Abstract
Osteoarthritis (OA) is characterized by the breakdown of articular cartilage that is mediated in part by increased production of matrix metalloproteinases (MMPs) and aggrecanases (ADAMTS), enzymes that degrade components of the cartilage extracellular matrix. Efforts to design synthetic inhibitors of MMPs/ADAMTS have only led to limited clinical success. In addition to pharmacologic therapies, physiologic joint loading is widely recommended as a nonpharmacologic approach to improve joint function in osteoarthritis. Clinical trials report that moderate levels of exercise exert beneficial effects, such as improvements in pain and physical function. Experimental studies demonstrate that mechanical loading mitigates joint destruction through the downregulation of MMPs/ADAMTS. However, the molecular mechanisms underlying these effects of physiologic loading on arthritic joints are not well understood. We review here the recent progress on mechanotransduction in articular joints, highlighting the mediators and pathways in the maintenance of cartilage integrity, especially in the prevention of cartilage degradation in OA.
Keywords: mechanical loading, osteoarthritis, exercise, cartilage degradation
Introduction
Osteoarthritis (OA) is a progressive degenerate joint disease that affects the structural and functional integrity of joint tissues such as bone, tendons, and ligaments, which ultimately results in the destruction of articular cartilage. It is currently the leading cause of disability and pain in the United States,1 and there are currently no cures for OA, and no effective pharmacological treatments exist that slow or halt its progression.2 Physical activity is one of the most widely prescribed nonpharmacological therapies for OA management,3 based on its ability to limit pain and improve physical function.4,5 However, the mechanisms underlying these beneficial effects of exercise and physical therapy (referred in this paper as “mechanical treatment”) are largely unknown. In this review, we will discuss the recent progress regarding the effects of mechanical treatment on OA, and highlight a novel mechanotransduction pathway that mediates the anti-inflammatory and chondroprotective effects of physiologic joint loading.
Cartilage destruction in osteoarthritis
Osteoarthritis is characterized by cartilage degradation, synovial inflammation, and alterations within the subchondral bone, including bone remodeling, subchondral sclerosis, and osteophyte formation.2,6 Clinical features of osteoarthritis include joint pain, stiffness, and swelling, which together contribute to patient disability.7 The pathogenesis of OA is unclear, but risk factors for developing OA include aging, joint trauma, obesity, and heritable genetic factors.2 OA is the most common joint disease, affecting an estimated 15% of the U.S. population.8
At the molecular level, one of the most prominent features of OA is the imbalance between the anabolic and catabolic activities within chondrocytes, the sole cell population within cartilage. Breakdown of the cartilage extracellular matrix is mediated in part by upregulated expression of proteolytic enzymes, including matrix metalloproteinases (MMPs) or a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS).9 In cases of normal tissue turnover, levels of active MMPs and ADAMTS are suppressed, in part by tissue inhibitors of metalloproteinases (TIMPs).10 However, in osteoarthritis, the activities of proteolytic enzymes overwhelm those of TIMPs, resulting in cartilage breakdown.2
Due to the role upregulated MMPs/ADAMTS play in arthritis, inhibitors for these proteolytic enzymes have been explored as therapeutic strategies to treat OA. However, clinical trials so far have been met with limited success and resulted in side effects including musculoskeletal pain and inflammation.11–13 These adverse effects have been mainly attributed to the lack of selectivity of these inhibitors. Metalloproteinases share structural similarities and are susceptible to regulation by broad-spectrum inhibitors.14 Poor selectivity is problematic because in addition to matrix remodeling, MMPs/ADAMTS play important roles in wound healing, angiogenesis, development, morphogenesis, and bone remodeling.15,16 Therefore, it appears successful therapeutic strategies will require the specific inhibition and appropriate modulation of MMPs/ADAMTS involved in OA.
Physiologic joint loading and osteoarthritis
Nonpharmacologic therapies for OA, such as aerobic exercise, strength training, and passive motion therapy, have been reported to exert protective effects on the joint. At least 20 min of weekly rigorous physical exercise, defined as activities leading to shortness of breath or sweating, is protective against the development of cartilage defects in healthy adults.17,18 Less vigorous physical activities such as walking are also beneficial for joint health. Subjects who walk regularly (more than three times a week for at least 20 min each time) have a reduced risk of developing bone marrow lesions.17 Bone marrow lesions are associated with the development of chondral defects and may serve as a predictive biomarker of OA development.19
For people with OA, regular exercise also has been demonstrated to be of benefit. A Cochrane Review of 32 clinical trials comparing land-based therapeutic exercise (i.e., muscle strengthening, aerobics, manual therapy) to a nonexercise group found that exercise treatment resulted in moderate improvements in pain and physical function.20 Physical interventions that are less studied, including hydrotherapy and Tai Chi, have reported significant improvements in pain and physical function for at least 24 weeks after the start of these exercise programs.21 Although clinical trials evaluating the effect of exercise on joint structure in OA patients are limited, preliminary results from these studies are promising. A four-month exercise program consisting of aerobic and weight-bearing exercises increased proteoglycan content in the articular cartilage of OA subjects.22 Strength training, when compared to range of motion exercises for 30 months, decreased the mean rate of joint space narrowing, but the difference was not statistically significant.23 Together, the evidence support that moderate levels of exercise improve symptoms of OA, but whether exercise also has a disease modifying effect is still unclear.
Molecular effects of exercise in the synovial joint
Although the beneficial effects of exercise for osteoarthritis patients are well documented, the mechanisms are still largely unknown. In vitro and in vivo experiments have begun to identify the molecular effects of physiologic loading in chondrocytes, and determine factors mediating the beneficial actions of loading. In vitro, proinflammatory cytokine interleukin (IL)-1β stimulates the release of proinflammatory mediators such as nitric oxide (NO), prostaglandin (PG)E2, and cyclo-oxygenase (COX)-2.24 Dynamic compression of chondrocytes at 0–15% strain and 1 hertz (Hz) counteracts the production of these IL-1β–induced mediators, possibly through a p38 mitogen-activated protein kinase (MAPK)-dependent pathway.24 Mechanical stimulation of chondrocytes also antagonizes IL-1β-and TNF-α–induced inflammatory and catabolic responses, such as upregulated COX-2, inducible nitric oxide synthase (iNOS), and genes involved in cartilage catabolism, such as MMPs 9 and 13.25–27 This beneficial effect of mechanical loading was attributed to inhibiting transcription factor nuclear factor-kappa B (NF-κB) from translocating into the nucleus to activate target genes and also to interference with multiple signaling events upstream of NF-κB.28 Another mechanism through which mechanical loading acts is by preventing IL-1α–induced cartilage degradation.29 Bovine cartilage explants incubated with IL-1α led to the degradation of collagen and proteoglycans and resulted in aggrecan cleavage by MMPs and ADAMTS. Dynamic loading at 0.5 megapascals (MPa) and 0.5Hz of these explants inhibited the catabolic actions of IL-1α and prevented cartilage degradation.29
In vivo experiments have clearly demonstrated the anti-catabolic and anti-inflammatory effects of physiologic joint loading. Hindlimb immobilization of rodents resulted in catabolic changes, including reduced Safranin O staining, indicative of proteoglycan loss, and increases in MMP-3 and ADAMTS-5.30 However, 1 h of daily passive joint motion inhibited the increases in MMP-3 and ADAMTS-5 and prevented changes in proteoglycan loss.30 In animal models of antigen-induced arthritis, daily bouts of passive motion therapy decreased joint inflammation and maintained the structural integrity of the articular cartilage when compared to immobilized controls, demonstrating its potential for therapeutic use. Mechanistically, passive motion therapy exerted potent anti-inflammatory effects. Passive motion significantly decreased the levels of proinflammatory genes and mediators of matrix breakdown (IL-1β, COX-2, MMP-1) and induced anti-inflammatory cytokine IL-10.26,31 IL-10 has protective effects in cartilage,32 and its induction may be one mechanism by which mechanical signals render anti-inflammatory effects. Together, the in vitro and in vivo data suggest that a variety of loading conditions are sufficient to preserve cartilage integrity by counteracting cytokine-induced proinflammatory and catabolic effects.
In addition to the direct effects mechanical loading exerts on chondrocytes, exercise can affect the synovial cavity. One bout of exercise in female patients with OA increased the concentration of IL-10 in the synovial fluid and in the peri-synovial compartment when compared to a non-exercise group.33 Passive mobilization of knee joints in anesthetized rabbits increased hyaluronan (HA) secretion when compared to static controls.34 Hyaluronan is synthesized by synoviocytes and contributes to the lubricating capacity of synovial fluid.35 In patients with OA, the concentration of HA is reduced,36 and intra-articular injections of HA are widely used for the relief of knee pain associated with OA.37
Chondrocyte mechanotransduction
Mechanotransduction is the process by which biomechanical signals regulate cell activity and behavior. Chondrocytes are able to sense and react to mechanically induced changes within the cartilage matrix.38 Chondrocyte mechanotransduction is initiated at the interface between the cell membrane and extracellular matrix,39 and the processing of these mechanical signals involves mechanoreceptors such as ion channels and integrins. For example, membrane stretch, a condition that chondrocytes experience during compression or during hypo-osmotic conditions that cause swelling,40 activates potassium channels.41 The function of ion channels in chondrocyte membranes is not clear, but they may be involved in chondrocyte functions such as cell proliferation and matrix secretion.42,43 Integrins are heterodimeric transmembrane receptors consisting of α and β subunits44 and interact with cytoskeletal proteins such as fibronectin, vitronectin, and osteopontin.45–47 Mechanical stimulation of human chondrocytes increases expression of aggrecan and decreases MMP-3 gene expression in a pathway involving the α5β1 integrin and IL-4 release.48 However, this response to mechanical stimulation is absent in chondrocytes derived from OA cartilage, suggesting abnormal chondrocyte signaling may be involved in OA disease progression.49
Little is known of the joint loading-activated signaling pathways that help maintain cartilage integrity in OA. To identify the molecular basis of exercise in osteoarthritis, transcriptome-wide microarray analysis was performed in rodents experimentally induced with arthritis and either run on a treadmill daily for 21 days or subject to cage activity. Treadmill exercise initiated one day after arthritis induction significantly slowed progression of arthritis, while upregulating gene networks associated with matrix synthesis and suppressing proinflammatory gene networks.50 Of interest, treadmill exercise initiated five or nine days after arthritis induction, when cartilage destruction was more severe, was less effective in protecting articular cartilage from destruction.50
The NF-κB network was one of the gene clusters suppressed by treadmill exercise.50 NF-κB transcription factors are involved in immune and inflammatory responses and regulate expression of genes responsible for inflammation, apoptosis, cell cycle, and matrix breakdown.51,52 In response to various stimuli such as TNF-α, IL-1β, and lipopolysaccharides (LPS), NF-κB is activated and translocates to the nucleus to regulate transcription of its target genes.53 With treadmill exercise, expression of many genes required for NF-κB activity was suppressed, suggesting the suppression of NF-κB activation mediates the anti-inflammatory effects of exercise.50
CITED2-mediated mechanotransduction
One transcriptional regulator that appears to play a crucial role in cartilage homeostasis is CITED2 (CBP/p300-interacting transactivator with ED-rich tail 2). CITED2 is a transcriptional coregulator that does not bind DNA directly. It positively regulates transcription by recruiting CBP (cAMP-responsive element-binding protein) and p300 to interact with other DNA-binding transcription factors such as Lhx2, PPARα, PPARγ, Smad 2, and TFAP2.54 CITED2 also negatively regulates target genes by competing for CBP/p300 binding with transcription factors including Ets-1, NF-κB, HIF-1α, STAT2, and p53.55,56 Through these mechanisms, CITED2 is able to regulate many cellular processes such as embryonic development, cell proliferation, inflammation, and matrix turnover.54
With regard to cartilage integrity, CITED2 expression in chondrocytes in vitro is increased by moderate intensities of flow shear and intermittent hydrostatic pressure (IHP) and in chondrocytes in vivo by joint motion.57,58 Increased CITED2 expression in vivo correlated with the maintenance of cartilage integrity and the suppression of collagenase MMP-1, suggesting the anticatabolic effects of physiologic joint loading were mediated by CITED2.58 As demonstrated by competitive binding and transcriptional activity assays, CITED2 suppresses MMP-1 transcription by competing with MMP transactivator Ets-1 for binding to its coactivator p300. In addition to MMP-1, Ets-1 binds to the promoter regions of other MMPs including MMP-2, -3, -8, -9, and -13.59 Therefore, it is likely CITED2 may regulate additional MMPs through a similar manner.
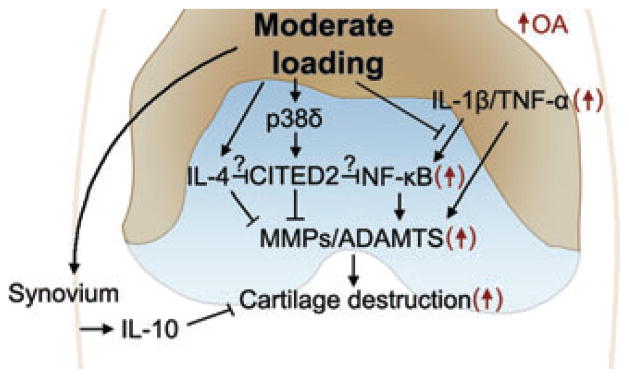
Upstream of CITED2, moderate IHP loading phosphorylated p38δ, which was required for the transactivation of CITED2.58 p38 belongs to the MAP kinase family, which is activated in response to mechanical stresses.60 While moderate loading activated p38δ and CITED2, high levels of IHP phorphorylated p38α and MMP-1, but not CITED2. This may explain why CITED2 is specifically activated by moderate loading and also suggests that p38α is involved in the upregulation of MMP-1. The data indicate different members of the p38 family may act as a “mechanosensitive switch” in chondrocytes, which act to upregulate or downregulate MMP expression based on the mechanical loading regimes. The evidence that CITED2 is inducible by IL-461 and may interact with components of NF-κB,56 suggests a potential role of CITED2 as a central mediator in these mechanotransduction pathways involved in maintaining cartilage integrity (Fig. 1).
Figure 1.
CITED2 as a central mediator of the hypothesized mechanotransduction pathways in the maintenance of cartilage integrity in healthy joints by balancing catabolic and anti-catabolic events, and in the reduction or prevention of cartilage degradation in diseased (OA) joints by suppressing upregulated proinflammatory networks in OA.
Conclusions
Physical activity is widely prescribed as a non-pharmacologic therapy for patients with OA. While moderate levels of physical activity are reported to exert beneficial clinical effects in patients with OA, the parameters for each type of exercise (i.e., intensity, duration, frequency) are not well characterized within the OA population. Furthermore, the mechanisms of how mechanical signals mediate these effects are not well understood. The recent identification of mechanotransduction pathways in response to physiological joint loading, such as the CITED2-mediated pathway, and its potential cross-talk to pathways mediated by NF-κB, contribute to our understanding of mechanisms underlying mechanical treatment, and may lead to novel therapeutic targets and strategies to treat or prevent cartilage destruction in arthritis.
Acknowledgments
The work has been supported by NIH Grants AR47628 and AR52743.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50. doi: 10.1111/j.1749-6632.2010.05808.x. [DOI] [PubMed] [Google Scholar]
- 3.Ng NT, Heesch KC, Brown WJ. Strategies for managing osteoarthritis. Int J Behav Med. 2011 May 26; doi: 10.1007/s12529-011-9168-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Fransen M, McConnell S, Bell M. Therapeutic exercise for people with osteoarthritis of the hip or knee. A systematic review. J Rheumatol. 2002;29:1737–1745. [PubMed] [Google Scholar]
- 5.Roddy E, Zhang W, Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis. 2005;64:544–548. doi: 10.1136/ard.2004.028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokota H, Leong DJ, Sun HB. Mechanical loading: bone remodeling and cartilage maintenance. Curr Osteoporos Rep. 2011;9:237–242. doi: 10.1007/s11914-011-0067-y. [DOI] [PubMed] [Google Scholar]
- 7.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 9.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224–240. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens LM, Fingleton LM, Matrisian B. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 12.Devy L, Dransfield DT. New strategies for the next generation of matrix-metalloproteinase inhibitors: selectively targeting membrane-anchored MMPs with therapeutic antibodies. Biochem Res Int. 2011;2011:191670–191681. doi: 10.1155/2011/191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skiles JW, Gonnella NC, Jeng AY. The design, structure, and clinical update of small molecular weight matrix metalloproteinase inhibitors. Curr Med Chem. 2004;11:2911–2977. doi: 10.2174/0929867043364018. [DOI] [PubMed] [Google Scholar]
- 14.Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.McQuibban GA, et al. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 16.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Racunica TL, et al. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis Rheum. 2007;57:1261–1268. doi: 10.1002/art.22990. [DOI] [PubMed] [Google Scholar]
- 18.Foley S, Ding C, Cicuttini F, Jones G. Physical activity and knee structural change: a longitudinal study using MRI. Med Sci Sports Exerc. 2007;39:426–434. doi: 10.1249/mss.0b013e31802d97c6. [DOI] [PubMed] [Google Scholar]
- 19.Wluka AE, et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis. 2009;68:850–855. doi: 10.1136/ard.2008.092221. [DOI] [PubMed] [Google Scholar]
- 20.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008;8:CD004376–CD004468. doi: 10.1002/14651858.CD004376.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Fransen M, Nairn L, Winstanley J, et al. Physical activity for osteoarthritis management: a randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Rheum. 2007;57:407–414. doi: 10.1002/art.22621. [DOI] [PubMed] [Google Scholar]
- 22.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–3514. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 23.Mikesky AE, et al. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum. 2006;55:690–699. doi: 10.1002/art.22245. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury TT, et al. Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther. 2008;10:R35–R48. doi: 10.1186/ar2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gassner R, et al. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 26.Ferretti M, et al. Biomechanical signals suppress proinflammatory responses in cartilage: early events in experimental antigen-induced arthritis. J Immunol. 2006;177:8757–8766. doi: 10.4049/jimmunol.177.12.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madhavan S, et al. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthritis Cartilage. 2006;14:1023–1032. doi: 10.1016/j.joca.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dossumbekova A, et al. Biomechanical signals inhibit IKK activity to attenuate NF-kappaB transcription activity in inflamed chondrocytes. Arthritis Rheum. 2007;56:3284–3296. doi: 10.1002/art.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torzilli PA, Bhargava M, Park S, Chen CT. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage. 2010;18:97–105. doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong DJ, et al. Matrix metalloproteinase-3 in articular cartilage is upregulated by joint immobilization and suppressed by passive joint motion. Matrix Biol. 2010;29:420–426. doi: 10.1016/j.matbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferretti M, et al. Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J Orthop Res. 2005;23:1165–1171. doi: 10.1016/j.orthres.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubberts E, et al. Intra-articular IL-10 gene transfer regulates the expression of collagen-induced arthritis (CIA) in the knee and ipsilateral paw. Clin Exp Immunol. 2000;120:375–383. doi: 10.1046/j.1365-2249.2000.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmark IC, et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther. 2010;12:R126–R137. doi: 10.1186/ar3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingram KR, Wann AK, Angel CK, et al. Cyclic movement stimulates hyaluronan secretion into the synovial cavity of rabbit joints. J Physiol. 2008;586:1715–1729. doi: 10.1113/jphysiol.2007.146753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell J, Bellamy N, Gee T. Differences between systematic reviews/meta-analyses of hyaluronic acid/hyaluronan/hylan in osteoarthritis of the knee. Osteoarthritis Cartilage. 2007;15:1424–1436. doi: 10.1016/j.joca.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Altman RD. Status of hyaluronan supplementation therapy in osteoarthritis. Curr Rheumatol Rep. 2003;5:7–14. doi: 10.1007/s11926-003-0077-6. [DOI] [PubMed] [Google Scholar]
- 37.Barron MC, Rubin BR. Managing osteoarthritic knee pain. J Am Osteopath Assoc. 2007;107:ES21–ES27. [PubMed] [Google Scholar]
- 38.Ramage L, Nuki G, Salter DM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports. 2009;19:457–469. doi: 10.1111/j.1600-0838.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 40.Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 41.Mobasheri A, et al. Characterization of a stretch-activated potassium channel in chondrocytes. J Cell Physiol. 2010;223:511–518. doi: 10.1002/jcp.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouw JK, Imler SM, Levenston ME. Ion-channel regulation of chondrocyte matrix synthesis in 3D culture under static and dynamic compression. Biomech Model Mechanobiol. 2007;6:33–41. doi: 10.1007/s10237-006-0034-1. [DOI] [PubMed] [Google Scholar]
- 43.Wu QQ, Chen Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp Cell Res. 2000;256:383–391. doi: 10.1006/excr.2000.4847. [DOI] [PubMed] [Google Scholar]
- 44.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–446. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 45.Kock LM, et al. RGD-dependent integrins are mechanotransducers in dynamically compressed tissue-engineered cartilage constructs. J Biomech. 2009;42:2177–2182. doi: 10.1016/j.jbiomech.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 46.Loeser RF. Integrins and cell signaling in chondrocytes. Biorheology. 2002;39:119–124. [PubMed] [Google Scholar]
- 47.Van der Kraan PM, Buma P, Van Kuppevelt T, Van den Berg WB. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthritis Cartilage. 2002;10:631–637. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 48.Wright MO, et al. Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: evidence of a role for alpha 5 beta 1 integrin as a chondrocyte mechanoreceptor. J Orthop Res. 1997;15:742–747. doi: 10.1002/jor.1100150517. [DOI] [PubMed] [Google Scholar]
- 49.Millward-Sadler SJ, Wright MO, Davies LW, et al. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000;43:2091–2099. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 50.Nam J, et al. Transcriptome-wide gene regulation by gentle treadmill walking during the progression of monoiodoacetate-induced arthritis. Arthritis Rheum. 2011;63:1613–1625. doi: 10.1002/art.30311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marcu KB, Otero M, Olivotto E, et al. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anghelina M, et al. Regulation of biomechanical signals by NF-kappaB transcription factors in chondrocytes. Biorheology. 2008;45:245–256. [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 54.Sun HB. CITED2 mechanoregulation of matrix metalloproteinases. Ann N Y Acad Sci. 2010;1192:429–436. doi: 10.1111/j.1749-6632.2009.05305.x. [DOI] [PubMed] [Google Scholar]
- 55.Dial R, Sun ZY, Freedman SJ. Three conformational states of the p300 CH1 domain define its functional properties. Biochemistry. 2003;42:9937–9945. doi: 10.1021/bi034989o. [DOI] [PubMed] [Google Scholar]
- 56.Lou X, et al. Negative feedback regulation of NF-kappaB action by CITED2 in the nucleus. J Immunol. 2011;186:539–548. doi: 10.4049/jimmunol.1001650. [DOI] [PubMed] [Google Scholar]
- 57.Yokota H, Goldring MB, Sun HB. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem. 2003;278:47275–47280. doi: 10.1074/jbc.M304652200. [DOI] [PubMed] [Google Scholar]
- 58.Leong DJ, et al. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011;25:182–191. doi: 10.1096/fj.10-164277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29–40. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzgerald JB, et al. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 2008;283:6735–6743. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
- 61.Sun HB, Zhu YX, Yin T, Sledge G, Yang YC. MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc Natl Acad Sci USA. 1998;95:13555–13560. doi: 10.1073/pnas.95.23.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]