Abstract
Background
This study investigated diabetes (DB) and heart disease (HD) family health history (FHH) knowledge and changes following provision of personalized disease risk feedback.
Methods
497 adults from 162 Mexican-origin families were randomized by household to conditions based on feedback recipient and content. Each provided personal and relatives’ DB and HD diagnoses and received feedback materials following baseline assessment. Multivariate models were fitted to identify factors associated with the rate of “don’t know” FHH responses.
Results
At baseline U.S. nativity was associated with a higher “don’t know” response rate (p=0.002). Though confounded by country of birth, younger age showed a trend toward higher “don’t know” response rates. Overall, average “don’t know” response rates dropped from 20% to 15% following receipt of feedback (p<0.001). An intervention effect was noted, as “don’t know” response rates decreased more in households where one family member (vs all) received supplementary risk assessments (without behavioral recommendations) (p=0.011).
Conclusions
Limited FHH knowledge was noted among those born in the US and younger participants, representing a key population to reach with intervention efforts. The intervention effect suggests that “less is more” indicating the potential for too much information to limit health education program effectiveness.
Keywords: Family health history, Mexican Americans, diabetes, heart disease
Introduction
Family health history (FHH) represents the combined effects of genetic, environmental, and social factors that contribute to disease risk.1 Consequently, FHH is a strong predictor of disease, and is an important clinical tool for identifying those at increased risk of common, complex conditions.2,3 Knowledge of FHH has important implications for health care delivery, including screening and lifestyle recommendations targeted to early detection and disease prevention.4,5 Indeed, research suggests that, for heart disease and diabetes, an individual with just one affected FDR or two affected SDRs is considered to be at increased risk for developing these diseases.3,6–9
Consequently, FHH knowledge is highly relevant to the assessment of diabetes and heart disease risk. Identifying those at increased risk for these chronic conditions enables health professionals to recommend appropriate preventive actions, which if applied by the patient, have the potential to prevent disease onset.10 But, in order for this personalization in health care to be effective, patients must have accurate FHH information; thus, not knowing one’s FHH can have serious consequences for understanding and assessing chronic disease risk and identification of appropriate preventive strategies. Unfortunately, FHH knowledge is limited in the United States, as active collection of FHH information from family members is generally infrequent and incomplete.11,12
The need for effective programs aimed at improving FHH knowledge is particularly relevant for immigrant and minority families, who more often experience language and communication barriers between family members as well as with health care providers; less access to or engagement in the medical system; and lack of medical and health knowledge regarding the role of family health history as a risk factor for many diseases.13–19 Mexican Americans, for instance, comprising almost 10% of the U.S. population, are almost twice as likely as non-Hispanic whites to develop diabetes. Further, diabetes is a known risk factor for heart disease, which is one of the leading causes of death in the United States.20–22 Thus, it is critical to identify effective approaches for improving FHH knowledge in this at-risk group.
In addition to engaging this often understudied population, the current report also improves upon the limited literature investigating factors associated with individual’s knowledge of their FHH. In contrast to the traditional clinical visit recruitment, a community-based recruitment approach was used, which allowed access to a more diverse set of participants who may not have been actively engaged in the health care system.23 Moreover, a more sensitive measure of FHH knowledge was used based on the gold standard - a detailed three-generation FHH assessment.24 This is distinct from the global assessments of perceived familiarity with FHH used in previous research.25
Despite the documented widespread lack of FHH knowledge and the importance of FHH information for assessing disease risk and tailoring of preventive strategies, very little research has examined ways to improve FHH knowledge, particularly among underserved minority populations at increased risk. Therefore, the current study aimed to assess FHH-based knowledge and evaluate improvement in knowledge following an intervention among a largely immigrant minority sample of Mexican origin families. Specifically, the study aimed to assess: 1) the demographic and health care-related characteristics associated with limited FHH knowledge for diabetes and heart disease at baseline assessment, 2) the demographic and health care-related characteristics associated with change in FHH knowledge at follow-up assessment, and 3) whether a family-based intervention providing FHH-based risk feedback can improve FHH knowledge.
Methods
Data collection
A total of 497 Mexican origin adults aged 18–75 years from 162 households in Houston, Texas were recruited for Project Risk Assessment for Mexican Americans (RAMA).23 Three or four members representing at least two generations from each household participated in a family-based intervention study that aimed to use FHH information to promote disease risk communication, encourage risk reducing behaviors, and motivate engagement in health promoting activities.23,26,27 Baseline and ten-month follow-up surveys were conducted between October 2007 and January 2010 with rolling accrual, and participants were compensated with a $20 gift card upon completion of each assessment. Approval for this research was obtained from the MD Anderson Cancer Center and the National Human Genome Research Institute at the National Institutes of Health (#NCT00469339).
Assessments were completed in the participants’ preferred language of either English or Spanish. Each participant enumerated all of his/her biological FDRs and SDRs via telephone survey prior to baseline data collection. The baseline survey was conducted in the home of each participating family. Each participating family member completed the survey independently on provided tablet computers; participants were asked not to discuss the survey questions with each other until after the assessment. Bilingual interviewers, blinded to household randomization, were available to answer any questions participants may have about survey items. Follow-up assessments were completed via telephone interview, on average, ten months after baseline to allow sufficient time to detect any long-term behavioral changes and allow for the diffusion of FHH or disease risk information within families. Assessments included participant socio-demographic characteristics, health care access and utilization, and a detailed three-generation FHH, including age at diagnosis, if known, for each enumerated family member. Participants were free to skip or refuse to answer any question on the survey or in the interview.
Household Randomization and Feedback
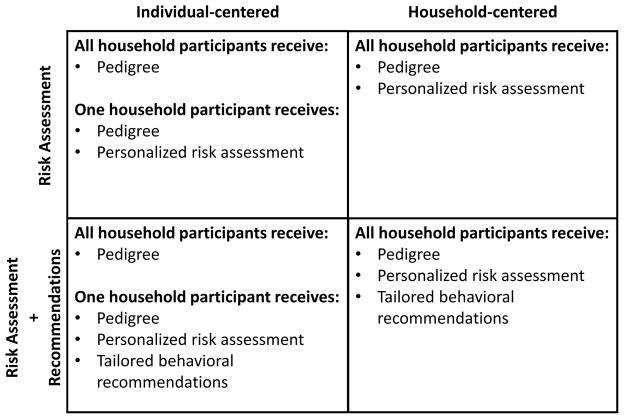
The primary goal of the study was to identify optimal approaches for introducing FHH-based risk information into family systems to improve FHH knowledge and engagement in health promoting behaviors. Households were randomized into one of four feedback conditions defined by a 2×2 factorial design (Figure 1). The first factor determined which household members, one or all participating family members, received supplemental personalized FHH-based risk assessments indicating their risk of developing DB and HD disease in their lifetime. The second factor determined whether additional behavioral recommendations would be provided in conjunction with the supplemental risk assessments. Behavioral recommendations focused on screening and lifestyle factors aimed at risk reduction.
Figure 1.
Baseline FHH information provided by each participant was used to develop individualized risk feedback packets, which were mailed to each participant within one week of the baseline assessment.23 Feedback content was generated using Family Healthware™, the Centers for Disease Control and Prevention’s FHH tool.5 While every participant received a feedback packet with a personal pedigree depicting their individually reported FHH, additional content of the feedback packet was determined by participants’ household randomization status. Participants who received supplemental personalized risk assessments were provided a packet with a pedigree and separate documents referring to their heart disease risk and diabetes risk. The risk messages were color-coded with red, yellow, green, and white, indicating strong risk, moderate risk, weak (population) risk, and currently affected by the disease, respectively. Risk messages were based on specific algorithms that incorporate the number of affected FDRs, SDRs, and their age at diagnosis.3 Behavioral recommendations included information about screening tests for blood glucose, blood pressure and cholesterol, as well as lifestyle behaviors such as weight management, physical activity, fruit and vegetable consumption, smoking, alcohol intake, and aspirin use. Each recommendation was personalized to the individual participant’s reported behaviors at baseline and was dependent on their identified FHH-based risk level.
Measures
FHH knowledge regarding diabetes and heart disease
At the baseline and ten-month assessments, participants indicated for each FDR and SDR whether he or she had been diagnosed with diabetes and/or heart disease. Available response options to these FHH questions were “yes,” “no,” or “don’t know.”
A measure of limited FHH knowledge was constructed based on the proportion of enumerated FDRs and SDRs for whom respondents selected a “don’t know” response to the diagnosis status questions. For instance, a participant with five FDRs and fifteen SDRs would have a total of twenty enumerated family members. If the participant selected the “don’t know” response for the diabetes diagnosis status of four of his relatives, he would be lacking 20% (4/20) of his FHH for diabetes resulting in a proportion of “don’t know” responses equal to.20. These proportions were then averaged across diabetes and heart disease to obtain an aggregate measure. This measure allows for the comparison of FHH knowledge while controlling for the differences in family size across participants. A difference score was computed where the baseline “don’t know” proportion was subtracted from the ten-month follow-up proportion. Negative values indicate improved knowledge, with a reduction in “don’t know” responses; positive values indicate an increase in “don’t know” responses.
Participant characteristics
Participant age was collected as a continuous variable and then categorized roughly by decades, with 18 to 29 year-olds as the youngest group and those 60+ years of age comprising the oldest group. Demographic characteristics included gender, country of birth, education level, marital status, parenthood, and a proxy for socio-economic status (owning both their home and car).28,29 Participants also reported their health insurance status and whether they had visited a health care provider in the last year. All participant characteristics were obtained through self-report at baseline assessment.
Statistical analysis
A conservative approach was used to estimate the required sample size for this study, one where family members’ FHH knowledge was assumed to be highly correlated. Power analyses indicated that 140 households would be required to detect a medium effect size (Cohen’s f =.15) using a Type I error rate of.05 and achieving a power equal to 0.80, controlling for covariates. Analyses presented utilize responses from 444 members of 157 families, indicating sufficient power to address the aims of this report.
Data were analyzed in SPSS using linear regression including Generalized Estimating Equations with an exchangeable covariance structure to control for the clustering of responses within families and household feedback condition.30 Analyses for Aim 1 involved fitting both bivariate and multivariate models to identify participant characteristics including age, gender, health insurance status, health care provider visit within the last year, marital status, parenthood, and car and home ownership on the outcome variable, proportion of “don’t know” responses. The randomization process did not result in intervention groups with an equal distribution of proportion of “don’t know” responses to the FHH questions. To address this issue, we modeled the difference, or change, in proportion of “don’t know” responses between baseline and 10-month follow-up. Thus, analyses for Aim 2 considered the association between these difference scores and participant characteristics in both bivariate and multivariate models. Finally, to address Aim 3, both the main effects and the interaction between the two factors defining feedback conditions (i.e., risk assessment recipient and inclusion of behavioral recommendations) were entered into the model, controlling for the participant characteristics.
Results
Demographics
Participant characteristics for 444 participants from 157 families are shown in Table 1. We excluded those lost to follow-up (N=38) and those with missing data on one or more key socio-demographic or health care access variables (N=15) from the analysis. On average, participants were 41 years old (SD=15 years). At baseline, three-quarters of the participants identified themselves as parents, 70% were married, and just over half of the participants were female. The majority of these participants were not born in US (70%) and 58% had not completed high school. Fifty four percent of participants indicated that they owned both their car and their home. Further, almost two-thirds of the participants indicated that they had health insurance, with 90% stating that they had seen a health care professional in the last year. With regard to family structure, the majority (77.7%) of participating families included nuclear families (i.e., parents and their adult child), followed by blended families (12.1%) and married couples with an elderly parent living in the home (7.6%). The remainder generally included adult children living with their extended family (e.g., grandparents, an aunt and uncle).
Table 1.
Socio-demographic characteristics of study participants completing both baseline and ten-month follow-up assessment (N=444)
| Characteristics | Total (%) |
|---|---|
| Gender | |
| Male | 196 (44.1%) |
| Female | 248 (55.9%) |
| Age Group | |
| 18 – 20 years | 138 (31.1%) |
| 30 – 39 years | 45 (10.1%) |
| 40 – 49 years | 110 (24.8%) |
| 50 – 59 years | 108 (24.3%) |
| 60 + years | 43 (9.7%) |
| Place of birth | |
| U.S. | 132 (29.7%) |
| Other country | 312 (70.3%) |
| Marital Status | |
| Married | 312 (70.3%) |
| Not married | 132 (29.7%) |
| Parental Status | |
| Parent | 333 (75.0%) |
| Not a parent | 111 (25.0%) |
| Socio-economic Status | |
| Owns house and car | 239 (53.8%) |
| Does not own house and car | 205 (46.2%) |
| Educational level | |
| < High school education | 258 (58.1%) |
| High school graduate/GED or above | 186 (41.9%) |
| Insurance Status | |
| Any health insurance | 280 (63.1%) |
| No Health Insurance | 164 (36.9%) |
| Healthcare Usage | |
| Healthcare visit in last year | 400 (90.1%) |
| No healthcare visit in last year | 44 (9.9%) |
Aim 1: FHH knowledge by participant characteristics at baseline
The mean proportion of “don’t know” responses by socio-demographic and health care access characteristics at the baseline assessment are presented in Table 2. On average, participants did not know about 20% of their FHH at the baseline assessment. Bivariate associations indicate that those who are married (16% vs. 29%; p<.001), parents (16% vs. 32%; p<.001), and those who owned both their car and their home (17% vs. 23%; p=.005) reported knowing more of their FHH. Participants in the youngest age group (18–29 years) reported a significantly higher proportion of “don’t know” responses to FHH questions (30%) when compared to each of the other age groups (14–17%, ps<0.001), while no significant differences were noted between the other age groups.
Table 2.
Mean (SD) proportion of “don’t know” responses at baseline and follow-up by socio-demographic characteristics with bivariate test of change in proportion of “don’t know” responses by characteristic (N=444)
| Characteristics | Baseline | Follow-up | Wald | p value |
|---|---|---|---|---|
| Overall | 0.199 (.240) | 0.155 (.179) | 15.505 | <0.001 |
| Gender | 0.093 | 0.761 | ||
| Male | 0.215 (.263) | 0.168 (.192) | ||
| Female | 0.185 (.219) | 0.145 (.167) | ||
| Age Group | 25.99 | 0.001 | ||
| 18 – 29 | 0.304 (.299) | 0.181 (.212) | ||
| 30 – 39 | 0.174 (.211) | 0.135 (.155) | ||
| 40 – 49 | 0.149 (.194) | 0.123 (.145) | ||
| 50 –59 | 0.145 (.187) | 0.148 (.167) | ||
| 60 + | 0.142 (.164) | 0.190 (.179) | ||
| Place of Birth | 10.58 | 0.001 | ||
| U.S. | 0.299 (.275) | 0.197 (.208) | ||
| Other | 0.156 (.210) | 0.137 (.161) | ||
| Marital Status | 16.923 | <0.001 | ||
| Married | 0.158 (.200) | 0.149 (.169) | ||
| Other | 0.292 (.295) | 0.170 (.199) | ||
| Parental Status | 15.04 | <0.001 | ||
| Parent | 0.159 (.194) | 0.145 (.164) | ||
| Not a parent | 0.317 (.314) | 0.183 (.215) | ||
| SES | 4.42 | 0.035 | ||
| Owns both house and car | 0.172 (.201) | 0.148 (.158) | ||
| Does not own house and car | 0.229 (.275) | 0.164 (.200) | ||
| Educational Level | 10.34 | 0.001 | ||
| < High school education | 0.161 (.210) | 0.147 (.163) | ||
| High school graduate/GED or above | 0.249 (.268) | 0.167 (.199) | ||
| Insurance Status | 1.011 | 0.315 | ||
| Any health insurance | 0.207 (.236) | 0.173 (.184) | ||
| No health insurance | 0.182 (.246) | 0.124 (.166) | ||
| Healthcare Use | 0.235 | 0.628 | ||
| Healthcare visit in past year | 0.195 (.234) | 0.154 (.173) | ||
| No healthcare visit in past year | 0.228 (.283) | 0.167 (.224) |
Note: GEE adjustment with exchangeable covariance structure to account for clustering within the family looking at change from baseline to follow-up for each variable separately (i.e. bivariate associations).
Table 3 presents the multivariate models for the proportion of “don’t know” responses at baseline regressed on the socio-demographic characteristics of the participants. At baseline, US nativity is the only significant factor in the model (p=0.002), with those in the younger age group showing a trend towards higher “don’t know” response rates.
Table 3.
Multivariate Regression. Baseline proportion of “don’t know” responses and ten-month change in proportion of “don’t know” responses regressed on covariates. Ten-month change in proportion of “don’t know” responses considers household randomization. (N=444)
| Baseline “Don’t know” responses [B (SE)] | p value | Change in “Don’t know” responses at follow-up [B (SE)] | p value | |
|---|---|---|---|---|
| Intercept | 0.178 (0.057) | 0.002 | 0.012 (.060) | 0.845 |
| Gender | ||||
| Female | −0.031 (0.023) | 0.181 | 0.013 (0.024) | 0.610 |
| Male | reference | reference | ||
| Place of Birth | ||||
| U.S. | 0.086 (0.028) | 0.002 | −0.046 (.030) | 0.132 |
| Other | reference | reference | ||
| Marital Status | ||||
| Married | 0.019 (0.044) | 0.665 | 0.035 (0.039) | 0.364 |
| Other | reference | reference | ||
| Parental Status | ||||
| Parent | −0.068 (0.050) | 0.181 | 0.026 (0.046) | 0.577 |
| Not a parent | reference | reference | ||
| Age Group | ||||
| 18 – 29 | 0.103 (0.056) | 0.065 | −0.111 (0.045) | 0.014 |
| 30 – 39 | 0.039 (0.040) | 0.332 | −0.075 (0.046) | 0.106 |
| 40 – 49 | 0.022 (0.033) | 0.513 | −0.069 (0.034) | 0.042 |
| 50 –59 | 0.014 (0.034) | 0.675 | −0.040 (0.035) | 0.253 |
| 60 + | reference | reference | ||
| SES | ||||
| Owns both house and car | 0.006 (0.024) | 0.805 | −0.021 (0.022) | 0.335 |
| Does not own house and car | reference | reference | ||
| Educational Level | ||||
| < HS Education | reference | reference | ||
| HS Diploma + | 0.011 (0.024) | 0.634 | −0.018 (0.026) | 0.484 |
| Insurance Status | ||||
| Health Insurance | reference | reference | ||
| No Health Insurance | −0.008 (0.025) | 0.737 | −0.034 (0.024) | 0.154 |
| Healthcare System Use | ||||
| In past year | reference | reference | ||
| Not in past year | −0.002 (0.044) | 0.965 | 0.026 (0.039) | 0.514 |
| Intervention Effect | ||||
| One received RAs | 0.044 (0.028) | 0.117 | ||
| All received RAs | reference | |||
| No Behavioral Recs | 0.020 (0.030) | 0.513 | ||
| Behavioral Recs | reference | |||
| One received RAs x | −0.104 (0.041) | 0.011 | ||
| No Behavioral Recs | ||||
| Other (all no recs, all+recs, one+recs) | reference | |||
Note: GEE adjustment with exchangeable covariance structure to account for clustering within the family looking at change from baseline to follow-up for each variable separately (i.e. bivariate associations). RAs = Risk Assessments; Recs = Recommendations
Aims 2: FHH knowledge by participant characteristics at follow-up
Table 2 presents the mean “don’t know” responses by socio-demographic and health care access variables at ten-month follow-up and tests the change in “don’t know” responses between baseline and follow-up for each variable. As mentioned earlier, participants at baseline reported that, on average, they did not know about 20% of their FHH for diabetes and heart disease. At ten-month follow-up, average “don’t know” responses decreased significantly to 16% (p≤0.001).
On a bivariate level, significant decreases in “don’t know” responses were noted at follow-up by US nativity (p=.001), marital status (p<.001), parental status (p<.001), education (p=.001), and socio-economic status (p=.035), with the largest decrease seen amongst US-born participants, those who reported being unmarried, those without children, and those with higher education and socio-economic status. There were no significant differences at follow-up in percent of “don’t know” responses by gender, health insurance status, or whether the participant had visited a health care provider in the last year.
Table 3 presents the multivariate model for change in proportion of “don’t know” responses ten months after receipt of personalized disease risk feedback for heart disease and diabetes. As indicated by the negative coefficients, significant decreases in the percent of “don’t know” FHH responses were seen for the 18–29 (p=.014) and 40–49 (p=.042) year old groups compared to those over 60 years, when controlling for other socio-demographic factors and intervention feedback condition.
Aim 3: Risk information impact on FHH knowledge at follow-up
There was a significant interaction between the two factors characterizing the feedback conditions (Table 3; p=.011). Surprisingly, we noted significant improvement in FHH knowledge among participants randomized to the intervention condition wherein one participating family member (vs all participating family members) received his/her pedigree and risk assessment information without behavioral recommendations (vs with behavioral recommendations).
Discussion
The current report investigated factors associated with having limited FHH knowledge of heart disease and diabetes among members of Mexican origin families. At baseline, participants did not know about 20% of their FHH based on their first and second degree relatives. Younger participants and participants born in the U.S. had a greater proportion of “don’t know” responses. At follow-up, the proportion of “don’t know” responses significantly decreased, especially for those aged 18 to 29 years. Further, participants from households receiving minimal feedback information reported significantly improved FHH knowledge at the 10-month follow-up assessment when compared to those in the other feedback conditions.
At baseline, US nativity was significantly associated with reporting more “don’t know” responses to FHH questions when controlling for all other variables. However, nativity is strongly confounded with age in this sample of Mexican American immigrant families, with mostly younger participants being born in the US; consequently, younger participants reported less FHH knowledge. Considered together, younger age and nativity appear to play important roles in FHH knowledge, possibly due to geographical distance, social distance, or language barriers between the younger generations and those relatives still in Mexico or US-based relatives born in Mexico. This notion was validated, when age, rather than nativity, was found to be a significant predictor of improved FHH knowledge following intervention, suggesting that FHH interventions may have an important role in spurring intergenerational transfer of FHH information.
Increased FHH knowledge allows for the development of more accurate risk perceptions and assessment of appropriate screening and lifestyle recommendations, which play critical roles in the prevention of diabetes and other risk factors associated with heart disease.31,32 For example, according to the American Diabetes Association, individuals with an FHH indicating increased risk of diabetes should begin blood glucose screening starting at an earlier age (i.e. 18 years vs. 45 years for general population) and screen at more frequent intervals.33 Thus, given that age is a key factor related to FHH knowledge, targeting interventions to young individuals at increased risk for chronic diseases like heart disease and diabetes could be an important step in disease prevention. As well, family members’ disease diagnoses may be leveraged as “teachable moments” for increasing communication of FHH across generations.34 Recognition of risk and subsequent changes in lifestyle and health behaviors earlier in life is more effective in the prevention of chronic diseases, further highlighting the benefits of targeting younger individuals by mobilizing older generation family members.2,3
This study also assessed the impact of a family-based FHH intervention aimed at improving participants’ FHH knowledge. Participants, on average, exhibited a significant decrease in the proportion of their FHH that they “don’t know” from baseline to ten-month follow-up, particularly among the 18 to 29 year old participants. Unexpectedly, providing personalized pedigrees to each participating family member with only one family member receiving supplemental risk assessments with no lifestyle and screening recommendations was significantly more effective at improving FHH knowledge than all other intervention conditions. Perhaps the provision of risk assessments to all participating family members focused communication on shared disease risk, rather than identifying and reconciling discrepancies in their family health histories. As well, the provision of tailored behavioral recommendations may have focused family communication towards strategies for risk reduction.35,36 These results suggest that public health interventions might be improved if information is provided in phases so that people are not processing too much information at a given time. The first phase intervention would provide information aimed at increasing risk awareness, whereas the second phase intervention, occurring later in time, would provide behavioral recommendations aimed at health promotion and disease prevention. While promising, the intervention effect should be interpreted with the caveat that baseline “don’t know” responses were not equally distributed across intervention groups; as such, future research should investigate the robustness of these results.
Limitations
The current project has a few limitations. First, the results may not be generalizable beyond the Harris County-based, Mexican American population; however, this study offers an important foundation for further studies involving family disease risk assessment within other populations, including other minority and immigrant groups. Future research is warranted to evaluate whether these results replicate in families from different cultural contexts. While participants’ country of birth was known, the specific regions of birth was not known, thus potential regional affects cannot be evaluated herein. Also, the FHH information is self-reported and not verified by health care providers. However, the ascertained information reflects that which would be offered to providers in a clinical setting, with the added benefit of the ability to disentangle a “no” from a “don’t know” in FHH assessment. Finally, the interpersonal mechanisms that may have resulted in improved FHH knowledge, such as sharing of risk feedback or communication about FHH, were not explored in the current paper. Future research that identifies those interpersonal mechanisms within families that improve FHH knowledge will be imperative for designing effective family-based FHH interventions.
Conclusions
FHH knowledge of common chronic diseases may be limited among those who are at high risk for developing these conditions in their lifetime, consequently impeding the provision of appropriate screening and behavioral recommendations to at-risk individuals in clinical settings. In other words: what you don’t know can hurt you. The findings of the current study have important, novel implications for research and practice. Our results suggest that a family-based FHH feedback intervention implemented in the community setting has the potential to improve FHH knowledge, especially among younger family members, thereby resulting in more informed patients during clinical visits.2,4,37 Importantly, our findings show that “less is more” pointing to the potential for too much information to limit health education program effectiveness. Thus, family-based FHH interventions may be more effective in improving FHH knowledge when focused on reconciling discrepancies in members’ detailed FHH, rather than providing personalized risk assessments or tailored risk-reducing strategies. Given that the onset of chronic diseases, such as diabetes and heart disease, is occurring at progressively younger ages, reaching the young, healthy population at potentially increased risk of disease and improving their FHH knowledge may have important implications in disease prevention through improved risk assessment and tailored behavioral recommendations.38,39
Acknowledgments
We would like to extend our gratitude to our research participants, and everyone on the Project RAMA and Mano a Mano teams at The University of Texas M.D. Anderson Cancer Center. This study was supported by the Intramural Research Program of the National Human Genome Research Institute at the National Institutes of Health [Z01HG200335 to LMK]. Dr. Anna V. Wilkinson is funded by the National Cancer Institute [CA126988]. The authors have no conflicts of interest.
Footnotes
Clinicaltrials.gov: The Role of Family History and Culture in Communal Coping within Mexican-American Families-#NCT00469339
References
- 1.Guttmacher AE, Collins FS, Carmona RH. The Family History — More Important Than Ever. New England Journal of Medicine. 2004 Nov 25;351(22):2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- 2.Rich EC, Burke W, Heaton CJ, et al. Reconsidering the family history in primary care. J Gen Intern Med. 2004 Mar;19(3):273–280. doi: 10.1111/j.1525-1497.2004.30401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheuner MT, Wang S-J, Raffel LJ, Larabell SK, Rotter JI. Family history: A comprehensive genetic risk assessment method for the chronic conditions of adulthood. American Journal of Medical Genetics. 1997 Aug 22;71(3):315–324. doi: 10.1002/(sici)1096-8628(19970822)71:3<315::aid-ajmg12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi N, Wilson B, Santaguida P, et al. NIH State-of-the-Science Conference: Family History and Improving Health. Evidence report/technology assessment. 2009 Aug;(186):1–135. [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon PW, Scheuner MT, Jorgensen C, Khoury MJ. Developing Family Healthware, a family history screening tool to prevent common chronic diseases. Prev Chronic Dis. 2009 Jan;6(1):A33. [PMC free article] [PubMed] [Google Scholar]
- 6.JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005 Dec 01;91(suppl_5):v1–v52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall R, Saukko PM, Evans PH, Qureshi N, Humphries SE. Assessing family history of heart disease in primary care consultations: a qualitative study. Family Practice. 2007 Aug 14;24(5):435–442. doi: 10.1093/fampra/cmm037. [DOI] [PubMed] [Google Scholar]
- 8.Valdez R. Detecting Undiagnosed Type 2 Diabetes: Family History as a Risk Factor and Screening Tool. Journal of Diabetes Science and Technology. 2009 Jul 01;3(4):722–726. doi: 10.1177/193229680900300417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehly LM, Morris BA, Skapinsky KF, Goergen AF, Ludden A. Evaluation of the Families SHARE workbook: an educational tool for outlining disease risk and healthy guidelines to reduce risk of heart disease, diabetes, breast cancer, and colorectal cancer. BMC Public Health. doi: 10.1186/s12889-015-2483-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999 Apr 01;22(4):623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. [Accessed September, 2014];Evaluating family history for preventive medicine and public health. 2007 http://www.talkhealthhistory.org/pdf/CDC%20-%20Evaluating%20FHH%20for%20Preventive%20Medicine%20&%20Public%20Health.PDF.
- 12.Petruccio C, Mills Shaw KR, Boughman J, et al. Healthy choices through family history: a community approach to family history awareness. Community genetics. 2008;11(6):343–351. doi: 10.1159/000133306. [DOI] [PubMed] [Google Scholar]
- 13.Breen N, Rao SR, Meissner HI. Immigration, Health Care Access, and Recent Cancer Tests Among Mexican-Americans in California. Journal of Immigrant and Minority Health. 2008 Dec 04;12(4):433–444. doi: 10.1007/s10903-008-9198-3. [DOI] [PubMed] [Google Scholar]
- 14.Edelman D, Christian A, Mosca L. Association of Acculturation Status With Beliefs, Barriers, and Perceptions Related to Cardiovascular Disease Prevention Among Racial and Ethnic Minorities. Journal of Transcultural Nursing. 2009 Apr 22;20(3):278–285. doi: 10.1177/1043659609334852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harcourt N, Ghebre RG, Whembolua G-L, Zhang Y, Warfa Osman S, Okuyemi KS. Factors Associated with Breast and Cervical Cancer Screening Behavior Among African Immigrant Women in Minnesota. Journal of Immigrant and Minority Health. 2013 Jan 19;16(3):450–456. doi: 10.1007/s10903-012-9766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang W-C. Acculturative family distancing: Theory, research, and clinical practice. Psychotherapy: Theory, Research, Practice, Training. 2006;43(4):397–409. doi: 10.1037/0033-3204.43.4.397. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoudi E, Jensen GA. Diverging Racial and Ethnic Disparities in Access to Physician Care. Medical Care. 2012;50(4):327–334. doi: 10.1097/MLR.0b013e318245a111. [DOI] [PubMed] [Google Scholar]
- 18.Woloshin S. Language barriers in medicine in the United States. JAMA: The Journal of the American Medical Association. 1995 Mar 01;273(9):724–728. [PubMed] [Google Scholar]
- 19.Ye J, Mack D, Fry-Johnson Y, Parker K. Health Care Access and Utilization Among US-Born and Foreign-Born Asian Americans. Journal of Immigrant and Minority Health. 2011 Oct 30;14(5):731–737. doi: 10.1007/s10903-011-9543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dall TM, Zhang Y, Chen YJ, Quick WW, Yang WG, Fogli J. The Economic Burden Of Diabetes. Health Affairs. 2010 Jan 14;29(2):297–303. doi: 10.1377/hlthaff.2009.0155. [DOI] [PubMed] [Google Scholar]
- 21.Ennis S, Rios-Vargas M, Albert N. The Hispanic Population: 2010. 2010 Census Briefs. 2011 [Google Scholar]
- 22.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital and health statistics. Series 10, Data from the National Health Survey. 2012 Jan;(252):1–207. [PubMed] [Google Scholar]
- 23.Koehly LM, Ashida S, Goergen AF, Skapinsky KF, Hadley DW, Wilkinson AV. Willingness of Mexican-American Adults to Share Family Health History with Healthcare Providers. American Journal of Preventive Medicine. 2011 Jun;40(6):633–636. doi: 10.1016/j.amepre.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wattendorf DJ, Hadley DW. Family history: the three-generation pedigree. American Family Physician. 2005;72(3):441– 448. [PubMed] [Google Scholar]
- 25.Ashida S, Goodman MS, Stafford J, Lachance C, Kaphingst KA. Perceived familiarity with and importance of family health history among a medically underserved population. J Community Genet. 2012;3(4):285–295. doi: 10.1007/s12687-012-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashida S, Wilkinson AV, Koehly LM. Motivation for Health Screening. American Journal of Preventive Medicine. 2010 Apr;38(4):396–402. doi: 10.1016/j.amepre.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Heer HD, de la Haye K, Skapinsky KF, Goergen AF, Wilkinson AV, Koehly LM. Let’s move together: impact of family health history information on encouragement and co-engagement in physical activity of Mexican origin parents and children. Health Education and Behavior. in press. [Google Scholar]
- 28.Diemer MA, Ali SR. Integrating social class into vocational psychology: theory and practice implications. Journal of Career Assessment. 2009;17:247– 265. [Google Scholar]
- 29.Diemer MA, Mistry RS, Wadsworth ME, Lopez I, Reimers F. Best practices in conceptualizing and measuring social class in psychological research. Analyses of Social Issues and Public Policy. 2013;13(1):77– 113. [Google Scholar]
- 30.SPSS Statistics for Windows [computer program] Chicago: SPSS Inc; 2008. Version 17.0. [Google Scholar]
- 31.Silberberg J, Wlodarczyk J, Hensley M, et al. Accuracy of reported family history of heart disease: The impact of ‘don’t know’ responses. Australian and New Zealand Journal of Medicine. 1994 Aug;24(4):386–389. doi: 10.1111/j.1445-5994.1994.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 32.Zlot AI, Silvey K, Newell N, Coates RJ, Leman R. Family History of Colorectal Cancer: Clinicians’ Preventive Recommendations and Patient Behavior. Preventing Chronic Disease. 2011 Dec;2011 [PMC free article] [PubMed] [Google Scholar]
- 33.Standards of Medical Care in Diabetes--2009. Diabetes Care. 2008 Dec 31;32(Supplement_1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride CM, Blocklin M, Lipkus IM, Klein WMP, Brandon TH. Patient’s lung cancer diagnosis as a cue for relatives’ smoking cessation: evaluating the constructs of the teachable moment. Psycho-Oncology. 2015 doi: 10.1002/pon.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: An interdependence and communal coping approach. Social Science & Medicine. 2006 Mar;62(6):1369–1380. doi: 10.1016/j.socscimed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Lyons RF, Mickelson KD, Sullivan MJL, Coyne JC. Coping as a Communal Process. Journal of Social and Personal Relationships. 1998 Oct 01;15(5):579–605. [Google Scholar]
- 37.Bernhardt BA, Weiner J, Foster EC, Tumpson JE, Pyeritz RE. The economics of clinical genetics services. II. A time analysis of a medical genetics clinic. American journal of human genetics. 1987 Oct;41(4):559–565. [PMC free article] [PubMed] [Google Scholar]
- 38.Mosca L. National Study of Women’s Awareness, Preventive Action, and Barriers to Cardiovascular Health. Circulation. 2006 Jan 31;113(4):525–534. doi: 10.1161/CIRCULATIONAHA.105.588103. [DOI] [PubMed] [Google Scholar]
- 39.Tortolero SR, Goff DC, Nichaman MZ, Labarthe DR, Grunbaum JA, Hanis CL. Cardiovascular Risk Factors in Mexican-American and Non-Hispanic White Children: The Corpus Christi Child Heart Study. Circulation. 1997 Jul 15;96(2):418–423. doi: 10.1161/01.cir.96.2.418. [DOI] [PubMed] [Google Scholar]