Abstract
Vascular endothelial cell growth factor A (VEGF) is a biologically and therapeutically important growth factor because it promotes angiogenesis in response to hypoxia, which underlies a wide variety of both physiological and pathological settings. We report here that both VEGF receptor 2 (VEGFR2)-positive and -negative cells depended on VEGF to endure hypoxia. VEGF enhanced the viability of platelet-derived growth factor receptor α (PDGFRα)-positive and VEGFR2-negative cells by enabling indirect activation of PDGFRα, thereby reducing the level of p53. We conclude that the breadth of VEGF's influence extends beyond VEGFR-positive cells and propose a plausible mechanistic explanation of this phenomenon.
INTRODUCTION
Receptor tyrosine kinases (RTKs) govern many biological processes. They can be activated in multiple ways, including by their cognate ligands (direct activation), and indirectly, which has also been called transactivation. For instance, circulating autoantibodies and ligands of G protein coupled receptor induce tyrosine phosphorylation of platelet-derived growth factor receptors (PDGFRs) (1–10).
In the context of a blinding eye disease called proliferative vitreoretinopathy, indirect activation of PDGFRα drives pathogenesis in experimental animals and is associated with this disease in patients (11). Disease initiation involves mislocalization of cells into the vitreous of the eye, whereupon such cells are exposed to a plethora of growth factors that selectively and enduringly activate PDGFRα and thereby promotes the viability of the mislocalized cells by reducing the level of p53. The vitreal growth factors that are responsible for indirectly activating PDGFRα are outside the PDGF family and hence called non-PDGFs.
Attempts to identify which non-PDGFs are responsible for indirectly activating PDGFRα led to the discovery of a hierarchy among the three classes of growth factors that engage PDGFRα (12, 13). These three classes of growth factors include PDGFs (direct activators), non-PDGFs (indirect activators), and vascular endothelial cell growth factor A (VEGF), which competitively antagonizes PDGF-dependent activation of PDGFRα (14). The hierarchy between these three classes of growth factors is shown in Fig. 1A and termed the VEGF/PDGF/non-PDGF paradigm. This diagram illustrates how VEGF promotes the survival of cells via PDGFRα.
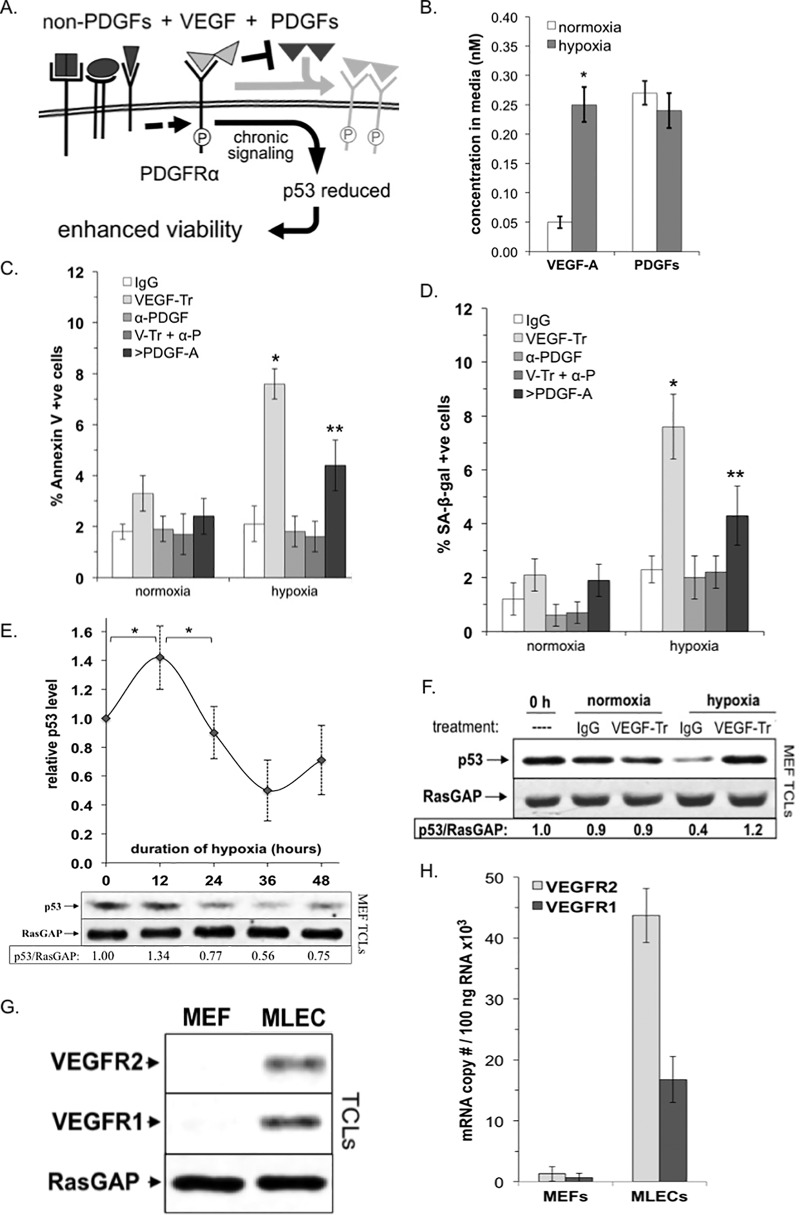
FIG 1.
VEGF promoted the viability of fibroblasts enduring hypoxia. (A) VEGF/PDGF/non-PDGF paradigm. When all three types of growth factors that engage PDGFRα are present, VEGF antagonizes PDGF-dependent activation of PDGFRα and thereby enables indirect activation of PDGFRα by non-PDGFs. This indirect mode of activation results in persistent signaling that reduces p53 and thereby enhances survival of cells (23, 26). (B) Hypoxia elevated the level of VEGF in the conditioned medium. Equal numbers of MEFs were seeded on plates at low density in 0.5% serum-containing medium supplemented with PDGFs (0.1 nM [each] PDGF-A, PDGF-AB, and PDGF-B). Cells were subjected to either normoxic (∼21% O2) or hypoxic (∼0.5% O2) conditions as detailed in Materials and Methods. After 48 h, the culture medium was subjected to multiplex analysis to determine the concentration of VEGF and PDGFs (the total of the A, AB, and B isoforms). Data representing the mean concentration under normoxic and hypoxia conditions from four independent experiments ± the SD were compared using a paired t test, where an asterisk denotes P < 0.01. (C) VEGF protected hypoxic cells from apoptosis. MEFs were seeded and placed under normoxic or hypoxic conditions as described for panel B. As part of the conditioning, cells at ∼50% confluence were switched to low-serum media (0.5%) 24 h prior to treatment in order to induce quiescence (i.e., to synchronize cells in G0 and reduce signaling background). Cells were treated with control (human IgG, 26 μg/ml) or VEGF-TRAP (V-Tr, 20 μg/ml), a formulation of neutralizing antibodies against all five PDGF isoforms (α-PDGF; 5 μg/ml of antibody against each of the PDGF isoforms), a combination of both VEGF-TRAP and anti-PDGF, as described above (V-Tr + α-P), and lastly a saturating dose (200 ng/ml or 7.1 nM) of PDGF-A (>PDGF-A). At 36 h, the percentage of apoptotic cells was determined by FITC-annexin V staining. Data are presented as the mean percentages ± the SD obtained for three independent experiments; an asterisk denotes P < 0.01 using a paired t test. (D) VEGF protected hypoxic cells from senescence (same as panel C, except the cells were analyzed for β-galactosidase [SA-β-Gal] activity). The graph presents the means ± the SD from three independent experiments; an asterisk denotes P < 0.01 using a paired t test. (E) MEFs were seeded at low density and grown under normoxic or hypoxic conditions as described for panel B. At the times indicated, the cells were lysed and clarified, and the resulting total cell lysates (TCLs) were subjected to quantitative Western blot analysis with anti-p53 and anti-RasGAP antibodies. The top of this panel shows data from three independent experiments, reported as the mean fold change in p53/RasGAP signal ± the SD as a function of hypoxic duration; an asterisk denotes P < 0.01 using a paired t test. The bottom of the panel is a Western blot from a representative experiment. (F) VEGF reduce p53 in hypoxic cells. MEFs were seeded at low density, allowed to attach overnight, and then conditioned for 36 h under normoxic or hypoxic conditions as described for panel B. The cells were treated with control (human IgG, 26 μg/ml) or VEGF-TRAP (VEGF-Tr, 20 μg/ml). PDGFs (0.1 nM [each] PDGF-A, PDGF-AB, and PDGF-B) were added to all treatments except 0 h. After treatment, the cells were harvested and lysed, and the resulting TCLs were subjected to Western blot analysis with the indicated antibodies. The signal intensities were quantified, and ratios representing band intensities normalized to the untreated control at 0 h are presented below the immunoblots. The data shown are representative of three independent experiments. (G) MEFs lacked VEGFR2 and VEGFR1 protein. Equal numbers of primary MEFs and mouse lung endothelial cells (MLECs) were harvested, and TCLs were prepared and subjected to Western blot analysis with anti-VEGFR2, anti-VEGFR1, and anti-RasGAP antibodies (the latter to assess protein loading). Unlike MLECs, where both receptors were readily observable, VEGFRs were undetectable in MEFs. (H) MEFs expressed very low levels of VEGFR2 and VEGFR1 mRNA. Real-time RT-PCR was used to determine mRNA copy number (per 100 ng of total RNA) for VEGFR2 and VEGFR1 transcripts extracted from primary MEFs and MLECs grown for 36 h under hypoxic conditions. MLECs were used as a positive control. The copy number for each VEGFR transcript was determined based on comparison to a standard curve using purified plasmids for each receptor type.
As mentioned above, the VEGF/PDGF/non-PDGF paradigm was discovered in a pathological context. In the course of testing its relevance in a physiological setting, which is the focus of this report, the following novel discoveries were made. VEGF promoted survival of VEGF receptor 2 (VEGFR2)-negative cells (such as fibroblasts) during hypoxia in vitro, ex vivo, and in vivo. Furthermore, the VEGF/PDGF/non-PDGF paradigm was a plausible explanation for this phenomenon.
MATERIALS AND METHODS
Cell culture.
Primary mouse embryonic fibroblasts (MEFs) were obtained from American Type Culture Collection (Manassas, VA) at passage 2, used between passages 3 to 7, and maintained in high-glucose-containing Dulbecco modified Eagle medium (DMEM; Gibco BRL) containing 10% fetal bovine serum. Primary mouse lung endothelial cells (MLECs) were a generous gift from P. A. D'Amore (Schepens Eye Research Institute) and used as a positive control for VEGFR mRNA expression. These cells were used at low passage (4–9) and maintained on collagen-coated plates in EGM-2 medium containing 10% horse serum and 0.1% bovine brain extract (Lonza, Allendale, NJ). The cells were passaged using 0.5% trypsin with 0.05 mM EDTA and cultured in medium supplemented with 500 U/ml penicillin and 500 μg/ml streptomycin, followed by incubation at 37°C in a humidified 5% CO2.
For hypoxic conditioning of the MEFs, the cells were first cultured in low-serum media (0.5%) for 36 h to synchronize cell cycles in G0. Fresh low-serum media supplemented with PDGFs (0.1 nM [each] PDGF-A, -AB, and -B) plus any treatment were thoroughly degassed by flushing with a 0.5% O2 gas mixture from a hypoxia tank at a flow rate of 10 liters/min for 5 min. This medium replaced the starvation medium in each culture dish, and the cells were quickly placed into a modular incubator chamber (Stem Cell Technologies, Inc., Tukwila, WA), which was flushed with the same hypoxia gas mixture (flow rate of 20 liters/min for 10 min) and then sealed shut to prevent further gas exchange with the external atmosphere. At 24 h, the modular chamber was flushed again in the same manner. At 36 h after hypoxic induction, the cells were harvested for analysis. Parallel cultures were grown outside the hypoxia chamber (at 21% O2) using the same medium (without degassing) to serve as normoxic controls.
Quantification by real-time PCR.
The absolute number of VEGFR1 and VEGFR2 mRNA transcripts was determined as previously described (12). Briefly, standard curves for absolute reverse transcription-PCR (RT-PCR) quantification were first generated using T7 RNA polymerase (Life Technologies, Woburn, MA) to generate homogenous pools of cRNA transcripts from purified pGEM (3.0-kb) plasmids containing cDNA sequence inserts corresponding to mouse mRNA for VEGFR1 (AF063657) or VEGFR2 (AF063658). The cRNA products were purified and quantified by UV absorbance at 260 nM, and concentrations were converted to the copy number for each transcript using that transcript's molecular weight. Three separate cRNA dilution series were prepared in duplicate over 5 orders of magnitude (spanning 105- to 1010-fold dilutions) and then subjected to RT-PCR amplification, along with unknowns and controls. For each standard, the average threshold cycle (CT) values were determined from each dilution series and used to generate a standard curve, from which absolute copy number could be interpolated using CT values determined for each unknown. To quantify mRNA from cells, ∼2.5 μg of total RNA was extracted using TRIzol reagent (Invitrogen) from 2 × 105 cells and quantified by determining the UV absorbance at 260 nm. The cDNA was generated using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. One microliter of the resulting cDNA product was subjected to real-time RT-PCR amplification alongside standards and controls using the SYBR green system in conjunction with a 96-well plate LightCycler (Roche, San Francisco, CA). Forward and reverse PCR primers corresponded to each receptor cDNA, and each primer pair was chosen to generate amplicons of similar size to each other. VEGFR1 mRNA primers (sense, 5′-GTC ACA GAA GAG GAT GAA GGT GTC-3′; antisense, 5′-CAC AGT CCG GCA CGT AGG TGA TT-3′) and VEGFR2 mRNA primers (sense, 5′-CAC CAC TCA AAC GCT GAC ATG TA-3′; antisense, 5′-GCT CGT TGG CGC ACT CTT-3′) were used. A negative control (no template) was run to exclude contamination of buffers, primers, and enzymes. Amplification was carried out as follows: 50°C for 2 min once, 95°C for 10 min once, followed by 30 cycles of 95°C for 15 s and 60°C for 30 s (the annealing temperatures of all primers were the nearly the same) and 72°C for 45 s, followed finally by 72°C for 10 min on the last cycle. A 30-cycle limit was established based on amplification of nonsense products in the no-template control. Dissociation curve analysis was performed, and the RT-PCR data were analyzed using SDS 7000 software. The transcript abundances interpolated from standard-curve CT values are expressed as the absolute receptor mRNA copy number per 100 ng of total RNA (100 ng of total RNA approximates 5,000 to 10,000 cells).
Biological reagents.
VEGF-TRAP (V-Tr, Aflibercept; Eylea, Tarrytown, NY), a dimeric, recombinant fusion glycoprotein comprising portions of the VEGFR1 and VEGFR2 extracellular domains fused to the Fc region of human IgG1, was used as the VEGF neutralizing agent in all experiments. Human IgG1 control antibody (BD Biosciences, San Jose, CA) was used at an equimolar concentration to VEGF-TRAP. Antibodies to PDGFRα, p-PDGFRα, VEGFR1, VEGFR2, p53, Rab5a, GFAP, NG-2, IB-4, and the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 were obtained from Cell Signaling Technology (Beverly, MA). Nutlin-3 was obtained from Cayman Chemical (Ann Arbor, MI). The Ras GTP-activating protein (RasGAP) antibody was a crude rabbit antiserum raised against a glutathione S-transferase (GST) fusion protein of human RasGAP (15). Secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescent substrate for detection of horseradish peroxidase came from Pierce Research Products (Rockford, IL). FlowTACS (TUNEL-based) apoptosis detection kit was from Trevigen (Gaithersburg, MD), and the ApoAlert annexin V-FITC apoptosis detection kit was from Clontech Laboratories (Mountain View, CA). Alexa Fluor 488-wheat germ agglutinin (WGA-488) was obtained from Molecular Probes (Thermo Fisher, Hanover Park, IL). CY3-conjugated anti-rabbit (emitting red fluorescence) and CY5-conjugated anti-goat (emitting far red fluorescence) antibodies were obtained from Abcam (Cambridge, MA). All recombinant growth factors were purchased from PeproTech (Rocky Hill, NJ); all other antibodies were from Santa Cruz (Santa Cruz, CA). Quantification of growth factors/cytokines in conditioned media of cells or explants was performed using the Milliplex multiplex cytokine detection system assays (Millipore, Billerica, MA), with the exception of PDGF-C, PDGF-D, hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), and connective tissue growth factor (CTGF) which were quantified by Western blot analysis using the appropriate standards (Peprotech, Rocky Hills, NJ). The C12FDG fluorescent substrate for β-galactosidase, used for assaying senescence, was from Molecular Probes (Thermo Fisher). SU6656 (SignaGen Laboratories, Rockville, MD) was used at 1.0 μM, which is sufficient to inhibit the tyrosine kinase activity of SKFs (Fyn, Yes, Lck, and Lyn) but not the other tyrosine kinases that it is known to target (16). The two PDGFR-targeting kinase inhibitors used were AG1296 at 50 nM (Millipore) and imatinib mesylate (Gleevec) at 10 μM (Novartis Pharmaceuticals, East Hanover, NJ).
Western blotting.
After treatment and conditioning, the cells were washed twice with ice-cold phosphate-buffered saline (PBS) and then lysed in sample buffer (50 mM Tris-HCl [pH 6.8], 10% glycerol, 2% sodium dodecyl sulfate [SDS], 1% β-mercaptoethanol, 10 mM EDTA, and 0.02% bromophenol blue) for 15 min on ice. Total cell lysates (TCLs) were heated to 95°C for 5 min, clarified by centrifugation at 14,000 × g for 15 min at 4°C, and then run on 10% acrylamide SDS-PAGE gel. Electrophoretically resolved proteins were transferred to polyvinylidene difluoride membranes by semidry transfer, blocked, and blotted with the appropriate detection and loading control antibodies. The enhanced chemiluminescent substrate used with horseradish peroxidase (HRP) detection (via HRP–anti-IgG from Pierce Research, Rockford, IL). Each immunoblot area shown was representative of at least three independent experiments. Immunoblot signals were captured and analyzed by scanning densitometry in conjunction with Quantity One software (Bio-Rad). Experimental signal intensities were adjusted for background and loading based on signal intensity of the loading control and then normalized to a negative control or subcellular fraction marker, as indicated. Where signals were quantified, normalized values are given underneath.
Viability assays.
The percentage of apoptotic cells was determined by staining with FITC-conjugated annexin V. Cells were counterstained with propidium iodide (PI) to exclude nonviable cells. Annexin V-positive cells were measured from a total viable population of 50,000 cells by flow cytometry (17). The percentage of senescent cells was determined by quantifying senescence-associated β-galactosidase (SA-β-Gal) activity using C12FDG, a β-Gal substrate that fluoresces upon being cleaved (18). Senescent cells were measured from a total viable population of 50,000 cells by flow cytometry. Cell cycle engagement was determined by DAPI (4′,6′-diamidino-2-phenylindole) staining, followed by DNA content analysis using a flow cytometer, as previously described (19). Only cells with DNA content >N, where N equals the diploid DNA content, were included in the analysis (i.e., the S, G2, and M phases) since this DNA content-based approach cannot distinguish the G0 phase (quiescence) from the G1 phase. Approximately 50,000 events were counted per experiment.
Preparation and processing of retinal explants.
Healthy P12 murine pups were sacrificed, their retinas physically were isolated and cut into segments, and approximately 1/6 of each total retinal area was isolated. Excised tissue segments were sandwiched between collagen and covered with medium containing 0.1% serum. The level of growth factors that were produced by the explants under these conditions was quantified and is listed in Table 1. After we allowed the explants to acclimate overnight, they were conditioned and treated as indicated for 36 h and then enzymatically dispersed into a single cell suspension using 0.01% trypsin and 0.05% papain for subsequent analysis by flow cytometry.
TABLE 1.
Growth factor levels in conditioned media of murine retinal explantsa
| Growth factor | Concn (nM) ± SD |
|
|---|---|---|
| Normoxic (36 h) | Hypoxic (36 h) | |
| VEGF | 0.03 ± 0.01 | 0.18 ± 0.04 |
| PDGF-A | 0.03 ± 0.00 | 0.02 ± 0.00 |
| PDGF-AB | 0.03 ± 0.00 | 0.04 ± 0.01 |
| PDGF-B | 0.05 ± 0.02 | 0.04 ± 0.01 |
| PDGF-C* | 0.02 ± 0.01 | 0.03 ± 0.02 |
| PDGF-D* | ND | ND |
| EGF | 0.08 ± 0.02 | 0.07 ± 0.02 |
| FGF-2 | 0.06 ± 0.02 | 0.06 ± 0.03 |
| HGF | 0.17 ± 0.05 | 0.24 ± 0.08 |
| IGF-1 | 0.65 ± 0.18 | 0.48 ± 0.16 |
| IL-6 | 0.06 ± 0.08 | 0.11 ± 0.08 |
| TGF-α | ND | 0.02 ± 0.00 |
| CTGF | 0.06 ± 0.02 | 0.04 ± 0.01 |
| TGF-β1 | 0.13 ± 0.04 | 0.16 ± 0.07 |
| TGF-β2 | 0.88 ± 0.16 | 0.65 ± 0.10 |
| G-CSF | ND | ND |
Retinas were removed from P12 mouse pups, sectioned, and placed between two collagen layers in the presence of DMEM containing 0.1% serum. Explanted tissue was left to incubate overnight at 37¡C before conditioning the samples under normoxic (21% O2) or hypoxic (0.5% O2) conditions as described in Fig. 1. No exogenous growth factors were added during conditioning. At 36 h, 100 μl of the conditioned media was withdrawn from each explant and subjected to multiplex and semiquantitative Western blot analyses to determine the concentrations of a panel of 16 growth factors, chosen based on their ability to modulate (directly or indirectly) PDGFRα activation (5, 22). PDGF-D and G-CSF were not detected (ND) in the conditioned media from explants subjected to normoxic or hypoxic conditions. *, active form only.
Induction of hypoxia in neonatal mice.
The procedure to induce hypoxia in neo-natal mice is a modification of the standard oxygen-induced retinopathy protocol (20). Along with their nursing dams, age-matched CD-1 litters were left in room air at 21% O2 or transferred to 75% O2 from P7 to P12. During 5 days of 75% O2 (hyperoxia), the developing retinal vasculature ceases to mature and undergoes vaso-obliteration. Daily dam rotation between normoxic and hyperoxic litters was performed during hyperoxia to reduce the risk of respiratory failure for dams exposed to excess oxygen (this method was established at the Schepens animal facility). On P12, pups were returned to room air, i.e., relative hypoxia. Both hypoxic pups and pups not undergoing oxygen flux (normoxic) received treatment for 2 days, beginning on P12. Treatments were injected intravitreally in a volume of 0.5 μl with a 34-gauge microsyringe. Animals were sacrificed on P14, after which the retinas were extracted, physically isolated, and cut into segments (roughly 1/6 of the total retinal area), followed by enzymatic dispersal into single cell suspensions for subsequent flow cytometry.
Retinal cell analysis by flow cytometry.
Retinal cell suspensions (prepared from explants or from freshly harvested retinas) were stained with PI to gate out nonviable cells, anti-PDGFRα–phycoerythrin (PE) and anti-VEGFR2–CY5.5 to mark receptor-specific subpopulations, and DAPI for cell cycle analysis (19). Samples were then split 50:50: one 50% fraction received FITC-annexin V for apoptotic detection (17), and the other 50% fraction was stained with C12FDG, an FITC-based substrate for detecting senescent cells (18). Both halves were then analyzed in parallel for each treatment and condition. All of the fluorescence markers used have emission spectra sufficiently distinct to distinguish each from the other by multichannel analysis. The estimated emission peaks for DAPI, FITC, PE, PI, and CY5.5 are 455, 520, 578, 617, and 694 nm, respectively. Samples prepared for analysis were neither permeabilized nor fixed. Flow cytometric analysis proceeded according to the following five-step gating strategy. (i) Cells are gated from debris based on flow cytometric parameters related to size (forward scatter) and granularity (side scatter [SSC]). (ii) Single cells are gated from cell aggregates, since the former resides within a distinct linear zone related to direct proportionality on an SSC area (SSC-A) versus the SSC-height (SSC-H) scatter plot. (iii) Viable cells are gated from dead/necrotic cells (nonviable cells), as well as nondescript nucleic acid aggregates, using PI, a DNA-binding dye excluded from living cells. (iv) By simultaneous detection of PE (orange-red) and Cy5.5 (single viable cells are further gated into subpopulations expressing PDGFRα (detected via PE emission), VEGFR2 (detected via CY5.5), or neither receptor (PE and CY5.5 emission equal to or less than unlabeled background). Cells expressing both receptors were also detected but not analyzed. (v) Each receptor subpopulation is subjected to a three-parameter viability analysis to detect cells that are apoptotic, senescent, or proliferating (in the S, G2, and M phases). Gating trees were constructed using this multistep strategy; data from each subsequent step “down” the tree represent a percentage of cells from the previous step “up.” For instance, “% Parent” indicates the percentage of gated events relative to the preceding gate (all parent gates show black values). Data acquisition was conducted at 4°C using a Beckman XL flow cytometer (Beckman Coulter, Indianapolis, IN) running FACSCalibur software (BD Bioscience, Franklin Lakes, NJ).
Histology.
To obtain vertical sections of the neural retina for analysis, whole pup eyes were enucleated after sacrifice and fixed in 4% paraformaldehyde overnight, then in increasing amounts of sucrose (in PBS) over the next 48 h. Using OCT embedding compound (R&D Systems, Minneapolis, MN), the fixed eyes were rapidly frozen on dry ice and vertically sectioned with a cryostat to obtain 8-μm-thick cross sections of the entire retinal circumference. Sections were mounted on Colorfrost mounting slides (Thermo Scientific, Hanover Park, IL), excess OCT compound washed away, and subjected to immunofluorescence analysis.
Immunofluorescence.
Indirect immunofluorescence was performed as described previously (21). Briefly, cells on glass coverslips were conditioned and treated as indicated, and then the coverslips were washed, transferred to separate wells, and fixed in 4% paraformaldehyde. After fixation (of cells or frozen sections), the samples were permeabilized with 0.2% Triton X-100 when required, washed, blocked with 10% donkey serum (2 h), and incubated overnight with primary antibody at 4°C, followed by fluorescence-labeled secondary antibodies for 1 h at room temperature. Unless indicated otherwise, mounting medium containing DAPI counterstain was used to mount coverslips on glass slides or to overlay sections prior to microscopy. Immunofluorescence for cells/sections was visualized at a ×40 to ×60 magnification using an Axio Scope.A1 inverted microscope (Zeiss, Thornwood, NY) or a TCS SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL) when higher resolution and subcellular clarity were desired.
Subcellular fractionation.
Separation of plasma membrane (PM), endosome (EN), and cytosol (CY) fractions from MEF homogenates was carried out as previously described (21). The procedure, in brief, is as follows. Immediately after treatment, the cells were rapidly cooled to 4°C and maintained at this temperature until the addition of SDS-containing sample buffer. Cells (108 MEFs) were scraped into homogenization buffer (HB; 0.25 M sucrose, 20 mM [pH 7.1], Tris-HCl, 1 mM MgCl2, 4 mM NaF, 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM 4-[2-aminoethyl]-benzenesulfonyl fluoride, 10 μg/ml aprotinin, and 1 μM pepstatin A) and homogenized by mechanical disruption using a frosted-glass micropestle and mortar (VWR). The homogenates were centrifuged at 200 × g for 5 min to remove cellular debris and whole nuclei (P1). The aspirated postnuclear supernatant (S1) was centrifuged at 1,500 × g for 10 min to yield a supernatant (S2) and a pellet (P2). P2 was suspended in 0.25 M sucrose HB, overlaid upon an equal volume of 1.42 M sucrose HB, and centrifuged at 82,000 × g for 1 h. A foggy white pellicule at the 0.25 to 1.42 M sucrose interface was collected as the PM fraction. S2 was aspirated and centrifuged at 100,000 × g for 30 min to yield the soluble CY fraction and a microsomal pellet (P3). This pellet was suspended in 0.25 M sucrose HB and overlaid upon a discontinuous sucrose gradient containing equal volumes of HB at 1.00 and 1.15 M sucrose. The gradient was centrifuged at 200,000 × g for 1.5 h to obtain the purified EN fraction, which was located as a faint pellicule at the 0.25 to 1.00 M sucrose interface. PM, CY, and EN fractions were diluted 1:1 into 2× sample buffer containing 1% SDS in preparation for SDS-PAGE. A typical experiment using 108 MEFs leads to total yields of 50 to 100 μg of plasma membrane protein, 30 to 50 μg of endosomal/light microsomal protein, and up to 500 μg of CY protein.
Statistical analysis.
Unless otherwise mentioned, all experiments were performed independently at least three times. Data are reported as means ± the standard deviations (SD) unless stated otherwise. All statistical data were analyzed using by SigmaPlot analysis software. When two groups were compared, the Student t test was used. P values of <0.05 or <0.01 were considered statistically significant.
RESULTS
VEGF promoted viability of fibroblasts enduring hypoxia.
Figure 1A illustrates our recent discovery that VEGF promotes viability of cells by reducing the level of p53. VEGF accomplishes this by persistently activating monomeric PDGFRα; this process is independent of VEGFRs and even proceeds in fibroblasts, which express little few any VEGFRs (12, 13, 22). Although these discoveries were made in the context of a blinding eye disease, we speculated that this VEGF-driven survival mechanism was also relevant to physiology. To this end, we tested whether VEGF promoted viability of fibroblasts enduring hypoxia.
If VEGF is required for cells to survive hypoxia, then neutralizing it will be detrimental. We found that this was indeed the case. There was more VEGF in the conditioned medium of hypoxic cells (Fig. 1B), and neutralizing it compromised three measures of viability: apoptosis, senescence, and proliferation (compare the white and light gray bars in Fig. 1C and D; also data not shown). Although the decline in viability appeared to affect only a small percentage of the total population, it was observed even though the cells were in medium containing serum (0.5%) and PDGFs (PDGF-A, -AB, and -B; each at 0.1 nM, or 2.8 ng/ml). In contrast, the viability of normoxic cells was unaffected by neutralizing VEGF (Fig. 1C and D).
Figure 1A shows that VEGF promotes viability by competitively preventing PDGF from binding to PDGFRα. If this mechanism is relevant to the hypoxic setting, then PDGF is responsible for compromised viability. Three types of findings supported this possibility. First, there was a similar level of PDGF and VEGF in the conditioned medium of hypoxic cells (Fig. 1B). Second, boosting the level of PDGF-A increased the percentage of apoptotic and senescent cells (Fig. 1C and D). Third, neutralizing PDGFs overcame the decline in viability that was caused by neutralizing VEGF (Fig. 1C and D). Neutralizing PDGFs alone had no effect (Fig. 1C and D). Taken together the data support the mechanism shown in Fig. 1A, i.e., VEGF promotes viability of hypoxic cells by antagonizing PDGFs.
We performed additional experiments to assess whether VEGF promoted the viability of hypoxic cells according to the mechanism illustrated in Fig. 1A. Silencing the expression of PDGFRα or pharmacologically inhibiting the PDGFRs reduced viability of cells enduring hypoxia, whereas there was no impact on normoxic cells (data not shown). These results indicate that PDGFRα was an essential player and are consistent with the effect of PDGF-A (the PDGFRα-specific isoform) in Fig. 1C and D.
Non-PDGFs indirectly activate PDGFRα by triggering a reactive oxygen species (ROS)- and Src family kinase (SFK)-dependent signaling pathway. This concept emerged from our previously published findings, which indicated that pharmacological and/or genetic approaches to antagonize ROS or SFKs prevented indirect activation of PDGFRα (5, 23). Similarly, antagonizing either ROS, or SFKs reduced viability (increased apoptosis and senescence) in hypoxic cells (data not shown).
There is an extensive body of literature demonstrating that reducing p53 enhances viability of cells (24). This is also the case for a blinding eye disease in which VEGF-mediated suppression of p53 is central to pathogenesis (25). For these reasons, we considered whether VEGF regulated the level of p53 in fibroblasts enduring hypoxia. Consistent with the fact that p53 rises in response to stress (24), we observed that the level of p53 increased within 12 h of switching cells to hypoxic conditions (Fig. 1E). The level of p53 subsequently declined, and the timing of this decline coincided with the peak of VEGF accumulation (Fig. 1E and data not shown). Importantly, neutralizing VEGF prevented the decline in p53 (Fig. 1F). Furthermore, antagonizing signaling enzymes (PI3K and Mdm2) that are required for the decline in p53 (26) compromised the viability of hypoxic cells (data not shown). These inhibitors also compromised the viability of normoxic cells, albeit to a smaller extent (data not shown), likely due to perturbation of signaling events not uniquely downstream of indirectly activated PDGFRα. These experiments indicate that VEGF suppressed p53 in cells experiencing hypoxia.
Instead of the mechanism diagramed in Fig. 1A, we considered whether VEGF suppressed p53 by directly activating PDGFRα. The finding that VEGF binds to PDGFRα (14, 27) supports this possibility. However, stimulating normoxic or hypoxic cells with a low (0.5 nM or 19 ng/ml) or high (5 nM or 190 ng/ml) dose of VEGF failed to activate PDGFRα or reduce p53 (data not shown).
For this series of experiments, we chose primary MEFs because they express PDGFRα and have no or barely detectable levels of VEGFRs when assayed by Western blotting or RT-PCR, respectively (Fig. 1G and H). Even in cell that express both PDGFRα and VEGFRs (such as retinal pigment epithelial cells), VEGF enables indirect activation of PDGFRα and thereby promotes survival of cells (12).
Taken together, these data indicate that VEGF fosters viability of cells enduring hypoxia, and that the mechanism appears to be the same one that drives pathology of a blinding eye disease (Fig. 1A).
The viability of PDGFRα-positive/VEGFR-negative cells was dependent on VEGF in retinal explants enduring hypoxia.
The conditions in the preceding series of experiments were deliberately chosen to test whether the VEGF/PDGF/non-PDGF was a plausible explanation for why fibroblasts develop a dependence on VEGF during hypoxia. Serum was included as a source of non-PDGFs, and PDGFs were added to match the level of VEGF that accumulated during hypoxia. Figures 2 and 3 document our efforts to test the relevance of the VEGF/PDGF/non-PDGF paradigm in increasingly physiological settings.
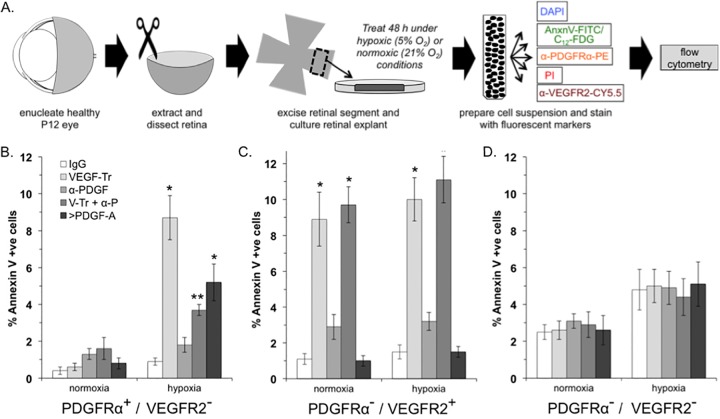
FIG 2.
VEGF promoted viability of PDGFRα+/VEGFR2− cells in retinal explants enduring hypoxia. (A) Overview of the experimental procedure, which is described in detail in Materials and Methods. (B to D) Percentages of FITC-annexin V-positive (apoptotic) cells that were PDGFRα positive and VEGFR2 negative (B), PDGFRα negative and VEGFR2 positive (C), or PDGFRα negative and VEGFR2 negative (D). The data are reported as the means ± the SD from five eyes (n = 5), comprising 50,000 events in total. Significant differences compared to IgG for each condition was determined using a paired t test, where a single asterisk denotes P < 0.01 and two asterisks denote P < 0.05.
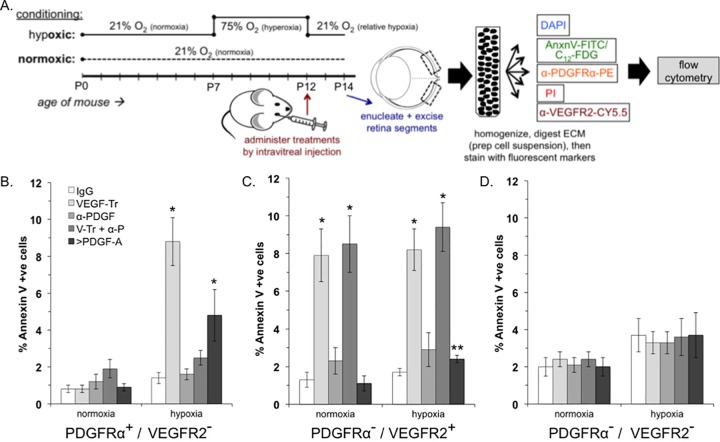
FIG 3.
VEGF promoted the viability of PDGFRα+/VEGFR2− retinal cells in mice enduring relative hypoxia. (A) Overview of the experimental procedure, which is described in detail in Materials and Methods. (B to D) Percentages of FITC-annexin V-positive (apoptotic) cells that were PDGFRα+/VEGFR2− (B), PDGFRα−/VEGFR2+ (C), or PDGFRα−/VEGFR2− (D). The data are reported as the means ± the SD from five eyes (n = 5), comprising 50,000 events in total. Significant differences compared to IgG for each condition was determined using a paired t test, where a single asterisk denotes P < 0.01 and double asterisks denote P < 0.05.
We sought to determine whether PDGFRα-positive/VEGFR-negative cells within tissues that contained multiple cell types depended on VEGF to endure hypoxia. We chose retinal explants from neonatal mice because the retina is a tissue that experience hypoxia in the natural course of development (20). Retinal explants were isolated, placed between two layers of collagen, overlaid with medium containing 0.1% serum and no purified growth factors, and then incubated for 36 h under normoxic (21% O2) or hypoxic (0.5% O2) conditions. The explants were recovered, dissociated into a single cell suspension, stained for PDGFRα, VEGFR2, and apoptosis or senescence markers, and quantified by fluorescence-activated cell sorting (FACS) analysis (Fig. 2A). Neutralizing VEGF during the period of hypoxia increased the percentage of apoptotic and senescent cells (Fig. 2B and data not shown). In contrast, VEGF was not required for viability under normoxic conditions (Fig. 2B and data not shown). Furthermore, neutralizing PDGFs had no impact (Fig. 2B and data not shown). However, neutralizing PDGFs significantly reduced (for apoptosis) or eliminated (for senescence) the detrimental effect of removing VEGF (Fig. 2B and data not shown). Finally, increasing the concentration of PDGF increased the percentage of apoptotic and senescent cells (Fig. 2B and data not shown). These results indicate that PDGFRα-positive, VEGFR-negative cells rely on VEGF to survive hypoxia in a multicellular tissue and suggest that the underlying mechanism involves the VEGF/PDGF/non-PDGF paradigm illustrated in Fig. 1A.
Because the retina consists of multiple cell types, this experimental system provided the opportunity to assess the dependence on VEGF and PDGF in other cell populations, such as PDGFRα-negative, VEGFR2-positive cells (e.g., endothelial cells; data not shown). Neutralizing VEGF increased apoptosis in both normoxic and hypoxic conditions (Fig. 2C), which is consistent with previous work that endothelial cells depend on VEGF for their viability (28). In contrast, eliminating PDGF had no effect, even when combined with neutralization of VEGF (Fig. 2C). Similarly, increasing the concentration of PDGF did not alter the percentage of apoptotic cells under normoxia or hypoxia (Fig. 2C).
We also analyzed a third population of cells, namely, those that were negative both PDGFRα and VEGFR2. Hypoxia modestly increased overall apoptosis in this population, and neutralizing VEGF, PDGF, or both or increasing the concentration of PDGF had no effect (Fig. 2D).
We conclude that distinct cell types within a tissue differed in their reliance on VEGF for optimal viability during hypoxia. As expected from previous publications, VEGFR2-positive cells such as endothelial cells were dependent on VEGF. The novel finding is that VEGF promoted viability of VEGFR2-negative cells. Furthermore, the mechanism illustrated in Fig. 1A is a plausible explanation for the reliance of PDGFRα-positive, VEGFR2-negative cells on VEGF. Finally, these results, obtained using a tissue explant and endogenously produced factors, largely mirrored the in vitro data collected from a single cell type responding to a deliberately defined set of conditions.
VEGF promoted viability of PDGFRα-positive, VEGFR-negative retinal cells in mice subjected to hypoxia.
We also tested the relevance of the VEGF/PDGF/non-PDGF paradigm in mice as diagramed in Fig. 3A. Seven days after birth, neonatal mice were exposed to 75% oxygen until day 12, whereupon the oxygen concentration was reduced to 21%. This is an established approach to induce relative hypoxia of the retina (20). Control mice were exposed to room air (21% O2) for the entire 14-day period. Specific agents were injected into the vitreous at the onset of relative hypoxia (day 12), and mice were sacrificed after another 36 h, which precedes the onset of neovascularization (20). The retina was isolated, dissociated into a single cell suspension, stained for PDGFRα, VEGFR2, and apoptosis markers, and quantified by FACS analysis. Neutralizing VEGF or increasing the concentration of PDGF-A increased the apoptosis and senescence of PDGFRα-positive, VEGFR-negative cells (Fig. 3B and data not shown). Furthermore, neutralizing PDGFs overcame the effect of the VEGF trap, whereas eliminating only PDGFs had no impact. The PDGFRα-negative, VEGFR-positive population of cells depended on VEGF under both hypoxic and normoxic conditions (Fig. 3C). Finally, the viability of double-negative cells was independent of VEGF and PDGFs (Fig. 3D). These observations indicate that the VEGF/PDGF/non-PDGF paradigm was relevant to the retina undergoing hypoxia in living mice.
Although the results with living mice largely reflected the data obtained using tissue explants, there were also some differences. For instance, apoptosis of PDGFRα-negative, VEGFR2-positive cells increased after the elevation of PDGF in mice but not in explants (Fig. 2C and 3C). Endothelial cells produce PDGF and thereby recruit pericytes, with which they engage in a functional relationship (29). Elevating PDGF may thus disrupt pericyte recruitment and thereby increase the fragility of endothelial cells during hypoxia. Pericyte recruitment is defective in mice that mislocalize PDGF (29).
The subcellular location of phosphorylated PDGFRα (pPDGFRα) indicated its mode of activation.
To further address the in vivo relevance of the VEGF/PDGF/non-PDGF paradigm, we sought to determine whether VEGF enabled indirect activation of PDGFRα in vivo. Existing approaches to detect activated PDGFRα in vivo (e.g., phosphospecific antibodies) do not distinguish the mode of activation. Consequently, the first step was to develop such an approach.
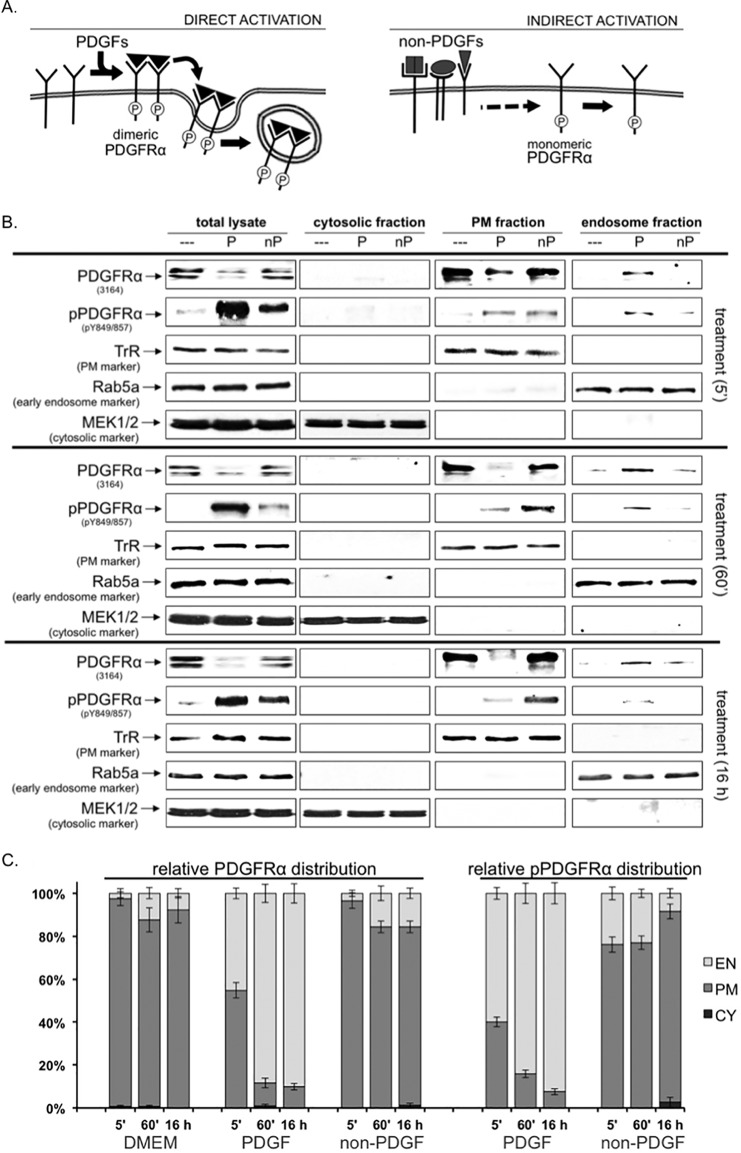
In order to distinguish between direct and indirect modes of activation, we focused on the subcellular location of the activated PDGFRα. Directly activated PDGFRα leaves the cell surface in less than 5 min because PDGF promotes its rapid internalized into the endosome or lysosome compartment, where it is degraded (30). In contrast, the indirectly activated PDGFRα is expected to spend more time on the cell surface because its half-life is at least 24 times longer than directly activated PDGFRα (>2 h versus <5 min) (26, 31). This line of reasoning predicts that directly and indirectly activated PDGFRα should populate distinct subcellular locations: vesicular (directly activated) versus cell surface (indirectly activated) (Fig. 4A).
FIG 4.
The subcellular location of pPDGFRα indicated its mode of activation: a biochemical approach. (A) Diagram illustrating the direct and indirect modes of activation of PDGFRα. The left panel shows that the direct, PDGF-mediated mode of activation promotes rapid transit of the activated receptor from the cell surface to endosomes. The right panel shows that non-PDGFs engage their own receptors and thereby trigger an intracellular signaling cascade that activates monomeric PDGFRα, which persist on the plasma membrane (5, 26). (B) Western blot analysis on fractionated cells. Serum-deprived MEFs were treated for the indicated times with DMEM alone (- - -), 0.5 nM PDGF-A (P), or 0.5 nM total of non-PDGFs (nP, comprising epidermal growth factor (EGF), fibroblast growth factor 2 (FGF-2), hepatocyte growth factor (HGF), IGF-1, interleukin-6 (IL-6), transforming growth factor α (TGF-α), CTGF, TGF-β1, TGF-β2, and G-CSF at 0.05 nM each). After treatment, the cells were harvested, homogenized, and subjected to subcellular fractionation to obtain endosomal (EN), plasma membrane (PM), and cytosolic (CY) fractions. The fractions were subjected to Western blot analysis with anti-PDGFRα, anti-pPDGFRα, and antibodies to three fraction-specific markers (to assess the purity of each fraction): Rab5a (early EN marker), TrR (PM marker), and MEK1/2 (CY marker). The immunoblots shown are representative of three independent experiments. (C) Graphical summary of the Western blot data. The sum of detectable receptor signal in all three fractions (endosomal (EN), plasma membrane (PM), and cytosolic (CY) fractions was designated as 100%. The data are presented as distribution graphs in which each column contains the distribution of PDGFRα or pPDGFRα relative to all three fractions (totaling 100%). The percent relative distribution was calculated as follows: % distribution = PDGFRα (or pPDGFRα) band intensity in fraction (EN*, PM*, or CY*)/sum of PDGFRα (or pPDGFRα) band intensities from all fractions (EN* + PM* + CY*). The “*” denotes that the signal value has been normalized to a fraction-specific marker.
To test this prediction, we compared the amount of activated PDGFRα in biochemically fractioned cells that had been exposed to the two types of agonists. As expected, PDGFRα was predominantly in the plasma membrane fraction of nonstimulated cells (Fig. 4B and C). Within 5 min of exposure to PDGF, the majority of activated PDGFRα relocated to the endosomal compartment; >90% of directly activated PDGFRα was in this subcellular fraction at the 16-h time point (Fig. 4B and C). These results are consistent with the well-established dogma regarding trafficking of directly activated RTKs (32). The subcellular location of indirectly activated PDGFRα was diametrically opposed to the location of directly activated PDGFRα. At the 16-h time point, >90% of indirectly activated PDGFRα was in the plasma membrane fraction (Fig. 4B and C). The subcellular location of total PDGFRα was similar to the location of activated PDGFRα in response to both agonists (Fig. 4B and C). These biochemical studies indicated that the mode of activation determined the subcellular location of activated PDGFRα.
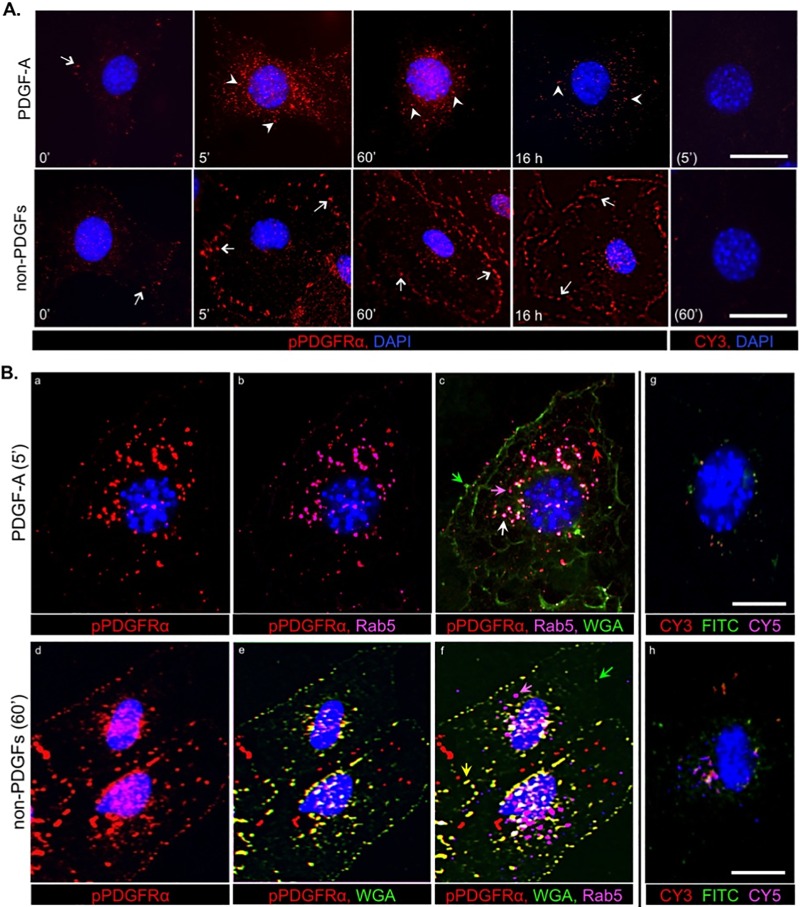
The prediction regarding the subcellular location of directly and indirectly activated PDGFRα was also evaluated by immunofluorescence. In cells exposed to PDGF, the phosphorylated PDGFRα (pPDGFRα) signal increased by 5 min and then declined substantially, although not completely, by the latest time point (top row of Fig. 5A). Directly activated PDGFRα was typically perinuclear and associated with vesicles, such as early endosomes, which express Rab5 (Fig. 5Bc, pink arrow). Although the vast majority of pPDGFR was observed in vesicles, not all colocalized with Rab5 (Fig. 5Bc, red arrow), which is likely because Rab5 is expressed only on a subset of vesicles through which directly activated RTKs such as PDGFRα traffic (33, 34). In contrast to the rapid clearance of directly activated pPDGFRα from the cell surface, indirectly activated pPDGFRα persisted on the cell surface (bottom row of Fig. 5A). Furthermore, indirectly activated pPDGFRα did not accrue in a perinuclear location, but accumulated into puncta at the periphery of cells (Fig. 5A; arrows versus arrowheads show the perinuclear location of the directly activated PDGFRα). Indirectly activated PDGFR colocalized with wheat germ agglutinin (WGA), which, under the conditions used in these experiments, decorates the cell surface (Fig. 5Bf, yellow arrow). The distinct subcellular locations of directly and indirectly activated PDGFR are apparent when comparing Fig. 5Bc and f; directly activated PDGFRα was predominantly in vesicles that are perinuclear (white arrow), whereas indirectly activated PDGFRα was on the cell surface and at the periphery (yellow arrow). The specificity controls (i.e., the use of a secondary antibody in the absence of a primary antibody) routinely indicated that nonspecific signals were low or undetectable (Fig. 5). The results of both of these experimental approaches indicate that the subcellular location of activated PDGFRα indicates its mode of activation.
FIG 5.
The mode of PDGFRα activation determined its subcellular localization: an immunofluorescence approach. (A) Analysis by indirect immunofluorescence of PDGFRα phosphorylation and trafficking in MEFs stimulated with PDGF-A or non-PDGFs. Equivalent numbers of MEFs were seeded on coverslips in a 24-well plate and allowed to reach no more than ∼50% confluence before serum was withdrawn for 16 h and then stimulated for the indicated times with DMEM alone (0′ control), PDGF-A (0.5 nM), or non-PDGFs (0.5 nM total, comprising EGF, FGF-2, HGF, IGF-1, IL-6, TGF-α, CTGF, TGF-β1, TGF-β2, and G-CSF at 0.05 nM each). After treatment, the cells were subjected to immunofluorescence analysis (Materials and Methods) using anti-pPDGFRα, followed by a CY3-conjugated secondary antibody (emitting red fluorescence) and counterstained with DAPI. Arrows indicate regions on the cell surface colocalizing with pPDGFRα (cell surface staining using WGA is shown in panel B); arrowheads indicate vesicular structures (e.g., endosomes) colocalizing with pPDGFRα. Controls without primary antibody (rightmost panels) were stained with DAPI and CY3-conjugated secondary antibody; only DAPI could be seen in the resulting images. The images are representative of three independent experiments. (B) Analysis by indirect triple immunofluorescence of PDGFRα phosphorylation and subcellular localization after direct and indirect activation. MEFs were seeded on coverslips, cultured, and starved as for panel A and then stimulated with PDGF-A (0.5 nM) or non-PDGFs (0.5 nM total), same mixture as for panel A for 5 min and 60 min, respectively. These times were chosen based on the time course analysis in panel A when fluorescent signal intensities were highest. After treatment, the cells were subjected to sequential immunofluorescence analysis with first Alexa Fluor 488-wheat germ agglutinin (WGA-488, emitting green fluorescence), a potent lipophilic dye for staining plasma membranes, and then, after an extensive rinse and a brief permeabilization step (see Materials and Methods) to expose intracellular antigens, further staining with antibodies to pPDGFRα (anti-rabbit antibody) and Rab5 (anti-goat antibody; Rab5 is an early endosome marker), followed by the appropriate secondary antibodies: CY3-conjugated anti-rabbit antibody (emitting red fluorescence) and CY5-conjugated anti-goat antibody (emitting far red fluorescence, which was pseudocolored pink in the images for ease of analysis). All coverslips were counterstained with DAPI. The arrows shown on the four-color overlay images (c and f) provide an example of a particular colocalization. Green arrows indicate regions of plasma membrane devoid of pPDGFRα, magenta arrows indicate early endosomes devoid of pPDGFRα, red arrows indicate pPDGFRα-positive/Rab5-negative vesicles (e.g., late endosomes), white arrows indicate pPDGFRα-positive early endosomes, and yellow arrows indicate pPDGFRα-positive regions of the plasma membrane. The no primary antibody controls (rightmost images) were stained with DAPI and secondary antibodies (the two mentioned above, plus FITC-conjugated anti-rat antibody to pseudotype WGA-488); these panels define the background fluorescence for pPDGFRα, WGA and Rab5. The images are representative of three independent experiments. All scale bars shown are 50 μm.
VEGF promoted indirect activation of PDGFRα during hypoxia.
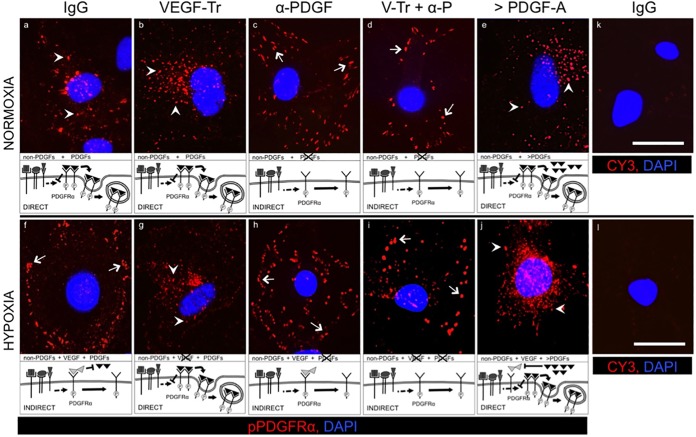
Having developed approaches to distinguish the mode of activation of PDGFRα, we used them to determine whether the relevance of the VEGF/PDGF/non-PDGF paradigm to hypoxic cells in an in vitro (Fig. 6) and in vivo (Fig. 7) setting. Said a different way, we tested the hypothesis that hypoxia switches the mode of activation of PDGFRα from direct to indirect. Indeed, we observed that PDGFRα was activated directly in cultured MEFs that were exposed to serum and PDGFs under normoxic conditions (pPDGFRα is in perinuclear vesicles; white arrowheads of Fig. 6a). In contrast, pPDGFRα was in puncta at the periphery of cells enduring hypoxia (Fig. 6f, arrows). Importantly, neutralizing VEGF switched the mode of activation from indirect to direct in hypoxic cells (Fig. 6f versus g), whereas it had no effect on normoxic cells (Fig. 6a versus b). That PDGF is essential to this switch in localization can be seen from a reversion to the peripheral punctate phenotype when neutralization of VEGF was combined with neutralization of PDGF (Fig. 6d). Furthermore, increasing the concentration of PDGFs to exceed VEGF switched the mode of activation in hypoxic cells from indirect to direct (Fig. 6f versus j). Thus, in cultured cells, hypoxia promoted indirect activation of PDGFRα. Furthermore, the underlying mechanism appeared to be the VEGF/PDGF/non-PDGF paradigm illustrated in Fig. 1A. Finally, the location of pPDGFRα enabled us to determine its mode of activation.
FIG 6.
Hypoxia triggered VEGF-mediated indirect activation of PDGFRα in cultured cells. Equal numbers of MEFs were seeded on coverslips in a 24-well plate and allowed to attach before replacing their media with DMEM containing 0.5% low serum supplemented with PDGF (0.1 nM [each] PDGF-A, PDGF-AB, and PDGF-B) for 36 h to synchronize cell cycles in G0. After this, the cells were subjected to normoxic or hypoxic conditioning (as described for Fig. 1 and in Materials and Methods) using fresh low-serum media (the same as above) supplemented using the following treatments for 36 h: control IgG (26 μg/ml), VEGF-TRAP (V-Tr, 20 μg/ml), neutralizing cocktail of antibodies against all five PDGF isoforms (α-PDGF, 5 μg/ml of antibody against each PDGF isoform), V-Tr plus anti-PDGF together (V-Tr + α-P), or a saturating dose (200 ng/ml, 7.1 nM) of PDGF-A (>PDGF-A). Media plus treatments were replaced every 12 h. No exogenous VEGF was added. With the exception of the “>PDGF-A” condition, no exogenous PDGFs were added. After treatment, the cells were subjected to immunofluorescence analysis (see Materials and Methods) with anti-pPDGFRα, followed by a CY3-conjugated secondary antibody (emitting red fluorescence) and counterstained with DAPI. Arrows indicate regions on the cell surface colocalizing with p-PDGFRα, and arrowheads indicate vesicular structures (e.g., endosomes) colocalizing with p-PDGFRα. For images a to j, the schematic at the bottom of each panel indicates the factors involved, the impact of treatment, and the predicted consequence for PDGFRα. For images k and l, there were no primary antibody controls, samples were stained with DAPI and CY3 conjugated secondary antibody, and only DAPI could be seen in the resulting images. The images are representative of three independent experiments. Scale bar, 50 μm.
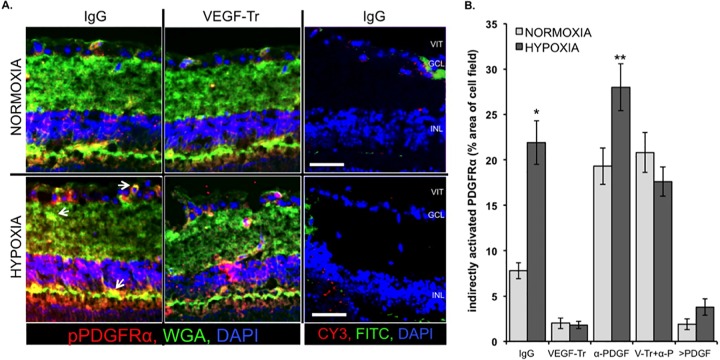
FIG 7.
VEGF enabled indirect activation of PDGFRα in mice enduring hypoxia. (A) VEGF promoted indirect activation of PDGFRα in the hypoxic mouse retina. Retinal cryosections (8 μm thick) were prepared from the eyes isolated from animals subjected to the protocol outlined in Fig. 3A. The sections were stained sequentially with WGA-488 (plasma membrane dye, green), anti-pPDGFRα plus CY3-conjugated secondary (red), and DAPI counterstain (blue) and imaged in three channels using a confocal microscope. The arrows indicate regions of WGA (green) plus pPDGFRα (red) colocalization (yellow), indicating an abundance of cell surface-localized pPDGFRα, i.e., indirectly activated PDGFRα. No primary antibody controls (the rightmost images) were stained with DAPI, CY3-conjugated secondary antibody, and a FITC-conjugated anti-rat antibody to pseudotype WGA-488. Apart from DAPI, there was minimal background fluorescence. VIT, vitreous; GCL, ganglion cell layer; INL, inner nuclear layer. Scale bars, 10 μm. (B) Quantification of the amount of indirectly activated PDGFRα. The percentages of indirectly activated PDGFRα per field of retina (×100 magnification) was determined by calculating the total sum of all yellow areas (regions of cell surface-localized pPDGFRα) in a field and dividing this amount by the total sum of all green, red, and yellow fluorescence in that field. Triple merged images (WGA green + pPDGFRα red + DAPI blue) were used for quantification. At least five contiguously adjacent fields (each 50 × 50 × 8 μm in volume) from three equally treated and conditioned retinas were quantified (n = 5 × 3). The resulting data are graphically presented as the mean % area of yellow (indirectly activated PDGFRα) per cell field ± the SD. Significant differences between normoxic and hypoxic-conditioned retinas were determined using a paired t test, where an asterisk denotes P < 0.01 and two asterisks denote P < 0.05.
Having established these methods and concepts in the well-defined in vitro setting, we applied them to live mice in order to determine whether hypoxia triggered indirect activation of PDGFRα. Mice were subjected to the same protocol used in experiments described in Fig. 3, except that their eyes were processed for immunofluorescence instead of FACS analysis. Figure 7 shows retinal sections that were stained for pPDGFR (red), WGA (green), and DAPI (blue). Dual labeling was indicative of indirect activation of PDGFRα (arrows indicate some examples of such yellow regions). Quantification of the yellow regions revealed a statistically significant increase in mice that experienced hypoxia (Fig. 7B). The hypoxia-driven increase in indirect activation of PDGFRα (yellow signal) was not observed in mice injected with VEGF-Tr (Fig. 7A, compare the left and middle panels of the bottom row). These results indicate that hypoxia triggered VEGF-mediated indirect activation of PDGFRα in vivo.
The results of additional experiments further supported the in vivo relevance of the VEGF/PDGF/non-PDGF paradigm. Neutralizing PDGFs increased the amount of indirectly activated PDGFRα (both in normoxic and in hypoxic conditions), presumably because they antagonized endogenous non-PDGFs (Fig. 7B and data not shown). This concept was reinforced by the observation that injecting PDGFs reduced the level of indirectly activated PDGFRα (Fig. 7 and data not shown). The data in Fig. 7 complement the data in Fig. 3B; under hypoxic conditions, the low levels of indirect activation of PDGFRα corresponded to a high level of apoptosis. We conclude that VEGF promotes indirect activation of PDGFRα in an in vivo setting and that the underlying mechanism involves the VEGF/PDGF/non-PDGF paradigm.
DISCUSSION
Periods of hypoxia are integral to both nonpathological processes (embryogenesis and vascular remodeling), as well as ischemia-associated pathologies (solid cancers and proliferative retinopathies) (35). Cells respond to hypoxia acutely by increasing production of many agents, including VEGF, which promotes the viability of VEGFR-positive cells such as endothelial cells. VEGF also instructs endothelial cells to undergo angiogenesis, which constitutes an enduring resolution to hypoxia (36–38). The action of VEGF on VEGFR-positive cells leaves open the question of how VEGFR-negative cell types within tissue endure periods of hypoxia. The results presented here begin to address this question. VEGF promotes viability of PDGFRα-positive cells (such as pericytes, fibroblasts, astrocytes, myofibroblasts, certain epithelial cells, etc.), and the underlying mechanism appears to involve the VEGF/PDGF/non-PDGF paradigm (Fig. 1A).
Our results demonstrate that PDGF reduces the viability of cells enduring hypoxia. This observation is somewhat of a surprise in light of PDGF's ability to engage signaling pathways that enhance survival, such as PI3K/Akt (39, 40). However, PDGF has also been reported to stimulate apoptosis (41). A resolution to this apparent paradox emerges when one considers the context under which the various experiments have been performed. To monitor PDGF-driven signaling events and cellular responses, investigators routinely attempt to eliminate all other growth factors and even subject cells to a period of growth factor deprivation prior to stimulation with PDGF. In contrast, many growth factors are present in hypoxic conditions, including all three classes of growth factors that engage PDGFRα (Table 1). Under these conditions, the indirect mode of activating PDGFRα lowers p53 and thereby promotes the viability of the cells (26). By antagonizing indirect activation of PDGFRα, PDGFs reduce the viability of cells enduring hypoxia. In other experimental settings, such as supraphysiological doses of PDGF (500 ng/ml or 17.9 nM), PDGFs reduce the level of p53, just as PDGFRα mutants that harbor constitutively activating mutations (26). These results expand our appreciation of how environmental conditions (hypoxia and the presence of growth factors) influences a cell's response to PDGF.
Long-lasting activation of PDGFRα appears to be prerequisite for it to reduce p53 and thereby enforce viability. As mentioned above, this can be accomplished with supraphysiological doses of PDGF or by introducing mutations that constitutively elevate the receptor's kinase activity. In contrast, the indirect mode engages a ROS-driven self-perpetuating loop (23). Non-PDGFs directly activate their cognate receptors to boost the level of NADPH oxidase-generated ROS, which stimulate SFKs that phosphorylate and thereby stimulate the kinase activity of monomeric PDGFRα (23). The half-life of these activated PDGFRα monomers is long because they are not shuttled into the endocytosis/degradation pathway as are activated PDGFRα dimers (14, 23). Furthermore, indirectly activated PDGFRα triggers signaling events that block autophagy, i.e., the clearance of mitochondria (14, 23). This increases the level of mitochondrially produced ROS and thereby perpetuates the ROS/SFK driven pathway to activate monomeric PDGFRα (23). The importance of this self-perpetuating loop for indirect mode of activating RTKs is underscored by the discovery that PDGFRβ, which does not undergo indirect activation, acquires the ability to do so once it becomes capable of elevating mitochondrial ROS (25, 42). These studies provide mechanistic insight into how PDGFRα is persistently activated. The VEGF/PDGF/non-PDGF paradigm is one explanation for how this could occur in hypoxic tissues where many growth factors are simultaneously present.
The results presented here expand the breadth of influence of VEGF. It acts not only on endothelial cells to promote their survival and stimulate angiogenesis but also on PDGFRα-positive cells to promote their survival during periods of stress such as hypoxia. VEGF even acts via PDGFRα to promote survival of cell types that express both VEGFRs and PDGFRα, such as retinal pigment epithelial cells (12).
Neutralizing VEGF is the standard of care for patients with the neovascular form of age-related macular degeneration (43). Many (but not all) such patients experience an improvement in vision, which is attributed to resolution of edema. The studies described here predict the PDGFRα-positive cells (such as pericytes, fibroblasts, astrocytes, myofibroblasts, and retinal pigment epithelial cells) perish in the hypoxic regions of the retina of patients treated in this way. Our studies also predict that neutralizing both VEGF and PDGFs will spare the PDGFRα-positive cells of the retina. Preclinical studies demonstrate that the combination therapy is more effective than either monotherapy (44). Ongoing clinical trials will reveal whether neutralizing both PDGF and VEGF is more beneficial than neutralizing VEGF alone. Should this indeed be the case, then determining whether combo therapy enhances survival of PDGFRα-positive cells would be the next logical step.
ACKNOWLEDGMENTS
We thank Schepens and Massachusetts Eye and Ear researchers who provided insight, technical assistance, and critical analysis of our data. Specifically, we thank Maximilian Gerhardt for assisting with immunofluorescence, Jorge Aranda for help establishing tissue explants, Ruta Motiejunaite and Jessica Lanzim for help with establishment of the in vivo experiments, and especially Dhanesh Amarnani, who assisted L.A.K. and S.P. with intravitreal pup injections. We also thank Magali Saint-Geniez for supplying the plasmids and primers required for real-time PCR. We particularly thank Sarah Jacobo for technical guidance, reviewing the manuscript, and constructive input.
This research was supported by the National Institutes of Health grants EY012509 and EY022979 (A.K.), Harvard-Vision Clinical Scientist Development Program Research grant 5K12EY016335 (L.A.K.), and fellowship support from the Canadian Institutes of Health Research (S.P.).
S.P. designed the experiments and wrote the article. L.A.K. and S.P performed the in vivo mouse experiments. A.K. assisted with experimental design, critical evaluation of the data, and writing of the manuscript.
We declare no competing financial interests.
REFERENCES
- 1.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, Fraticelli P, Sambo P, Funaro A, Kazlauskas A, Avvedimento EV, Gabrielli A. 2006. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 2.Dolloff NG, Russell MR, Loizos N, Fatatis A. 2007. Human bone marrow activates the Akt pathway in metastatic prostate cells through transactivation of the alpha-platelet-derived growth factor receptor. Cancer Res 67:555–562. doi: 10.1158/0008-5472.CAN-06-2593. [DOI] [PubMed] [Google Scholar]
- 3.Heeneman S, Haendeler J, Saito Y, Ishida M, Berk BC. 2000. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor: key role for the p66 adaptor protein Shc. J Biol Chem 275:15926–15932. [DOI] [PubMed] [Google Scholar]
- 4.Herrlich A, Daub H, Knebel A, Herrlich P, Ullrich A, Schultz G, Gudermann T. 1998. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc Natl Acad Sci U S A 95:8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei H, Kazlauskas A. 2009. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem 284:6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linseman DA, Benjamin CW, Jones DA. 1995. Convergence of angiotensin II and platelet-derived growth factor receptor signaling cascades in vascular smooth muscle cells. J Biol Chem 270:12563–12568. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. 2007. The 5-HT transporter transactivates the PDGFβ receptor in pulmonary artery smooth muscle cells. FASEB J 21:2725–2734. doi: 10.1096/fj.06-8058com. [DOI] [PubMed] [Google Scholar]
- 8.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884–888. [DOI] [PubMed] [Google Scholar]
- 9.Siegbahn A, Johnell M, Nordin A, Aberg M, Velling T. 2008. TF/FVIIa transactivate PDGFRbeta to regulate PDGF-BB-induced chemotaxis in different cell types: involvement of Src and PLC. Arterioscler Thromb Vasc Biol 28:135–141. [DOI] [PubMed] [Google Scholar]
- 10.Tanimoto T, Lungu AO, Berk BC. 2004. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ Res 94:1050–1058. doi: 10.1161/01.RES.0000126404.41421.BE. [DOI] [PubMed] [Google Scholar]
- 11.Pennock S, Haddock LJ, Eliott D, Mukai S, Kazlauskas A. 2014. Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res 40:16–34. doi: 10.1016/j.preteyeres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Pennock S, Haddock LJ, Mukai S, Kazlauskas A. 2014. Vascular endothelial growth factor acts primarily via platelet-derived growth factor receptor alpha to promote proliferative vitreoretinopathy. Am J Pathol 184:3052–3068. doi: 10.1016/j.ajpath.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennock S, Kim D, Mukai S, Kuhnle M, Chun DW, Matsubara J, Cui J, Ma P, Maberley D, Samad A, Van Geest RJ, Oberstein SL, Schlingemann RO, Kazlauskas A. 2013. Ranibizumab is a potential prophylaxis for proliferative vitreoretinopathy, a nonangiogenic blinding disease. Am J Pathol 182:1659–1670. doi: 10.1016/j.ajpath.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennock S, Kazlauskas A. 2012. Vascular endothelial growth factor A competitively inhibits platelet-derived growth factor (PDGF)-dependent activation of PDGF receptor and subsequent signaling events and cellular responses. Mol Cell Biol 32:1955–1966. doi: 10.1128/MCB.06668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenkranz S, DeMali KA, Gelderloos JA, Bazenet C, Kazlauskas A. 1999. Identification of the receptor-associated signaling enzymes that are required for platelet-derived growth factor-AA-dependent chemotaxis and DNA synthesis. J Biol Chem 274:28335–28343. doi: 10.1074/jbc.274.40.28335. [DOI] [PubMed] [Google Scholar]
- 16.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. 2000. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20:9018–9027. doi: 10.1128/MCB.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozarowski P, Grabarek J, Darzynkiewicz Z. 2004. Flow cytometry of apoptosis. Curr Protoc Cell Biol Chapter 18:Unit 18. doi: 10.1002/0471143030.cb1808s21. [DOI] [PubMed] [Google Scholar]
- 18.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. 2009. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 19.Pozarowski P, Darzynkiewicz Z. 2004. Analysis of cell cycle by flow cytometry. Methods Mol Biol 281:301–311. [DOI] [PubMed] [Google Scholar]
- 20.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. 1994. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35:101–111. [PubMed] [Google Scholar]
- 21.Wang Y, Pennock S, Chen X, Wang Z. 2002. Internalization of inactive EGF receptor into endosomes and the subsequent activation of endosome-associated EGF receptors: epidermal growth factor. Sci STKE 2002:pl17. [DOI] [PubMed] [Google Scholar]
- 22.Pennock S, Rheaume MA, Mukai S, Kazlauskas A. 2011. A novel strategy to develop therapeutic approaches to prevent proliferative vitreoretinopathy. Am J Pathol 179:2931–2940. doi: 10.1016/j.ajpath.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei H, Kazlauskas A. 2014. A reactive oxygen species-mediated, self-perpetuating loop persistently activates platelet-derived growth factor receptor alpha. Mol Cell Biol 34:110–122. doi: 10.1128/MCB.00839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aylon Y, Oren M. 2007. Living with p53, dying of p53. Cell 130:597–600. doi: 10.1016/j.cell.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Lei H, Rheaume MA, Velez G, Mukai S, Kazlauskas A. 2011. Expression of PDGFRα is a determinant of the PVR potential of ARPE19 cells. Invest Ophthalmol Vis Sci 52:5016–5021. doi: 10.1167/iovs.11-7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei H, Velez G, Kazlauskas A. 2011. Pathological signaling via platelet-derived growth factor receptor α involves chronic activation of Akt and suppression of p53. Mol Cell Biol 31:1788–1799. doi: 10.1128/MCB.01321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ball SG, Shuttleworth CA, Kielty CM. 2007. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol 177:489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. 2007. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. 2003. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldin CH, Wasteson A, Westermark B. 1982. Interaction of platelet-derived growth factor with its fibroblast receptor: demonstration of ligand degradation and receptor modulation. J Biol Chem 257:4216–4221. [PubMed] [Google Scholar]
- 31.Rosenkranz S, Ikuno Y, Leong FL, Klinghoffer RA, Miyake S, Band H, Kazlauskas A. 2000. Src family kinases negatively regulate platelet-derived growth factor alpha receptor-dependent signaling and disease progression. J Biol Chem 275:9620–9627. doi: 10.1074/jbc.275.13.9620. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter G. 1987. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem 56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- 33.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. 1990. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62:317–329. doi: 10.1016/0092-8674(90)90369-P. [DOI] [PubMed] [Google Scholar]
- 34.Mohrmann K, van der Sluijs P. 1999. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol Membr Biol 16:81–87. doi: 10.1080/096876899294797. [DOI] [PubMed] [Google Scholar]
- 35.Pouyssegur J, Dayan F, Mazure NM. 2006. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 36.Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. 2006. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci 63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara N. 2004. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 38.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. 1998. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343. [DOI] [PubMed] [Google Scholar]
- 39.Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. 1989. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57:167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- 40.Coughlin SR, Escobedo JA, Williams LT. 1989. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science 243:1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- 41.Kim HR, Upadhyay S, Li G, Palmer KC, Deuel TF. 1995. Platelet-derived growth factor induces apoptosis in growth-arrested murine fibroblasts. Proc Natl Acad Sci U S A 92:9500–9504. doi: 10.1073/pnas.92.21.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei H, Qian CX, Lei J, Haddock LJ, Mukai S, Kazlauskas A. 2015. RasGAP promotes autophagy and thereby suppresses platelet-derived growth factor receptor-mediated signaling events, cellular responses, and pathology. Mol Cell Biol 35:1673–1685. doi: 10.1128/MCB.01248-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfeld PJ, Heier JS, Hantsbarger G, Shams N. 2006. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology 113:623 e621. doi: 10.1016/j.ophtha.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. 2006. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]