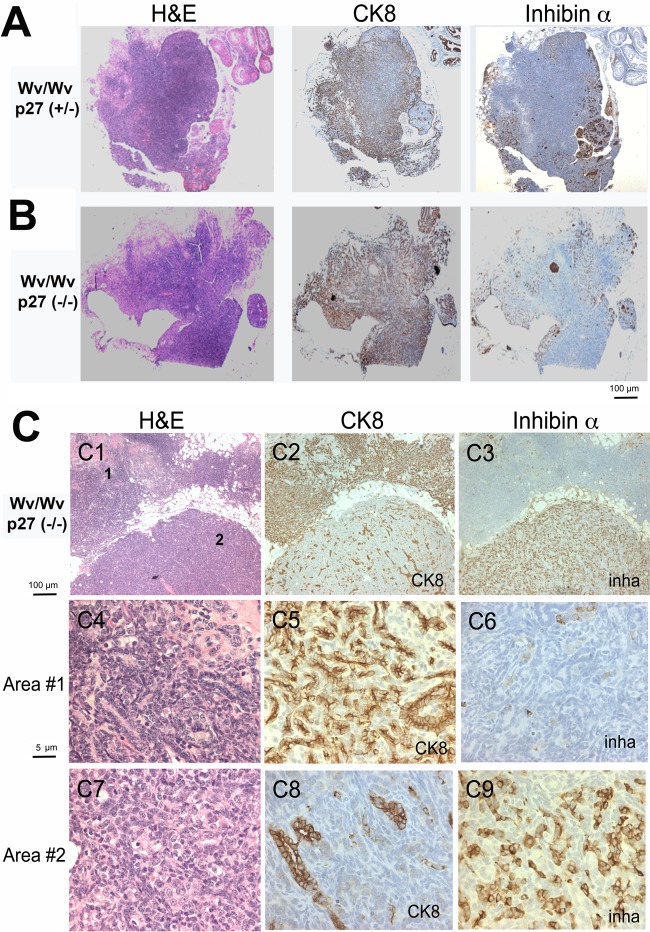
FIG 4.
Inverse correlation between the presence of granulosa cells and epithelial lesions in Wv/Wv; p27+/− and Wv/Wv; p27−/− ovaries. (A) Representative tissue sections stained with H&E, the granulosa cell marker inhibin α, and the epithelial marker CK8 in adjacent sections of ovarian tubular adenomas from a 12-month-old Wv/Wv; p27+/− mouse. (B) Staining of a representative ovarian tubular adenoma from a 12-month-old Wv/Wv; p27−/− mouse. (C) An ovarian tubular adenoma from an 8-month-old Wv/Wv; p27−/− mouse was analyzed in more detail (C1 to C3) for H&E, CK8, and inhibin α (inha) staining. Images at higher magnification (numbered in panel C1) are shown for area 1, which is CK8 positive (C4, H&E; C5, CK8; C6, inhibin α), and area 2, which is inhibin α positive (C7, H&E; C8, CK8; C9, inhibin α).
The presence of epithelial tubular adenoma is inversely correlated with inhibin α expression in p27-deficient Wv/Wv ovarian tumors.
Previously, we had observed that in ovarian tubular adenomas from Wv/Wv mice, the presence of inhibin α-positive granulosa cells was inversely correlated with the presence of cytokeratin-positive epithelial cells (45). Here, we found that the same correlation existed in the ovarian tumors from Wv/Wv; p27+/− and the Wv/Wv; p27−/− mice (Fig. 4), which developed much larger ovarian tumors. In all ovarian tumors from either Wv/Wv; p27+/− or Wv/Wv; p27−/− mice, CK8-positive epithelial lesions, mostly presenting as infiltrating adenomas, occupied the ovary and surrounding tissues. These tumors also contained patches of inhibin α-positive granulosa cells scattered in the tissues. In all of these ovarian tumors, the tumor mass was largely epithelial (CK8 positive) and often contained a minor component (10% or less) of granulosa cells (inhibin α positive) (Fig. 4). These granulosa cells were likely the remnants of the degenerated follicles. In 18 cases of tumors analyzed, we observed that, within a tumor where CK8-positive staining was sparse, inhibin α staining was positive in the same area of an adjacent section, and areas that were cytokeratin 8 positive were inhibin α negative (Fig. 4). An example of a Wv/Wv; p27−/− tumor is shown in detail in Fig. 4C: tumor area 1 is cytokeratin 8 positive and inhibin α negative (Fig. 4C, C4 to C6), and tumor area 2 is inhibin α positive while the growth and expansion of the cytokeratin 8-positive epithelial glands appears restricted (Fig. 4C, C7 to C9). Thus, a strict inverse correlation between cytokeratin 8 and inhibin α exists in the tumors from these Wv/Wv; p27+/− and Wv/Wv; p27−/− mice. The inverse correlation supports the suggestion that a paracrine factor(s) produced by follicles or granulosa cells suppresses epithelial growth in ovaries (45).
Restricted p53 deletion in ovarian surface epithelial cells augments ovarian tumor growth in Wv/Wv mice.
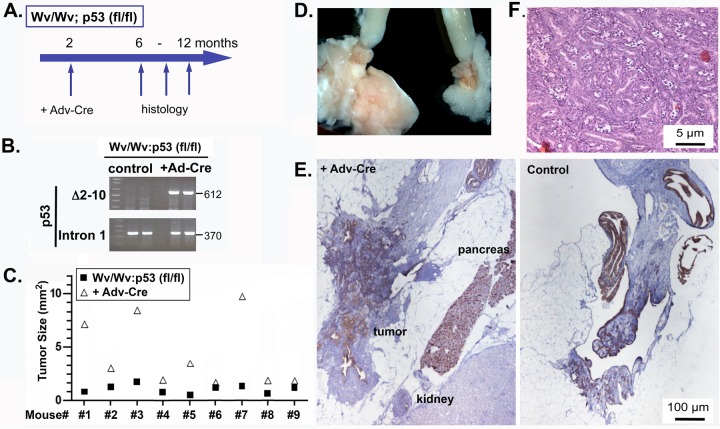
Since global deletion of p53 suppressed the development of ovarian tubular adenomas in the Wv/Wv mice (45), we explored another strategy for a restricted p53 deletion in ovarian epithelial cells using a flox-p53 line. By deleting p53 in ovarian surface epithelial cells through injection of adenovirus carrying a Cre transgene, we established an ovarian mouse model referred to as Wv/Wv; p53fl/fl; Adv-Cre, mimicking both reproductive factors (postmenopause) and genetic inactivation (p53) in human serous ovarian cancer. Female Wv/Wv; p53fl/fl mice were readily generated from Wv/+; p53fl/fl parents and were collected for the experiments. Using a mini-surgical procedure to gain access (see Fig. S1A and B in the supplemental material), Adv-Cre was injected into the ovarian bursa of 2-month-old Wv/Wv; p53fl/fl mice, at a time when ovarian follicles were nearly depleted and tubular adenoma formation had just started (Fig. 5A). The adenovirus (Adv-Cre) was injected on one side only, and the noninjected (or injected with Adv-LacZ or Adv-GFP as controls in some cases) ovary from the same mouse was used for comparison. Using Adv-LacZ as a reporter, this procedure was efficient at expressing the transgene carried by the adenoviral vector, and both the ovarian surface and, to a lesser extent, the inner epithelial lining of the ovarian bursa were transfected by the adenoviral vector (see Fig. S1C and D in the supplemental material). This procedure was also sufficient to transfect the ovarian surfaces of Wv/Wv mice at 2 months of age, when the ovaries were much smaller than those of the wild type and surface epithelial proliferation and the formation of tubular adenoma were about to start (see Fig. S1E in the supplemental material). The procedure of injection of Adv-Cre into ovarian bursa was able to delete the p53 gene in the Wv/Wv ovaries, and cells with p53 deleted persisted in the ovarian tumors, as detected by PCR genotyping of the DNA extracted from the ovarian tumors 4 months after infection with Adv-Cre (Fig. 5B). The mice were sacrificed 4 to 10 months after Adv-Cre injection to analyze for development of ovarian tumors. Adv-Cre-mediated p53 gene deletion generally enhanced the growth of ovarian tumors in the Wv/Wv mice (Fig. 5C; see Fig. S4 in the supplemental material), while p53 deletion did not cause obvious changes in control (Wv/+; p53fl/fl) ovaries (see Fig. S5 in the supplemental material), suggesting follicle depletion is required for tumor growth prompted by p53 inactivation. Comparison of the two ovaries from the same Wv/Wv; p53fl/fl mouse showed that Adv-Cre injection enlarged the ovarian tumors to various degrees, ranging from minimal to 10-fold (Fig. 5C). Adv-Cre-injected ovaries commonly contained cysts of hemorrhage appearance (see Fig. S4 in the supplemental material), and CK8 staining indicated that these tumors were largely epithelial in origin (see Fig. S6 in the supplemental material). Nevertheless, in most cases, the tumors were still restricted to the ovaries, and p53 deletion did not lead to metastasis or spreading of the tubular adenomas in the Wv/Wv mice.
FIG 5.
Ovarian tumors in Wv/Wv; p53fl/fl; Adv-Cre mice. (A) Schematic of the experimental protocol, in which 2-month-old Wv/Wv; p53fl/fl female mice were injected with Adv-Cre in the ovarian bursa of one side; the mice were then analyzed 4 to 10 months later (at 6 to 12 months of age). (B) DNA extracted from the ovarian or tumor tissues was used for PCR genotyping for the wild-type p53 allele and the delta floxed (df) allele (with deletion of exons 2 to 10). Adv-LacZ or Adv-GFP was used in the controls. (C) Tumor sizes were quantified based on a histological slide near the widest cross section of the ovary/tumor. The ovary/tumor pairs from injected and uninjected control sides were plotted for comparison. The difference between the two groups was statistically significant based on paired two-tailed Student t tests (P < 0.001). (D) Typical gross morphology of ovaries and uterine horns of a 10-month-old Wv/Wv; p53fl/fl; Adv-Cre mouse. The left ovary was injected with Adv-Cre. (E) Sections of a typical pair from uninjected or Adv-Cre-injected ovarian tissue or derived tumors were stained with CK8. The control (uninjected) ovary is shown for comparison. (F) H&E staining showing the tumor cell morphology of the ovarian adenoma from a 10-month-old Wv/Wv; p53fl/fl; Adv-Cre mouse.
In 3 out of 12 cases, aggressive tumors resulted from the Wv/Wv; p53fl/fl; Adv-Cre mice. The injected ovary developed into a tumor mass fused with surrounding tissues (Fig. 5D; see Fig. S7 and S8 in the supplemental material), and tumor lesions histologically adjacent to the pancreas were identified (Fig. 5E). In these tumors, the ovarian bursa was not recognizable and the tumor lesions occupied the entire space and spread along the connective tissues. As a result, the core tumor masses surgically dissected contained surrounding organs (Fig. 5E; see Fig. S7 and S8 in the supplemental material). Thus, we were able to induce ovarian tumors using a single oncogenic mutation (p53 deletion) in these germ cell-deficient mice, modeling the genotype of human serous ovarian cancer (29).
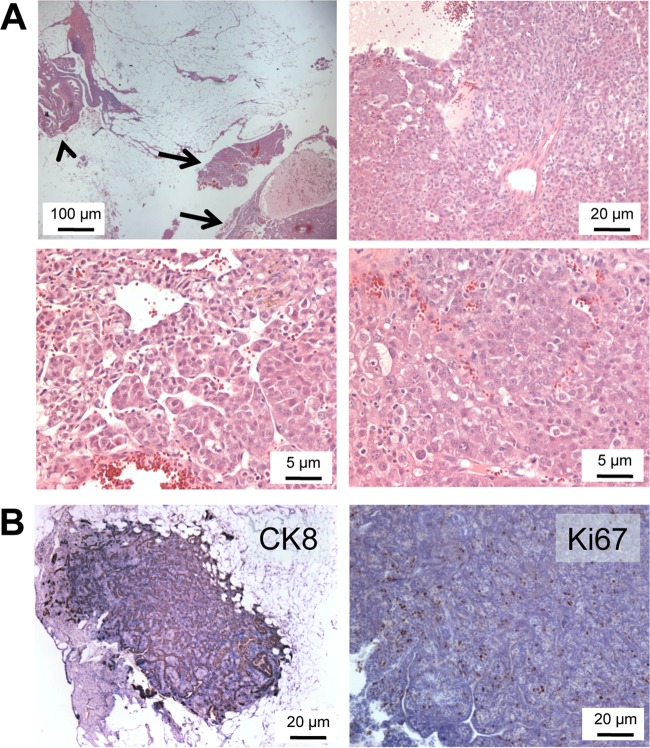
Restricted p53 deletion through MISR2-Cre produces epithelial and granulosa cell ovarian tumors in Wv/Wv mice.
To test if the MISR2-derived epithelial cells were capable of producing ovarian tumors and to explore an alternative approach for ovarian gene deletion other than the surgical procedure and injection of adenoviral vectors, the MISR2-Cre transgene was used to mediate p53 gene deletion in ovaries of Wv/Wv mice. With several steps of crossing, we produced Wv/Wv; p53fl/df; MISR2-Cre mice for analysis, expecting p53 deletion in ovarian tubular adenomas, since we had shown that these tumor cells were mostly derived from the MISR2 lineage. In 6 mutant mice analyzed at an age of 3 to 12 months, ovarian tumors were observed in all cases, as shown by examples at various magnifications (Fig. 6A). Generally, the tumors were bilateral and larger than those of Wv/Wv ovaries at the same stages. Many of the tumors were entirely positive for cytokeratin 8, suggesting epithelial origin, and also were highly Ki67 positive, indicating active proliferation (Fig. 6B). However, about half of the Wv/Wv; p53 fl/df; MISR2-Cre ovaries contained mixed epithelial and granulosa cell tumors (see Fig. S9 in the supplemental material), suggesting MISR2-Cre-mediated p53 deletion occurred in both epithelial and granulosa cells. Since MISR2-Cre is expressed in rete ovarii and in only a fraction of ovarian surface epithelial cells, the epithelial tumors were likely generated from both these sources, and p53 deletion also promoted tumorigenesis in the granulosa cells that likely were derived from degenerated follicles in the Wv/Wv ovaries. In the limited number of mutant mice produced and analyzed, we have not observed lesions originating from oviduct fimbria, where p53 deletion might have occurred in the fraction of epithelial cells derived from the MISR2 lineage.
FIG 6.
Ovarian tumors in Wv/Wv; p53fl/fl; MISR2-Cre mice. (A) Typical morphology of an ovarian tumor from a 12-month-old Wv/Wv; p53fl/fl; MISR2-Cre mouse with H&E staining at various magnifications. The tumor (arrows) was located adjacent to oviduct fimbria (arrowhead). (B) Representative images of immunostaining of ovarian tumors from Wv/Wv; p53fl/fl; MISR2-Cre mice for CK8 and Ki67.
Cell proliferation is limited by compensatory regulation of cyclin inhibitors.
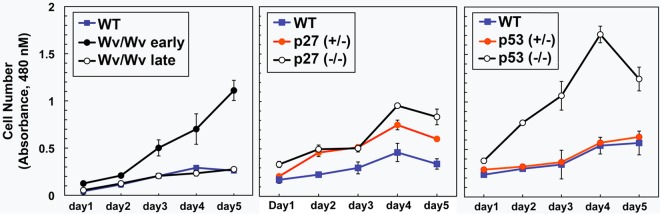
We examined the effects of p27 and p53 deletion on the growth and signaling of primary ovarian surface epithelial cells in culture. Compared to the wild type, ovarian epithelial cells isolated from Wv/Wv mice had an increased proliferation rate in culture at their early passages, though the growth rate returned to the basal state after culturing for a few more passages (Fig. 7). Both heterozygous and homozygous deletions of p27, but only homozygous p53 deletion, enhanced cell proliferation (Fig. 7).
FIG 7.
Increased proliferation of p27−/− and p53−/− mouse ovarian surface epithelial cells in culture. Primary ovarian surface epithelial cells were prepared from ovaries of mice with various genotypes. The growth rates of wild-type, Wv/Wv (early [passage 2] and late [passage 7] passages), p27+/−, p27−/−, p53+/−, and p53−/− primary ovarian surface epithelial cells were measured by WST-1 assay (n = 3) from days 1 to 5. The results are shown as means ± standard deviations (SD). The differences were found to be statistically significant between p27+/− or p27+/− and wild-type and between p53−/− and wild-type or p53+/− cells.
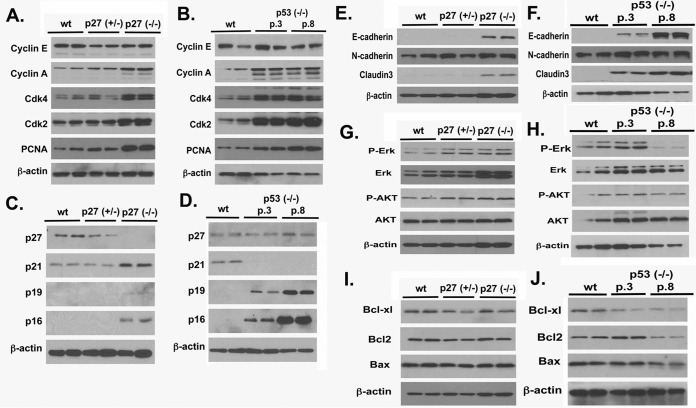
We also determined the cell cycle parameters of the cells in culture. Homozygous deletion of p27 and p53 increased cyclin A, Cdk4, Cdk2, and PCNA (Fig. 8A and B). Homozygous p27 deletion induced a compensatory increase in the expression of other cyclin-dependent kinase inhibitors, such as p21, p19 (slightly), and p16, though heterozygous deletion did not (Fig. 8C). p53 deletion led to the loss of p21 and an increase in p19 and p16 but not p27; the increases in p19 and p16 were greater in later passages (Fig. 8D). Deletion of p27 and p53 also caused an increase in the cell adhesion proteins E-cadherin and claudin 3 (Fig. 8E and F). Lastly, deletion of p27 or p53 did not significantly impact the activation of Erk1/2 and Akt (Fig. 8G and H) or the expression of the apoptotic regulators Bcl-XL, Bcl2, and Bax (Fig. 8I and J). Thus, loss of p27 or p53 likely impacts cell growth through the expression of cyclin inhibitors but does not significantly affect signaling or apoptosis. We reason that addition of p27 or p53 deletion augmented the ovarian adenomas in the Wv/Wv mice by enhancing tumor cell proliferation, though impacts on malignant properties were not apparent.
FIG 8.
Analysis of markers in p27+/−, p27−/−, and p53−/− mouse ovarian surface epithelial cells. Primary ovarian surface epithelial cells were prepared from ovaries of 3-month-old mice with wild-type, p27+/−, p27−/−, p53+/−, and p53−/− genotypes. Early (passage 3) and late (passage 8) p53−/− cells were used. The cells were analyzed for various markers by Western blotting. (A) Cell cycle and proliferation markers were analyzed in wild-type (wt), p27+/−, and p27−/− cells. (B) Cell cycle and proliferation markers were analyzed in wild-type and early (passage 3 [p.3]) and late (passage 8) passages of p53−/− cells. (C and D) Cyclin inhibitors. (E and F) Epithelial-to-mesenchymal transition markers. (G and H) Markers of cellular signaling. (I and J) Apoptosis and cell survival markers. The aligned panels of images from the immunoblots were derived from the same cell lysate, but more than one gel was used to obtain all the images. Thus, the beta-actin loading controls are representative but not necessarily from the same gel.
DISCUSSION
Modeling serous ovarian cancer in mice with genetic mutations found in human cancer remains a challenge, and how genetic events lead to the development of ovarian cancer has not been satisfactorily established. Here, we attempted to model epithelial ovarian cancer in mice by using an oncogenic mutation(s) to convert benign ovarian epithelial tubular adenomas to more malignant tumors. We were able to enhance tumor development by adding p27 or p53 deficiency, resulting in larger and more proliferative tubular adenomas that often resemble human borderline serous tumors or florid papillomatosis. Thus, we achieved the modeling of human ovarian epithelial tumors by introducing just a single oncogenic mutation into germ cell-deficient ovaries (mirroring a postmenopausal state), although the tumors were not overtly malignant.
Mutation of p27 is rare in cancer, though reduced or cytoplasmic mislocation of p27 is common (55, 60). Both p27-null and heterozygous mutant mice often develop tumors, indicating p27 is a haploinsufficient tumor suppressor (61, 62). Reduction of p27 expression predicts the ovarian prognosis in human ovarian cancer (55–60). In the current study, we found that both null and heterozygous p27 loss increased the development of ovarian epithelial tumors in Wv/Wv mice.
p53 inactivation is the only common genetic mutation found in high-grade ovarian serous cancer (29). However, in previous modeling attempts, p53 deletion by adenovirus delivery of Cre in p53 floxed mice often produced stromal tumors rather than epithelial tumors (31–33). In the previous works, combinations of two or more oncogenic mutations were needed to produce epithelial ovarian tumors in mice. Here, we were able to produce large ovarian epithelial tumors with p53 deletion alone in Wv/Wv mice. In control experiments using Wv/+ mice, deletion of p53 in ovarian epithelial cells either by adenoviral delivery of Cre or by crossing into MISR2-Cre mice did not cause significant growth or tumors of the ovaries. This result supports the idea that p53-stimulated proliferation of ovarian epithelial cells is restrained in follicle-containing ovaries and that ovarian epithelial cells are capable of proliferation only when follicles are depleted, such as in Wv/Wv mice after 2 months of age.
Analysis of ovarian epithelial cells in culture suggested that loss of p27 or p53 did not significantly affect signaling or apoptosis; rather, the mutations impacted cell growth through the expression of a cyclin inhibitor, p27 or p21, respectively. The alteration of the cell cycle is consistent with that found in human ovarian cancer (59, 63–65). Loss of either p53 or p27 in the cells induced compensatory expression of cyclin inhibitors, p16 and p19. This may be one reason for the still limited tumor aggressiveness we found occurring with either p27 or p53 deletion in the Wv/Wv mice.
Although the growth of ovarian epithelial tumors in the Wv/Wv mice was enhanced, often involved adjacent tissues, and caused fatalities, the histology and cytology of the tumors were not overtly malignant compared to human high-grade serous ovarian carcinomas. Several potential reasons were considered. First, serous cancer may originate from fallopian tube fimbria rather than ovarian surface epithelial cells. Although, Adv-Cre-induced p53 deletion also occurred in the fallopian tube epithelial cells in our procedure, the gene deletion was not as efficient as in ovarian surface epithelial cells, and no tumors or lesions from fallopian tube fimbria were observed. Additionally, p53 gain of function by point mutation may be critical for tumor malignancy, but the mouse models here used only a loss-of-function allele. The analysis in cell culture also indicated that the growth of the tumors likely was restrained by a compensatory mechanism in the cell cycle and expression of cyclin inhibitors. Lastly, chromosomal instability, in addition to p53 deletion, may be the driving force, since unique patterns of chromosomal loss occur in high-grade cancer (29). Thus, we speculate that additional chromosomal instability may be needed to promote the tumor cells to high-grade serous tumors. Limitations of using mice to model pathology, especially ovarian cancer, in humans include differences in life span, tissue scale, and anatomy. Preneoplastic microscopic lesions may persist for many years to develop fully genomic changes and aneuploidy in human patients while remaining undetected prior to diagnosis. They would be difficult to replicate in mice in the current laboratory setting.
For many years, ovarian surface and inclusion cyst epithelial cells were thought to be the cells of origin for epithelial ovarian cancer, of which high-grade serous cancer is the major histological subtype (66–68). Considering their possible Mullerian origin, an alternative idea is that epithelial cells of Mullerian remnants in ovaries or surrounding tissues, such as the rete ovarii epithelia, may be the true precursor cells of serous ovarian cancer (69, 70). Furthermore, in recent years, the cell of origin question has been reassessed, and now it is commonly accepted that the epithelial cells of the fallopian tube fimbria are the precursors of many or even the majority of ovarian serous cancers (19–22). Using MISR2-Cre as a marker for lineage tracing, we found that rete ovarii and only a small fraction of ovarian surface and oviduct fimbria epithelial cells are derived from the Mullerian lineage. Nevertheless, these Mullerian-derived ovarian surface epithelial cells have higher potential for growth and expansion to form tumors in the Wv ovaries, and these cells may be the precursors of ovarian serous cancer. Indeed, MISR2 expression is commonly detectable in ovarian cancer cell lines and primary cancer ascites cells (71), and the ovarian cancer cells are often responsive to recombinant Mullerian inhibitory substance (MIS) (72, 73).
The view that the loss of ovarian function and tissue homeostasis is a risk factor for ovarian cancer has been previously considered (74, 75). Overall, the data reported here support the idea that a reproduction-competent ovary offers an environment that limits proliferation of epithelial cells (45). However, when follicles are depleted, such as in a postmenopausal state, the constraint on ovarian epithelial cell proliferation is rescinded, particularly for the epithelial cells derived from the MISR2 lineage. Thus, in postmenopausal ovaries, epithelial cells of the ovarian surface, cysts, or rete ovarii become proliferative. The postmenopausal ovarian environment may also be permissive for implantation and growth of epithelial cells derived from fallopian tube fimbria.
In this study, we demonstrated that the addition of oncogenic mutations, such as loss of p27 or p53, promotes ovarian tumor development from the benign adenomas of germ cell-deficient ovaries (see Fig. S10 in the supplemental material). Otherwise, these oncogenic mutations have little impact on epithelial growth of ovaries containing follicles. The results establish that the postmenopausal ovarian environment is permissive for epithelial proliferation and transformation by oncogenic mutations, and the study offers a mechanistic explanation for the etiology of ovarian cancer risk in menopause.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the gift of a founder pair of p27 knockout mice from Andrew Koff via A. Di Cristofano (Fox Chase Cancer Center) and a breeding pair of p53fl/fl mice from Denise Connolly (Fox Chase Cancer Center). The project was started at Fox Chase Cancer Center (Philadelphia, PA) and was continued at the Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine (Miami, FL). Over the years, several present and prior laboratory members contributed work related to the project, including Dong-Hua Yang, Wensi Tao, Jennifer Smedberg, Malgorzata Rula, and several summer work students. We also thank our present and past laboratory members for discussion during the course of the project and reading of and suggestions and comments on the manuscript.
The work was supported by grants R01 CA095071, CA079716, and CA075389 from NCI, NIH, and also a grant from the Department of Defense (DOD) (W81XWH-06-1-0095; Ovarian Cancer Research Program Idea Award, A Mouse Model to Investigate Postmenopausal Biology as an Etiology of Ovarian Cancer Risk [11/01/2005-10/31/2008]).
Y.W. and X.-X.X. were responsible for study design, data analysis, and preparing the early draft of the manuscript. A.K.G., A.J.K.-S., and T.C.H. contributed to the development of concepts and approaches. Y.W., K.Q.C., E.R.S., T.M.Y., and R.M. participated in data acquisition and analysis. P.G.-A. and A.J.K.-S. contributed to data analysis and consultation. Y.W. wrote the first draft, and X.-X.X. worked on several subsequent draft revisions. We all participated in and contributed to data analysis and interpretation and the writing, revising, and editing of the manuscript, and all approved the final version.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00202-16.
REFERENCES
- 1.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, Xu XX, Hamilton TC. 2004. Focus on epithelial ovarian cancer. Cancer Cell 5:19–24. doi: 10.1016/S1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Lukanova A, Kaaks R. 2005. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev 14:98–107. [PubMed] [Google Scholar]
- 3.Tortolero-Luna G, Mitchell MF. 1995. The epidemiology of ovarian cancer. J Cell Biochem Suppl 23:200–207. [DOI] [PubMed] [Google Scholar]
- 4.Fathalla MF. 1971. Incessant ovulation—a factor in ovarian neoplasia? Lancet ii:163. [DOI] [PubMed] [Google Scholar]
- 5.Godwin AK, Testa JR, Handel LM, Liu Z, Vanderveer LA, Tracey PA, Hamilton TC. 1992. Spontaneous transformation of rat ovarian surface epithelial cells: association with cytogenetic changes and implications of repeated ovulation in the etiology of ovarian cancer. J Natl Cancer Inst 84:592–601. doi: 10.1093/jnci/84.8.592. [DOI] [PubMed] [Google Scholar]
- 6.Cramer DW, Welch WR. 1983. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst 71:717–721. [PubMed] [Google Scholar]
- 7.Mohle J, Whittemore A, Pike M, Darby S. 1985. Gonadotrophins and ovarian cancer risk. J Natl Cancer Inst 75:178–180. [PubMed] [Google Scholar]
- 8.Riman T, Persson I, Nilsson S. 1998. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin Endocrinol (Oxford) 49:695–707. doi: 10.1046/j.1365-2265.1998.00577.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi JH, Wong AS, Huang HF, Leung PC. 2007. Gonadotropins and ovarian cancer. Endocr Rev 28:440–461. doi: 10.1210/er.2006-0036. [DOI] [PubMed] [Google Scholar]
- 10.Gosden RG. (ed). 1985. Biology of menopause: the causes and consequences of ovarian aging. Academic Press, Inc, San Diego, CA. [Google Scholar]
- 11.Huhtaniemi I. 2010. Are gonadotrophins tumorigenic—a critical review of clinical and experimental data. Mol Cell Endocrinol 329:56–61. doi: 10.1016/j.mce.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Rulli SB, Huhtaniemi I. 2005. What have gonadotrophin overexpressing transgenic mice taught us about gonadal function? Reproduction 130:283–291. doi: 10.1530/rep.1.00661. [DOI] [PubMed] [Google Scholar]
- 13.Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE. 2002. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1:53–62. doi: 10.1016/S1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. 2003. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res 63:1389–1397. [PubMed] [Google Scholar]
- 15.Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY. 2003. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res 63:3459–3463. [PubMed] [Google Scholar]
- 16.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. 2005. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 17.Mullany LK, Fan HY, Liu Z, White LD, Marshall A, Gunaratne P, Anderson ML, Creighton CJ, Xin L, Deavers M, Wong KK, Richards JS. 2011. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene 30:3522–3536. doi: 10.1038/onc.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, Fearon ER, Cho KR. 2007. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell 11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. 2007. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol 19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 20.Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. 2008. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol 109:168–173. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. 2007. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 22.Nik NN, Vang R, Shih IEM, Kurman RJ. 2014. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol 9:27–45. doi: 10.1146/annurev-pathol-020712-163949. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi I, Takahashi K, Kon Y, Okamura T, Mototani Y, Araki Y, Kasai N. 2002. Mouse transgenic for murine oviduct-specific glycoprotein promoter-driven simian virus 40 large T-antigen: tumor formation and its hormonal regulation. Mol Reprod Dev 63:168–176. doi: 10.1002/mrd.10175. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. 2012. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A 109:3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, Karst AM, Hirsch MS, Setlur SR, Crum CP, Dinulescu DM, Drapkin R. 2013. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong MY, Kakar SS. 2009. Ovarian cancer mouse models: a summary of current models and their limitations. J Ovarian Res 2:12. doi: 10.1186/1757-2215-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lengyel E, Burdette JE, Kenny HA, Matei D, Pilrose J, Haluska P, Nephew KP, Hales DB, Stack MS. 2014. Epithelial ovarian cancer experimental models. Oncogene 33:3619–3633. doi: 10.1038/onc.2013.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullany LK, Richards JS. 2012. Minireview: animal models and mechanisms of ovarian cancer development. Endocrinology 153:1585–1592. doi: 10.1210/en.2011-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research Network. 2011. Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CM, Chang JL, Behringer RR. 2004. Tumor formation in p53 mutant ovaries transplanted into wild-type female hosts. Oncogene 23:7722–7725. doi: 10.1038/sj.onc.1208037. [DOI] [PubMed] [Google Scholar]
- 31.Clark-Knowles KV, Senterman MK, Collins O, Vanderhyden BC. 2009. Conditional inactivation of Brca1, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS One 4:e8534. doi: 10.1371/journal.pone.0008534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn BA, Brake T, Hua X, Baxter-Jones K, Litwin S, Ellenson LH, Connolly DC. 2009. Induction of ovarian leiomyosarcomas in mice by conditional inactivation of Brca1 and p53. PLoS One 4:e8404. doi: 10.1371/journal.pone.0008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing D, Scangas G, Nitta M, He L, Xu X, Ioffe YJ, Aspuria PJ, Hedvat CY, Anderson ML, Oliva E, Karlan BY, Mohapatra G, Orsulic S. 2009. A role for BRCA1 in uterine leiomyosarcoma. Cancer Res 69:8231–8235. doi: 10.1158/0008-5472.CAN-09-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith ER, Yeasky T, Wei JQ, Miki RA, Cai KQ, Smedberg JL, Yang WL, Xu XX. 2012. White spotting variant mouse as an experimental model for ovarian aging and menopausal biology. Menopause 19:588–596. doi: 10.1097/gme.0b013e318239cc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith ER, Wang Y, Xu XX. 2014. Development of a mouse model of menopausal ovarian cancer. Front Oncol 4:36. doi: 10.3389/fonc.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubreuil P, Rottapel R, Reith AD, Forrester L, Bernstein A. 1990. The mouse W/c-kit locus. A mammalian gene that controls the development of three distinct cell lineages. Ann N Y Acad Sci 599:58–65. [DOI] [PubMed] [Google Scholar]
- 37.Mintz B. 1957. Embryological development of primordial germ-cells in the mouse: influence of a new mutation, Wj. J Embryol Exp Morphol 5:396–406. [Google Scholar]
- 38.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. 1990. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J 9:1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reith AD, Rottapel R, Giddens E, Brady C, Forrester L, Bernstein A. 1990. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev 4:390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- 40.Murphy ED. 1972. Hyperplastic and early neoplastic changes in the ovaries of mice after genic deletion of germ cells. J Natl Cancer Inst 48:1283–1295. [PubMed] [Google Scholar]
- 41.Yang WL, Cai KQ, Smedberg JL, Smith ER, Klein-Szanto A, Hamilton TC, Xu XX. 2007. A reduction of Cox-2 gene dosage counters the menopausal ovarian morphological aging and tumor phenotype in Wv mice. Am J Pathol 170:1325–1336. doi: 10.2353/ajpath.2007.060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai KQ, Klein-Szanto A, Karthik D, Edelson M, Daly MB, Ozols RF, Lynch HT, Godwin AK, Xu XX. 2006. Age-dependent morphological alterations of human ovaries from populations with and without BRCA mutations. Gynecol Oncol 103:719–728. doi: 10.1016/j.ygyno.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 43.Nicosia SV. 1987. The aging ovary. Med Clin North Am 71:1–9. doi: 10.1016/S0025-7125(16)30878-1. [DOI] [PubMed] [Google Scholar]
- 44.Salazar H, Godwin AK, Daly MB, Laub PB, Hogan WM, Rosenblum N, Boente MP, Lynch HT, Hamilton TC. 1996. Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst 88:1810–1820. doi: 10.1093/jnci/88.24.1810. [DOI] [PubMed] [Google Scholar]
- 45.Cai KQ, Wang Y, Smith ER, Smedberg JL, Yang DH, Yang WL, Xu XX. 2015. Global deletion of Trp53 reverts ovarian tumor phenotype of the germ cell-deficient white spotting variant (Wv) mice. Neoplasia 17:89–100. doi: 10.1016/j.neo.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith ER, Yang WL, Yeasky T, Smedberg J, Cai KQ, Xu XX. 2013. Cyclooxygenase-1 inhibition prolongs postnatal ovarian follicle lifespan in mice. Biol Reprod 89:103. doi: 10.1095/biolreprod.113.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721–732. doi: 10.1016/S0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 48.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. 1994. Tumor spectrum analysis in p53-mutant mice. Curr Biol 4:1–7. [DOI] [PubMed] [Google Scholar]
- 49.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. 2001. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 50.Jonkers J, Berns A. 2002. Conditional mouse models of sporadic cancer. Nat Rev Cancer 2:251–265. doi: 10.1038/nrc777. [DOI] [PubMed] [Google Scholar]
- 51.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. 2003. Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol Cell Endocrinol 211:15–19. doi: 10.1016/j.mce.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. 2008. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 53.Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 54.Clark-Knowles KV, Garson K, Jonkers J, Vanderhyden BC. 2007. Conditional inactivation of Brca1 in the mouse ovarian surface epithelium results in an increase in preneoplastic changes. Exp Cell Res 313:133–145. doi: 10.1016/j.yexcr.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 55.Chu IM, Hengst L, Slingerland JM. 2008. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 56.Duncan TJ, Al-Attar A, Rolland P, Harper S, Spendlove I, Durrant LG. 2010. Cytoplasmic p27 expression is an independent prognostic factor in ovarian cancer. Int J Gynecol Pathol 29:8–18. doi: 10.1097/PGP.0b013e3181b64ec3. [DOI] [PubMed] [Google Scholar]
- 57.Jordan RC, Bradley G, Slingerland J. 1998. Reduced levels of the cell-cycle inhibitor p27Kip1 in epithelial dysplasia and carcinoma of the oral cavity. Am J Pathol 152:585–590. [PMC free article] [PubMed] [Google Scholar]
- 58.Masciullo V, Ferrandina G, Pucci B, Fanfani F, Lovergine S, Palazzo J, Zannoni G, Mancuso S, Scambia G, Giordano A. 2000. p27Kip1 expression is associated with clinical outcome in advanced epithelial ovarian cancer: multivariate analysis. Clin Cancer Res 6:4816–4822. [PubMed] [Google Scholar]
- 59.Milde-Langosch K, Hagen M, Bamberger AM, Loning T. 2003. Expression and prognostic value of the cell-cycle regulatory proteins, Rb, p16MTS1, p21WAF1, p27KIP1, cyclin E, and cyclin D2, in ovarian cancer. Int J Gynecol Pathol 22:168–174. doi: 10.1097/00004347-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Rosen DG, Yang G, Cai KQ, Bast RC Jr, Gershenson DM, Silva EG, Liu J. 2005. Subcellular localization of p27kip1 expression predicts poor prognosis in human ovarian cancer. Clin Cancer Res 11:632–637. [PubMed] [Google Scholar]
- 61.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85:733–744. doi: 10.1016/S0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 62.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. 1998. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Havrilesky LJ, Alvarez AA, Whitaker RS, Marks JR, Berchuck A. 2001. Loss of expression of the p16 tumor suppressor gene is more frequent in advanced ovarian cancers lacking p53 mutations. Gynecol Oncol 83:491–500. doi: 10.1006/gyno.2001.6464. [DOI] [PubMed] [Google Scholar]
- 64.Lee YH, Heo JH, Kim TH, Kang H, Kim G, Kim J, Cho SH, An HJ. 2011. Significance of cell cycle regulatory proteins as malignant and prognostic biomarkers in ovarian epithelial tumors. Int J Gynecol Pathol 30:205–217. doi: 10.1097/PGP.0b013e3182063e71. [DOI] [PubMed] [Google Scholar]
- 65.Nam EJ, Kim YT. 2008. Alteration of cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol Cancer 18:1169–1182. doi: 10.1111/j.1525-1438.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 66.Aoki Y, Kawada N, Tanaka K. 2000. Early form of ovarian cancer originating in inclusion cysts. A case report. J Reprod Med 45:159–161. [PubMed] [Google Scholar]
- 67.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. 2001. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22:255–288. [DOI] [PubMed] [Google Scholar]
- 68.Scully RE. 1995. Pathology of ovarian cancer precursors. J Cell Biochem Suppl 23:208–218. [DOI] [PubMed] [Google Scholar]
- 69.Dubeau L. 1999. The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gynecol Oncol 72:437–442. doi: 10.1006/gyno.1998.5275. [DOI] [PubMed] [Google Scholar]
- 70.Dubeau L. 2008. The cell of origin of ovarian epithelial tumours. Lancet Oncol 9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller AF Jr, Shah PC, Kehas DJ, Kenneally MK, Dombkowski DM, Ha TU, Preffer FI, Donahoe PK. 1999. Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res 5:3488–3499. [PubMed] [Google Scholar]
- 72.Pieretti-Vanmarcke R, Donahoe PK, Pearsall LA, Dinulescu DM, Connolly DC, Halpern EF, Seiden MV, MacLaughlin DT. 2006. Mullerian inhibiting substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer. Proc Natl Acad Sci U S A 103:17426–17431. doi: 10.1073/pnas.0607959103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, McLaughlin DT, Donahoe PK. 2006. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proc Natl Acad Sci U S A 103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanderhyden BC. 2005. Loss of ovarian function and the risk of ovarian cancer. Cell Tissue Res 322:117–124. doi: 10.1007/s00441-005-1100-1. [DOI] [PubMed] [Google Scholar]
- 75.Smith ER, Xu XX. 2008. Ovarian aging, follicle depletion, and cancer: a hypothesis for the etiology of epithelial ovarian cancer concerning follicle depletion. Lancet Oncol 9:1108–1111. doi: 10.1016/S1470-2045(08)70281-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.