Abstract
Personalizing intravenous (IV) busulfan doses in children using therapeutic drug monitoring (TDM) is an integral component of hematopoietic cell transplant. The authors sought to characterize initial dosing and TDM of IV busulfan, along with factors associated with busulfan clearance, in 729 children who underwent busulfan TDM from December 2005 to December 2008. The initial IV busulfan dose in children weighing ≤12 kg ranged 4.8-fold, with only 19% prescribed the package insert dose of 1.1 mg/kg. In those children weighing >12 kg, the initial dose ranged 5.4-fold, and 79% were prescribed the package insert dose. The initial busulfan dose achieved the target exposure in only 24.3% of children. A wide range of busulfan exposures were targeted for children with the same disease (eg, 39 target busulfan exposures for the 264 children diagnosed with acute myeloid leukemia). Considerable heterogeneity exists regarding when TDM is conducted and the number of pharmacokinetic samples obtained. Busulfan clearance varied by age and dosing frequency but not by underlying disease. The authors’ group is currently evaluating how using population pharmacokinetics to optimize initial busulfan dose and TDM (eg, limited sampling schedule in conjunction with maximum a posteriori Bayesian estimation) may affect clinical outcomes in children.
Keywords: busulfan, pediatric, hematopoietic cell transplant, prescribing patterns, pharmacokinetics, therapeutic drug monitoring
The alkylating agent busulfan is often administered to children as part of their conditioning regimen prior to hematopoietic cell transplantation (HCT).1 Even with individualized dosing based on either body weight (mg/kg) or body surface area (BSA; mg/m2), considerable interpatient variability exists in the clinical outcomes of busulfan-containing conditioning regimens. The variability in the efficacy and toxicity is due in part to interpatient differences in busulfan clearance and the narrow therapeutic range of busulfan systemic exposure.2 Over the past 15 years, several HCT centers have personalized either oral (PO) or intravenous (IV) busulfan using therapeutic drug monitoring (TDM) (see Supplemental Figure S1).
Busulfan plasma concentrations, measured as area under the plasma-concentration-time curve (AUC) or steady state concentration (CSS, calculated as AUC/dosing interval), have been associated with rejection, relapse, and toxicity in HCT recipients conditioned with busulfan as we have previously reviewed.2 The early busulfan pharmacodynamic data were reported in patients conditioned with the oral busulfan/IV cyclophosphamide (oral BU/CY) regimen. Children treated with oral busulfan, based on either a body weight (mg/kg) or a BSA (mg/m2) basis, achieve lower plasma concentrations than adults.3 Consistently achieving engraftment was initially challenging in children,4 and pharmacodynamic studies revealed that low busulfan exposure (ie, CSS <600 ng/mL) increases the risk of graft rejection in children receiving oral BU/CY.5 Engraftment improved from 74% to 94% with the use of TDM (target busulfan CSS of 600–900 ng/mL) in children receiving oral BU/CY.6 In contrast to busulfan pharmacodynamic data from adult populations,5,7,8 high busulfan AUC has not been consistently associated with regimen-related toxicity in children conditioned with oral BU/CY.6,9,10 Busulfan AUC is not associated with relapse in children with acute myeloid leukemia (AML),11 although such pharmacodynamic studies have been hindered by small sample sizes.9 Therefore, when conducting TDM of busulfan, the optimal busulfan AUC for a child differs from that of an adult.2,12
The narrow therapeutic index of oral busulfan was recognized while IV busulfan was undergoing drug development in the 1990s. The Food and Drug Administration–approved (FDA-approved) labeling has clear recommendations for the initial weight-based doses and the procedures for conducting TDM for IV busulfan when used as part of an HCT conditioning regimen in children. Since the FDA approval of IV busulfan in February 1999, there has been a substantive amount of attention given to optimizing its dosing, pharmacokinetics, and monitoring.13–19 TDM to achieve a target busulfan exposure has been the standard of care for select HCT recipients at our center since 1996. Since then, many HCT centers have used our clinical busulfan TDM service to personalize busulfan doses. Clinicians often request guidance regarding the optimal initial dose, pharmacokinetic sampling times, and target exposure for pediatric HCT recipients. Therefore, we sought to (1) describe current clinical practice of IV busulfan dosing and TDM in children and (2) characterize the pharmacokinetics of IV busulfan in the largest cohort of children (N = 729) undergoing HCT.
Materials and Methods
Study Population
This was a retrospective study in children who received HCT conditioning with IV busulfan at 1 of 51 institutions (see appendix) who paid for TDM services (ie, quantitation and pharmacokinetic modeling of busulfan pharmacokinetic samples) at the Seattle Cancer Care Alliance (SCCA) Busulfan Pharmacokinetics Laboratory from December 2005 to December 2008. During the time period, this laboratory was the reference laboratory for 2 Children’s Oncology Group (COG) trials in children with acute myelogenous leukemia (AML): COG AAML03P1 and AAML0531. All patients had their busulfan dose personalized to a target busulfan exposure (AUC or CSS) using TDM. Approval of the FHCRC Institutional Review Board and Children’s Oncology Group was obtained prior to data analysis. All data were anonymized prior to data analysis.
Inclusion criteria were as follows: age <21 years at the time of busulfan TDM. Records were examined for demographic data (ie, age, sex, height, weight, body surface area) and clinical data (ie, disease, treating institution) requested from the treating institution. Of note, the patient’s ethnicity and concomitant medications during busulfan-based HCT conditioning were not requested. The treating institution listed each child’s disease, which were categorized as follows: acute lymphoblastic leukemia (ALL); acute myeloid leukemia/myelodysplastic syndrome (AML/MDS; which includes acute leukemia, undifferentiated; biphenotypic leukemia; refractory anemia with excess blasts); secondary AML/MDS; aplastic anemia; bone marrow failure (includes amegakaryocytic thrombocytopenia; bone marrow failure syndrome; congenital amegakaryocytic thrombocytopenia; congenital anemia; Diamond Blackfan anemia; dyskeratosis congenita; Fanconi/aplastic anemia; hypoplasia anemia; idiopathic myelofibrosis; red cell aplasia; Schwachman-Diamond syndrome; sideroblastic anemia); chronic myeloid leukemia and juvenile myelomonocytic leukemia; combined immunodeficiency disorder (CID; includes bare lymphocyte syndrome; CD4 lymphopenia; CD40 ligand deficiency; gamma interferon deficiency; hypereosinophilic syndrome; immune deficiency; IPEX; leukocyte adhesion deficiency; NEMO deficiency; Omenn syndrome; reticular dysgenesis; SCID; Wiskott-Aldrich syndrome; X-linked hyper IgM syndrome; X-linked lymphoproliferative syndrome); granulocyte disorder (includes Chediak Higashi; chronic granulomatous disease; chronic neutropenia; congenital neutropenia; Kostmann syndrome); histiocytic disorder (includes familial hemophagocytic lymphoma; FEL/HLH; hemophagocytic lymphohistiocytosis; Langerhans cell histiocytosis; Griscelli syndrome); Hodgkin lymphoma; lymphoma (includes anaplastic large cell lymphoma; B-cell lymphoma; Burkitt lymphoma; T-cell lymphoma); metabolic storage disease (MSD; includes adrenoleukodystrophy; Hunter syndrome; Hurler syndrome; I-cell/mucolipidosis II; metachromatic leukodystrophy; neuroaxonal disorder; Wolman disease), myeloproliferative disorder (includes polycythemia vera); osteopetrosis; paroxysmal nocturnal hemoglobinuria; pediatric solid tumors (includes central nervous system atypical teratoid/rhabdoid tumor; desmoplastic small round tumor; Ewing sarcoma; medulloblastoma; pleuropulmonary blastoma; PNET; rhabdomyosarcoma; small round desmoid tumor; Wilm tumor); sickle cell anemia; thalassemia (includes alpha; beta; E/beta).
Intravenous Busulfan Dosing and Pharmacokinetic Sampling
The initial weight-based busulfan dose, the dosing frequency, the timing of the pharmacokinetic blood samples, and the target busulfan exposure were chosen at the discretion of the treating physician. Duration of busulfan therapy was not consistently recorded. AAML03P1 and AAML0531 stipulated that children with a matched family donor receive IV busulfan every 6 hours for 16 doses. Within these 2 COG protocols, the initial IV busulfan dose for children <10 kg was 0.8 mg/kg per dose, while children ≥10 kg but ≤4 years old received 1 mg/kg per dose, and children >4 years received 0.8 mg/kg per dose.
Quantitation and Pharmacokinetic Modeling of Busulfan Samples
Busulfan concentrations were determined by gas chromatography with mass spectrometry detection as previously described.9 The assay dynamic range was from 25 to 4500 ng/mL, and the interday CV was less than 8%. After quantitation of busulfan samples, the concentration-time data were fit using WinNonlin (version 5.0.1) via noncompartmental or compartmental modeling. The model was determined by visual inspection of the model fit to the individual concentration-time data. The AUC from time 0 to infinity (AUC0−∞) was calculated after the test dose (if given) and first busulfan dose of the conditioning regimen. The AUC from 0 to the end of the dosing interval (Τ) was calculated after the day 2 to 4 doses in patients receiving busulfan every 6, 8, or 12 hours, and the AUC0−∞ was calculated after the day 2 to 4 doses in patients receiving busulfan daily. The AUC0−∞ after the first dose equals the AUC0−Τ at steady state (ie, days 2 and 3). Clearance was calculated by dividing the dose by the AUC0−∞. After calculation of the patient’s clearance, the recommended dose for subsequent doses was calculated linearly to achieve the target busulfan exposure (AUC or CSS) that had been chosen by the treating physician. Subsequently, the technical staff verbally communicated the patient’s busulfan clearance, exposure, and the recommended busulfan dose. The technical staff addressed any questions regarding the results, and the treating physician chose the busulfan dose for the remainder of the HCT conditioning regimen. Upon completion of this discussion, a formal report was faxed summarizing the patient’s busulfan clearance, exposure, the recommended dose selected by the treating physician, and a predicted busulfan exposure over the entirety of the HCT conditioning regimen. Successful targeting was confirmed at the discretion of the treating institution, with further dose adjustments as needed. There was no standardization of dose adjustment practices, as the dose adjustment was at the discretion of the treating physician. The total busulfan dose administered over the entirety of HCT conditioning was not reported back to the Pharmacokinetics Laboratory.
Data Incorporation and Quality Assurance
The SCCA Busulfan Pharmacokinetic Laboratory uses a custom-built Microsoft Excel (Redmond, Washington) spreadsheet to document relevant patient information, analytic chemistry results, pharmacokinetic results from WinNonlin, and busulfan dose recommendations. Each patient had his or her own MS Excel worksheet. An MS Excel macro was created and validated to take data from these individual worksheets and incorporate them into a master spreadsheet. Upon incorporation, SAS (Cary, North Carolina) was used to identify the outliers for all data, and data were double-checked to ensure accurate data entry and reorganization.
Data Simulation
Using our previously published adult busulfan population pharmacokinetic model,20 we simulated busulfan concentrations for 2 hypothetical pediatric age-weight groups (ie, a 1-year-old infant and an 11-year-old child) to examine the expected exposure heterogeneity from IV busulfan dosing practices. The adult model was modified to account for expected differences due to size by using allometric relationships for all clearance and volume parameters. Additional modifications for enzyme ontogeny and organ maturation were not employed, as these factors were not expected to represent major influences given our target age range. Simulations were conducted using NONMEM version 6.2. For the 1-year-old, the busulfan concentrations were simulated using population averages of the pharmacokinetic characteristics at the minimum and maximum doses administered for children ≤12 kg (ie, 0.45–2.17 mg/kg). For the 11-year-old, the busulfan concentrations were simulated using population averages of the pharmacokinetic characteristics at the minimum and maximum doses administered for children >12 kg (ie, 0.41–2.2 mg/kg).
Statistical Methods
The association of various patient covariates with busulfan clearance was evaluated via analysis of variance and regression-based approaches. Patient covariates assessed included sex, age at the time of HCT, diagnosis (as categorized above), diagnosis (categorized as cancer or not cancer), treating institution, time of administration, and dosing frequency. The patients’ ethnic backgrounds and concomitant medications were not available and thus could not be included in this analysis. Two expressions of busulfan clearance were considered as the primary endpoints: busulfan clearance expressed by dosing weight (mL/min/kg), since the initial busulfan is most commonly calculated using body weight, and busulfan clearance expressed by BSA (mL/min/m2), since liver weight expressed relative to BSA (g/m2) is similar for children and adults.
Because the data were not normally distributed, we used a generalized additive model that can model the mean response as well as scale, location, and shape.21 After exploring a number of candidate distributions, we chose the Box-Cox t distribution. This distribution was chosen over a number of other candidate distributions (normal, t, gamma, generalized gamma) because it minimized generalized Akaike information criterion (GAIC). Rigby and Stasinopoulos21 define a positive random variable Y to have the Box-Cox t distribution (denoted BCT(μ, σ, ν, τ)) as follows: define the random variable Z by the following transformation of Y. Here Z has a truncated t distribution with τ degrees of freedom:
In the regression analyses, model selection was based on minimizing a GAIC where the penalty factor was chosen to be 3 (as opposed to the usual value of 2). This choice has been found to select models that are neither too complex nor too simple with respect to covariate choice. Furthermore, plots of clearance versus age and clearance versus BSA indicated nonlinear relationships. We modeled these variables using cubic splines with knots chosen at every point and 3 effective degrees of freedom over and above the 2 degrees of freedom for intercept and slope. Finally, plots indicated that the variability in clearance decreased with increasing age and BSA. So, we modeled the scale parameter as a linear function of age and BSA. The link functions for the mean and scale parameters were the log link. The 2 shape parameters were assumed to be constant. Regression analyses were carried out using the gamlss package in R.21
Results
Patient Characteristics
Patient pretransplant demographics and HCT characteristics are described in Table 1, with a more detailed description in Supplemental Table S1. The median age was 5.0 years (range, 0.1–20.0 years). Three hundred twenty-eight of the patients (45%) were less than 4 years of age, and 207 patients (28%) weighed less than 12 kg, at which weight higher initial IV busulfan doses are recommended per the package insert. The majority (55%, 402 of 729) of the patients were male. Dosing weight and BSA were calculated by each institution, so no equation can be provided for calculating dosing weight or BSA.
Table 1.
Description of patient population.a
| Total number of patients | 729 |
| Age, y | 7.1 ± 6.0 (0.1–20.0) |
| Dosing weight, kg | 28.7 ± 23.0 (2.65–117.8) |
| Sex | |
| Male | 402 |
| Female | 325 |
| Not reported | 2 |
| Diagnosis | |
| AML/MDS | 264 |
| CID | 88 |
| CML/JMML | 65 |
| Pediatric solid tumor | 43 |
| Histiocytic disorder | 39 |
| Acute lymphoblastic leukemia | 37 |
| Metabolic storage disease | 36 |
| Bone marrow failure | 30 |
| Sickle cell anemia | 28 |
| Thalassemia | 22 |
| AML/MDS, secondary | 16 |
| Aplastic anemia | 15 |
| Granulocyte disorder | 15 |
| Osteopetrosis | 12 |
| Lymphoma | 7 |
| Hodgkin lymphoma | 6 |
| Myeloproliferative disorder | 5 |
| Paroxysmal nocturnal hemoglobinuria |
1 |
| Initial dose for children ≤12 kg,b mg/kg | |
| 0.8 | 48 |
| 1.0 | 97 |
| 1.1c | 37 |
| Other | 25 |
| Initial dose for children >12 kg,b mg/kg | |
| 0.8c | 333 |
| 0.9 | 23 |
| 1.0 | 94 |
| Other | 28 |
Abbreviations: AML/MDS, acute myelogenous leukemia/myelodysplastic syndrome; CID, combined immunodeficiency disorders; CML/JMML, chronic myelogenous leukemia/juvenile myelomonocytic leukemia.
Data presented as n or as mean ± standard deviation (range).
For children receiving Q6hr busulfan; dose rounded to the nearest 0.1 mg/kg; more detailed description within Supplemental Table S2.
Dose per Busulfex package insert.
Initial IV Busulfan Dosing
The initial IV busulfan doses administered prior to subsequent pharmacokinetic modeling are presented in Table 1. Approximately 15% of all patients had more than 1 AUC estimation; only the first AUC estimation was included in this analysis. Supplemental Table S2 categorizes the initial prescribed doses with those of the package insert (1.1 mg/kg for children weighing ≤12 kg and 0.8 mg/kg for children weighing >12 kg22) and the Nguyen nomogram. Nguyen et al created an alternative weight-based nomogram for IV busulfan dosing in children that would achieve a mean busulfan AUC of 1125 µM×min based on population pharmacokinetic modeling.13
Excluding the 41 patients who only had pharmacokinetic sampling after a test dose, IV busulfan was administered every 6 hours (Q6hr) to 604 patients, every 8 hours (Q8hr) to 6 patients, every 12 hours (Q12hr) to 4 patients, and every 24 hours (ie, daily) to 74 patients.
Clinical Practice of TDM
Table 2 describes the characteristics of TDM in children. The majority (57%) of centers express their target busulfan exposure as AUC, which is typically presented in the units of µM×min. Therefore, all subsequent results are presented using AUC0−∞. There was considerable heterogeneity in the target busulfan AUC for each diagnosis; for example, there were 39 different target busulfan AUCs in the 264 children with AML. The number of different target AUCs for children with the same diagnosis was counted for the 5 most common diagnoses: AML, CID, pediatric solid tumors, MDS, and histiocytic disorders. The precise target busulfan AUCs for the most common diseases are presented in Supplemental Figure S2.
Table 2.
Practice patterns for therapeutic drug monitoring of intravenous busulfan.
| Characteristic | n (%) |
|---|---|
| Target busulfan exposure metric used to guide dosing | |
| AUC (µM × min) | 419 (57) |
| CSS (ng/mL) | 310 (43) |
| Number of different target busulfan AUCs for 5 most common diseases | |
| AML/MDS | 39 |
| CID | 31 |
| CML/JMML | 26 |
| Pediatric solid tumor | 21 |
| Histiocytic disorder | 17 |
| Number of AUCs drawn per patient | |
| 1 | 622 (85) |
| 2 | 94 (13) |
| 3 | 13 (2) |
| Number of samples obtained with initial AUC estimation | |
| 3 | 2 (0.3) |
| 4 | 72 (10) |
| 5 | 82 (11.3) |
| 6 | 44 (6.0) |
| 7 | 524 (71.9) |
| 8 | 5 (0.7) |
| Pharmacokinetic model to characterize busulfan AUC0−∞a | |
| Noncompartmental | 308 (42.6) |
| 1-compartment | 242 (33.4) |
| 2-compartment | 173 (24.0) |
| Dose recommendation for subsequent busulfan doses to achieve target AUCb | |
| Decrease | 183 (25.3) |
| No change | 176 (24.3) |
| Increase | 364 (50.3) |
n = 723 because 6 concentration-time profiles could not be modeled.
n = 723 because no dose was recommended for 6 patients either due to inability to model data or busulfan administration had finished by the time pharmacokinetic results were available.
Seven pharmacokinetic samples were obtained in the majority (71.9%) of children. The most common metric for busulfan exposure was AUC (57%). No centers use the package insert sampling schema, which states that pharmacokinetic sampling should occur at 2, 4, and 6 hours after the start of IV busulfan.22 No strict rules were in place regarding the pharmacokinetic modeling of each individual patient concentration-time profile. During this time, the majority (42%) of AUCs were modeled with noncompartmental analysis. The majority of patients had a dose adjustment recommended based on the TDM results, with no IV busulfan dose change recommended for only 24.3% of children. The acceptance of busulfan dose recommendations and the cumulative busulfan dose were not reported back by the treating institution to the Pharmacokinetics Laboratory. Because only a minority of children (~15%) had more than 1 AUC determination, we are unable to confirm that the target busulfan AUC was reached.
Busulfan Pharmacokinetics
For linear regression analyses, residual diagnostics gave no indication of lack of fit of the models. The diagnostics included normal plots of the residuals, standard residual plots, as well as worm plots. Stepwise model selection using GAIC indicated that for IV busulfan clearance (mL/min/kg), the best predictors were dosing frequency and BSA. For IV busulfan clearance (mL/min/m2), the best predictors were dosing frequency and age.
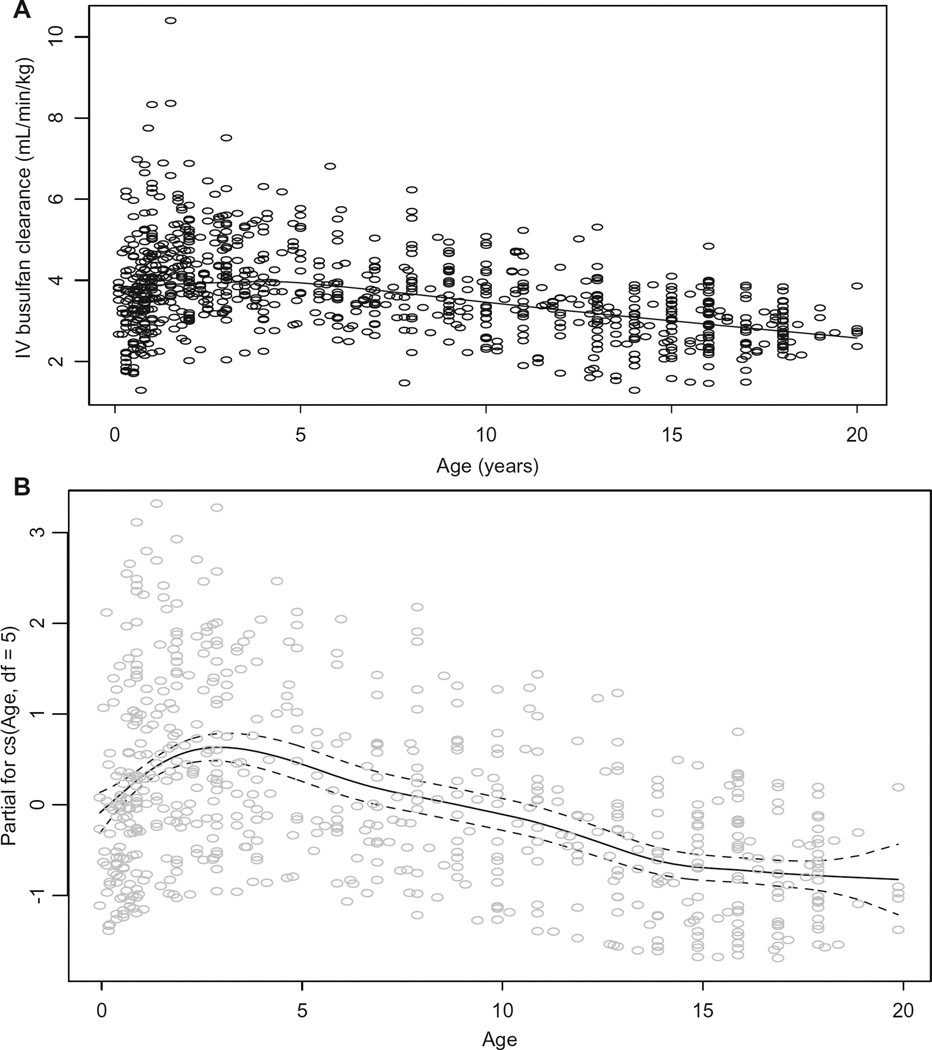
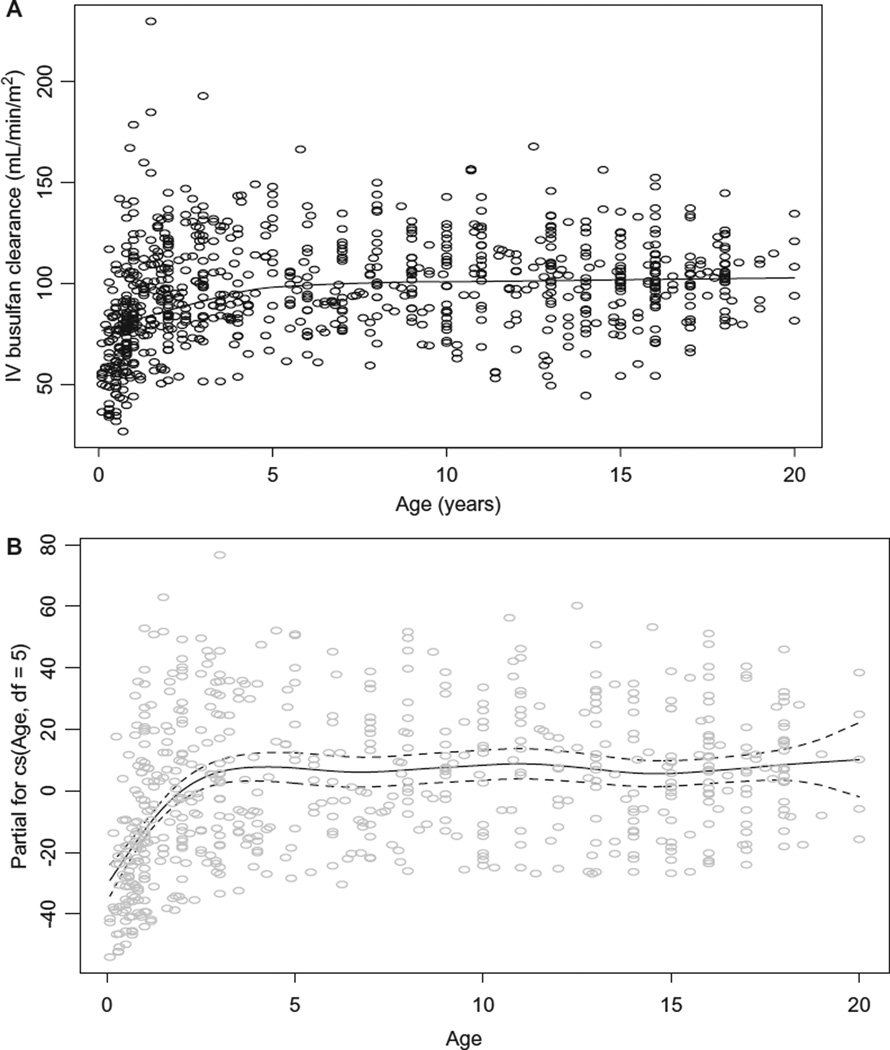
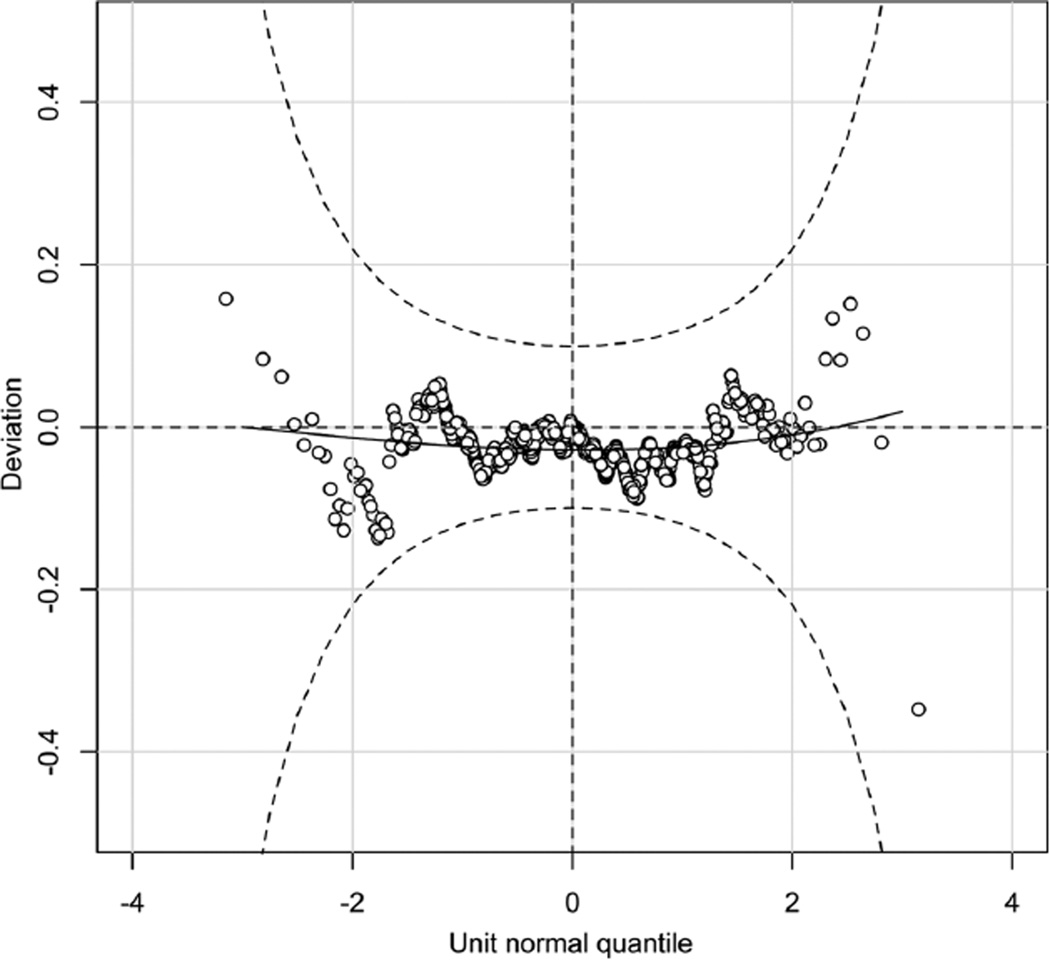
The average (± standard deviation) IV busulfan clearance was 3.7 ± 1.1 mL/min/kg and 97 ± 25 mL/min/m2, consistent with IV busulfan clearance in other pediatric HCT populations.13,15,16,23 Busulfan clearance differed by age when expressed by dosing weight (ie, mL/min/kg, Figure 1A), even with adjusting for dosing frequency. The plot of the influence of age upon the partial residuals of IV busulfan clearance expressed by weight is shown in Figure 1B. Busulfan clearance (mL/min/kg) peaked ~ age 3 years but fluctuated throughout maturation, indicating that linear modeling of busulfan clearance expressed by weight is not adequate such that nonlinear mixed effects modeling is needed to characterize factors associated with busulfan clearance expressed by mL/min/kg. Figure 2 shows the influence of age upon and the partial residuals of IV busulfan clearance expressed by BSA. Busulfan clearance (mL/min/m2) was low until age 4 years and then plateaued until age 17 years, at which time it slightly increased (Figure 2A, 2B). Subsequent regression modeling of clearance (mL/min/m2) focused on 4 parameters: μ, σ, ν, τ (Table 3). Each parameter can be modeled with its own link and variance function. Since the variability in clearance was increasing with younger age, we modeled σ as a linear function of age. The regression included age (modeled with a cubic spline [labeled cs in the output below; the degrees of freedom reflecting the flexibility of the spline]) to account for nonlinearity, dose frequency, and cancer (yes/no). The coefficient for age is only for the linear component and so is not directly interpretable. A better understanding of the relationship of clearance to age can be obtained graphically. Figure 3 is a detrended residual plot (a so-called worm plot) with confidence bands. The residuals all lie within the band, indicating no evidence of a poorly fitting model.
Figure 1.
Association of age with IV busulfan clearance expressed by dosing body weight, unadjusted for dosing frequency (A) and its partial residuals adjusted for dosing frequency (B). Dashed lines are 95% confidence band.
Figure 2.
Association of age with IV busulfan clearance expressed by BSA, unadjusted for dosing frequency and diagnosis (A) and its partial residuals adjusted for dosing frequency and diagnosis (B). Dashed lines are 95% confidence band.
Table 3.
Parameters from regression model of busulfan clearance (mL/min/m2).
| Estimate | Std. Error | t value | Pr(>|t|) | |
|---|---|---|---|---|
| μ coefficients | ||||
| (Intercept) | 87.1531 | 2.3504 | 37.081 | 2.987e-157 |
| cs (age, df = 5) | 1.2968 | 0.1708 | 7.592 | 1.216e-13 |
| Dose_freq | −0.5463 | 0.2365 | −2.309 | 2.126e-02 |
| Cancer | 3.6161 | 1.9616 | 1.843 | 6.577e-02 |
| σ coefficients | ||||
| (Intercept) | −1.36250 | 0.101853 | −13.377 | 6.749e-36 |
| Age | −0.02318 | 0.005435 | −4.264 | 2.329e-05 |
| ν coefficients | 0.474416 | 0.148967 | 3.184701 | 0.001524 |
| τ coefficients | 2.73489 | 1.22310 | 2.23604 | 0.02572 |
Figure 3.
Detrended normal plot of randomized quantile residuals with confidence band. Residuals all lie within the band indicating no evidence of lack of fit of the regression model.
Supplemental Figures S3A and S3B show that busulfan clearance differs slightly based on the dosing frequency. The pharmacokinetics of IV busulfan did not differ between children with an inherited disease and those with a cancer (Supplemental Table S3), which agrees with previous data from our laboratory24 but contradicts 2 other reports in the literature.25,26
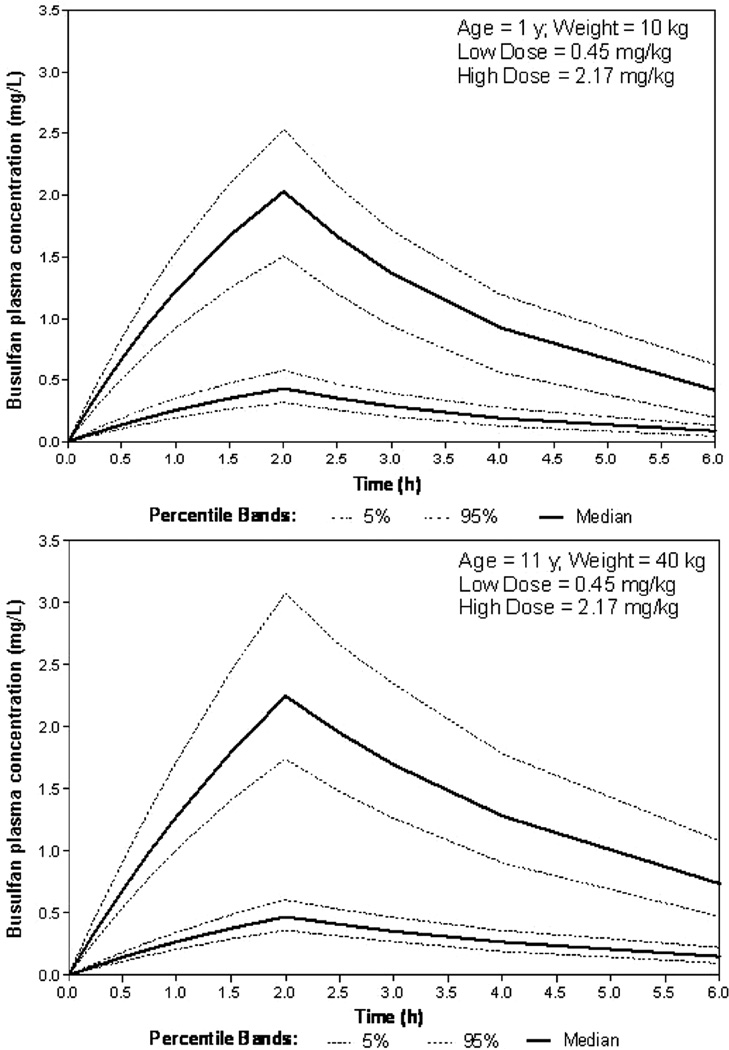
Simulations
Simulations confirm the effect of the heterogeneity in initial busulfan dosing practice upon the busulfan concentration-time profile. Figure 4 demonstrates the considerable impact of this variability in initial dosing upon busulfan pharmacokinetics. Upon the completion of our prospective population pharmacokinetic analyses, we will revisit the necessity of including ontogenic and maturational effects upon the age continuum. Our simulation model (Figure 4) uses a well-defined adult population pharmacokinetic model20 and represents an initial projection of pediatric exposures via allometric consideration for weight on key parameters consistent with FDA and industrial practices for designing initial pediatric trials.27 Given the wealth of clinical data and experience with busulfan, we expect to improve upon this with our own modeling attempts.
Figure 4.
Simulated busulfan plasma concentrations in a 1-year-old infant (top) and 11-year-old child (bottom) receiving IV busulfan at doses of 0.45 and 2.17 mg/kg. Pediatric population pharmacokinetic model modified from adult population pharmacokinetics.20
Discussion
We sought to characterize dosing patterns of IV busulfan administration and TDM in children undergoing HCT. Our long-range goal is to improve the outcomes for these children by more precise initial dosing of busulfan and less resource intensive methods for therapeutic drug monitoring in hopes of more efficiently achieving the target busulfan AUC. Our main findings are as follows: (1) There is considerable variation in the initial weight-based IV busulfan dose, with only 19% of infants and toddlers (ie, ≤12 kg) receiving the FDA–approved dose of 1.1 mg/kg; (2) the target busulfan AUC varies substantially among patients with the same disease, suggesting a need for additional pharmacodynamic data; (3) 7 samples are often obtained to estimate an individual child’s busulfan AUC and pharmacokinetic parameters, which is greater than expected for medication that typically exhibits 1-compartment pharmacokinetic behavior; and (4) IV busulfan clearance is dependent on age and dosing frequency but not diagnosis.
There is considerable variability in the initial (ie, before pharmacokinetic results are available) weight-based dosing of IV busulfan when administered every 6 hours (Suppl. Fig. S2). All patients appeared to be dosed based on body weight. Few patients weighing ≤12 kg received the FDA-approved dose of 1.1 mg/kg, although there was greater compliance with the package insert dosing in children weighing >12 kg (Table 1 and Suppl. Table S2). The labeled dosing guidance was based on simulations using a pediatric population pharmacokinetic model which indicated that ~60% of children would achieve a busulfan AUC between 900 to 1350 µM×min.22 Nguyen et al had developed a 5-category dosing nomogram that was expected to achieve a mean busulfan AUC of 1125 µM×min based on a pediatric IV busulfan population pharmacokinetic model.13 The success of the Nguyen nomogram in achieving a busulfan AUC of 900 to 1500 µM×min without TDM has been evaluated by 3 groups.10,28,29 Its success is variable, ranging from 56% in a cohort of 47 children10 to 91% in another cohort of 55 children.28 Recently, Trame et al created a busulfan population pharmacokinetic model from 94 children receiving oral (n = 54) or IV (n = 40) busulfan.29 Their simulations revealed that the Nguyen nomogram would result in only 44% of the children achieving a busulfan AUC of 900 to 1500 µM×min without TDM. Further simulations suggest that a higher proportion of children (ie, 70%–71%) achieve this target busulfan AUC with dosing IV busulfan based on BSA or allometric body weight. We are conducting a population pharmacokinetic modeling analysis to identify the optimal initial IV busulfan dose in hopes of more rapidly achieving the target busulfan AUC.
The pharmacodynamic associations of busulfan AUC vary based on patients’ ages, underlying diseases, and HCT conditioning regimens. In children, engraftment has been the only clinical endpoint consistently related to busulfan AUC such that engraftment rates are improved with busulfan TDM.5,6 No clear pharmacodynamic association exists between busulfan AUC and hepatotoxicity in children receiving targeted BU/CY or busulfan/melphalan.9,30,31 Similarly, no pharmacodynamic association exists between busulfan AUC and relapse risk in children with AML.11 Therefore, there is no clear rationale for the considerable heterogeneity in the target AUC per disease (Table 2 and Suppl. Fig. S2), particularly in children with AML. Interpretation of this finding is limited, in part, by the lack of available information regarding the other components of the conditioning regimen, the degree of myeloablation intended, and the organ function of the individual children. Unfortunately, pharmacodynamic outcomes are not available for this data set. A repository with such details is not available, so Supplemental Figure S2 presents the only data, to our knowledge, of the heterogeneity in busulfan target AUCs. A repository would allow for an adequate sample size to appropriately characterize busulfan pharmacodynamics in a homogenous population of children. Furthermore, the development of population pharmacokinetic models and limited sampling schedules could facilitate multi-institutional studies to better understand the concentration-effect relationship of busulfan in children. This data could then be used to provide data for clinical practice guidelines regarding the optimal busulfan target AUC for each disease.
Universal acceptance of busulfan dose targeting has been impeded by the time-intensive pharmacokinetic sampling schedule. This remains so, despite the recent increase in TDM of busulfan demonstrating the feasibility of this strategy (Suppl. Fig. S1). Most children had 7 pharmacokinetic samples drawn per AUC, which is high to characterize the pharmacokinetics of a 1-compartment drug.
More efficient methods of estimating busulfan AUC and clearance (as clearance = dose/AUC) are desirable. The demand for shorter busulfan courses for the purpose of reducing the intensity of conditioning is forcing an alternative method to targeted IV busulfan doses.19 Variable success in predicting IV busulfan clearance has been obtained with the use of test doses19 or pharmacogenetics of glutathione S-transferase (GST).32,33 The clearance of IV busulfan was slightly lower in children receiving busulfan daily; however, considerable overlap in busulfan clearance is seen between the various dosing frequencies (Suppl. Fig. S3).
The most promising method to improve TDM of IV busulfan is population pharmacokinetic modeling. Population pharmacokinetic modeling can identify covariates associated with busulfan pharmacokinetics. For instance, our data suggest that IV busulfan clearance is lower in young children (Figures 1 and 2). Children less than 4 years of age had lower busulfan clearance, even when expressed by BSA, which suggests lower GST activity in this population. Therefore, the expression of drug clearance relative to BSA appears to be the most appropriate method for comparing clearance in children of varying ages, as suggested recently.29 Part of the issue is that the current practice of linearly dividing dose by body weight does not reflect the true nature of the relationship between clearance and dosing weight.34 Dosing by body weight is a known systematic poor dosing practice, which is the rationale for many population pharmacokinetic models using allometric (nonlinear) relationships. Population pharmacokinetic models also facilitate development of optimal pharmacokinetic sampling schedules, which could lower the number of samples needed to characterize an individual child’s busulfan clearance. The risk of multiple blood samples in pediatric oncology patients is gaining increasing attention, suggesting a need for less intensive blood sampling schemas.35 Future research should address if a child’s busulfan AUC and pharmacokinetic parameters may be accurately estimated with a limited sampling schedule used in conjunction with maximum a posteriori Bayesian estimation, with a parameter prior based on population pharmacokinetic modeling.
Population pharmacokinetic-based approaches have already been applied to TDM of oral busulfan36 and IV cyclophosphamide37 in HCT recipients. Historically, such approaches have been inaccessible due to the paucity of adequately trained clinical pharmacology experts and software tools.38 The shortage of clinical pharmacologists with requisite direct patient care experience and pharmacometric expertise is in part due to lack of training programs and generally lower reimbursement for evaluative medical services.38 Barrett et al are developing novel decision support systems to improve the efficacy and safety of medications used to treat children.39 Such decision support systems incorporate relevant clinical data with a population pharmacokinetic model in a user-friendly interface to clearly communicate the optimal medication dose for each child. Busulfan is an ideal medication for the development of a decision support system because of the complexity of AUC-based dosing (compared to using a trough concentration, which is often done with other medications) and the need to personalize the busulfan target AUC based on the patient’s age, conditioning regimen, and underlying disease. An electronic clinical decision support system to apply consistent methods for TDM of IV busulfan is expected to improve clinical outcomes.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contributions of the patients whose IV busulfan was personalized by the Seattle Cancer Care Alliance Pharmacokinetics Laboratory, their families, and the clinical staff. They acknowledge the institutions, arranged in alphabetical order and listed in the appendix, which used the clinical services of the SCCA Pharmacokinetics Laboratory during this time period.
Funding
This study was supported by in part by NIH / NICHD, Pediatric Pharmacology Research Unit, Grant #HD037255-06, NICHD/NLM and Grant #1RC1LM010367-01, Decision Support System to Guide Pediatric Pharmacotherapy, Children’s Oncology Group Chair’s grant NIH U10 CA98543 and SDC U10 CA98413.
Appendix
Acknowledgment of the institutions that used SCCA Pharmacokinetics Service.
| Akron Children’s Hospital, Akron, OH |
| All Children’s Hospital, St Petersburg, FL |
| American Family Children’s Hospital, Madison, WI |
| Blair E. Batson Children’s Hospital, Jackson, MS |
| British Columbia Children’s Hospital, Vancouver, BC |
| Cardinal Glennon Children’s Medical Center, St Louis, MO |
| Children’s Hospital and Medical Center, Omaha, NE |
| Children’s Hospital and Research Center, Oakland, CA |
| Children’s Hospital of Alabama, Birmingham, AL |
| Children’s Hospital of Orange County, Orange, CA |
| Children’s Hospital of Pittsburgh, Pittsburgh, PA |
| Children’s Hospital of Richmond, Richmond, VA |
| Children’s Hospital of Wisconsin, Milwaukee, WI |
| Children’s Hospital, New Orleans, LA |
| Children’s Medical Center, Dallas, TX |
| Children’s Memorial Hospital, Chicago, IL |
| Children’s Mercy Hospital, Kansas City, MO |
| Children’s National Medical Center, Washington, DC |
| Cincinnati Children’s Hospital Medical Center, Cincinnati, OH |
| Cook Children’s Medical Center, Fort Worth, TX |
| Doernbecher Children’s Hospital, Oregon Health & Science University, Portland, OR |
| Floating Hospital for Children at Tufts Medical Center, Boston, MA |
| Health Sciences Centre, Winnipeg, MB |
| Helen DeVos Children’s Hospital, Grand Rapids, MI |
| Kapi’olani Medical Center for Women and Children, Honolulu, HI |
| Levine Children’s Hospital at Carolinas Medical Center, Charlotte, NC |
| Loma Linda University Children’s Hospital, Loma Linda, CA |
| Lucile Packard Children’s Hospital, Palo Alto, CA |
| Mattel Children’s Hospital UCLA, Los Angeles, CA |
| Medical City Children’s Hospital, Dallas, TX |
| Medical University of South Carolina Children’s Hospital, Charleston, SC |
| Memorial Sloan-Kettering Cancer Center, New York, NY |
| Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt, TN |
| Nationwide Children’s Hospital, Columbus, OH |
| North Carolina Children’s Hospital, Chapel Hill, NC |
| Penn State Hershey Children’s Hospital, Hershey, PA |
| Phoenix Children’s Hospital, Phoenix, AZ |
| Presbyterian/St Luke’s Medical Center, Denver, CO |
| Primary Children’s Medical Center, Salt Lake City, UT |
| Rady Children’s Hospital, San Diego, CA |
| Riley Hospital for Children, Indianapolis, IN |
| Seattle Children’s Hospital, Seattle, WA |
| St Louis Children’s Hospital, St Louis, MO |
| Steven and Alexandra Cohen Children’s Medical Center of New York, New Hyde Park, NY |
| Texas Transplant Institute/Methodist Children’s Hospital, San Antonio, TX |
| The Bristol-Myers-Squibb Children’s Hospital at Robert Wood Johnson University Hospital, New Brunswick, NJ |
| The Children’s Hospital at Montefiore, Bronx, NY |
| The Children’s Hospital, Denver, CO |
| The Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack, NJ |
| University of Michigan C. S. Mott Children’s Hospital, Ann Arbor, MI |
| Yale-New Haven Children’s Hospital, New Haven, CT |
Footnotes
Supplemental material for this article is available on the journal’s website at www.wileyonlinelibrary.com.
Declaration of Conflicting Interests
Dr McCune has received research funding from Otsuka Pharmaceutical of North America.
References
- 1.Deeg HJ, Maris MB, Scott BL, Warren EH. Optimization of allogeneic transplant conditioning: not the time for dogma. Leukemia. 2006;20(10):1701–1705. doi: 10.1038/sj.leu.2404327. [DOI] [PubMed] [Google Scholar]
- 2.McCune JS, Holmberg LA. Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol. 2009;5(8):957–969. doi: 10.1517/17425250903107764. [DOI] [PubMed] [Google Scholar]
- 3.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89(8):3055–3060. [PubMed] [Google Scholar]
- 4.Woods WG, Kobrinsky N, Buckley JD, et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: a report from the Children’s Cancer Group. Blood. 1996;87(12):4979–4989. [PubMed] [Google Scholar]
- 5.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16(1):31–42. [PubMed] [Google Scholar]
- 6.Bolinger AM, Zangwill AB, Slattery JT, et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant. 2001;28(11):1013–1018. doi: 10.1038/sj.bmt.1703264. [DOI] [PubMed] [Google Scholar]
- 7.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17(2):225–230. [PubMed] [Google Scholar]
- 8.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25(1):55–61. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 9.McCune JS, Gooley T, Gibbs JP, et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30(3):167–173. doi: 10.1038/sj.bmt.1703612. [DOI] [PubMed] [Google Scholar]
- 10.Schechter T, Finkelstein Y, Doyle J, et al. Pharmacokinetic disposition and clinical outcomes in infants and children receiving intravenous busulfan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(3):307–314. doi: 10.1016/j.bbmt.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Baker KS, Bostrom B, DeFor T, Ramsay NK, Woods WG, Blazar BR. Busulfan pharmacokinetics do not predict relapse in acute myeloid leukemia. Bone Marrow Transplant. 2000;26(6):607–614. doi: 10.1038/sj.bmt.1702590. [DOI] [PubMed] [Google Scholar]
- 12.Russell JA, Kangarloo SB. Therapeutic drug monitoring of busulfan in transplantation. Curr Pharm Des. 2008;14(20):1936–1949. doi: 10.2174/138161208785061382. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C. IV busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant. 2004;33(10):979–987. doi: 10.1038/sj.bmt.1704446. [DOI] [PubMed] [Google Scholar]
- 14.Shaw PJ, Nath C, Berry A, Earl JW. Busulphan given as four single daily doses of 150 mg/m2 is safe and effective in children of all ages. Bone Marrow Transplant. 2004;34(3):197–205. doi: 10.1038/sj.bmt.1704560. [DOI] [PubMed] [Google Scholar]
- 15.Gaziev J, Nguyen L, Puozzo C, et al. Novel pharmacokinetic behavior of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: a prospective evaluation of pharmacokinetic and pharmacodynamic profile with therapeutic drug monitoring. Blood. 2010;115(22):4597–4604. doi: 10.1182/blood-2010-01-265405. [DOI] [PubMed] [Google Scholar]
- 16.Wall DA, Chan KW, Nieder ML, et al. Safety, efficacy, and pharmacokinetics of intravenous busulfan in children undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2010;54(2):291–298. doi: 10.1002/pbc.22227. [DOI] [PubMed] [Google Scholar]
- 17.Dalal J, Neville KA. Busulfan in children: impact of development on dose-exposure-response relationship? Pediatr Blood Cancer. 2010;54(2):191–192. doi: 10.1002/pbc.22296. [DOI] [PubMed] [Google Scholar]
- 18.Bartelink IH, Bredius RG, Belitser SV, et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(2):231–241. doi: 10.1016/j.bbmt.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Kletzel M, Jacobsohn D, Duerst R. Pharmacokinetics of a test dose of intravenous busulfan guide dose modifications to achieve an optimal area under the curve of a single daily dose of intravenous busulfan in children undergoing a reduced-intensity conditioning regimen with hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(4):472–479. doi: 10.1016/j.bbmt.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Salinger DH, Vicini P, Blough DK, O’Donnell PV, Pawlikowski MA, McCune JS. Development of a population pharmacokinetics-based sampling schedule to target daily intravenous busulfan for outpatient clinic administration. J Clin Pharmacol. 2010;50(11):1292–1300. doi: 10.1177/0091270009357430. [DOI] [PubMed] [Google Scholar]
- 21.Rigby RA, Stasinopoulos DM. Using the Box-Cox t distribution in GAMLSS to model skewness and kurtosis. Statistical Modelling. 2006;6:209–229. [Google Scholar]
- 22.Otsuka America Pharmaceutical, Inc. Busulfex (busulfan) [package insert] Tokyo, Japan: [Accessed May 30, 2008]. http://www.ivbusulfex.com/Otsuka_IVBusulfex_v2AA.pdf. Revised February 2008. [Google Scholar]
- 23.Booth BP, Rahman A, Dagher R, et al. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol. 2007;47(1):101–111. doi: 10.1177/0091270006295789. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT. Up-regulation of glutathione S-transferase activity in enterocytes of young children. Drug Metab Dispos. 1999;27(12):1466–1469. [PubMed] [Google Scholar]
- 25.Hassan M, Fasth A, Gerritsen B, et al. Busulphan kinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplant. 1996;18(5):843–850. [PubMed] [Google Scholar]
- 26.Vassal G, Fischer A, Challine D, et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood. 1993;82(3):1030–1034. [PubMed] [Google Scholar]
- 27.Abernethy DR, Burckart GJ. Pediatric dose selection. Clin Pharmacol Ther. 2010;87(3):270–271. doi: 10.1038/clpt.2009.292. [DOI] [PubMed] [Google Scholar]
- 28.Vassal G, Michel G, Esperou H, et al. Prospective validation of a novel IV busulfan fixed dosing for paediatric patients to improve therapeutic AUC targeting without drug monitoring. Cancer Chemother Pharmacol. 2008;61(1):113–123. doi: 10.1007/s00280-007-0455-2. [DOI] [PubMed] [Google Scholar]
- 29.Trame MN, Bergstrand M, Karlsson MO, Boos J, Hempel G. Population pharmacokinetics of busulfan in children: increased evidence for body surface area and allometric body weight dosing of busulfan in children. Clin Cancer Res. 2011;17(21):6867–6877. doi: 10.1158/1078-0432.CCR-11-0074. [DOI] [PubMed] [Google Scholar]
- 30.Meresse V, Hartmann O, Vassal G, et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant. 1992;10(2):135–141. [PubMed] [Google Scholar]
- 31.Zwaveling J, Bredius RG, Cremers SC, et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant. 2005;35(1):17–23. doi: 10.1038/sj.bmt.1704707. [DOI] [PubMed] [Google Scholar]
- 32.Johnson L, Orchard PJ, Baker KS, et al. Glutathione S-transferase A1 genetic variants reduce busulfan clearance in children undergoing hematopoietic cell transplantation. J Clin Pharmacol. 2008;48(9):1052–1062. doi: 10.1177/0091270008321940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abassi N, Vadnais B, Knutson JA, et al. Pharmacogenetics of intravenous and oral busulfan in hematopoietic cell transplant recipients. J Clin Pharmacol. 2011;51(10):1429–1438. doi: 10.1177/0091270010382915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 35.Cole M, Boddy AV, Kearns P, et al. Potential clinical impact of taking multiple blood samples for research studies in paediatric oncology: how much do we really know? Pediatr Blood Cancer. 2006;46(7):723–727. doi: 10.1002/pbc.20463. [DOI] [PubMed] [Google Scholar]
- 36.Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28(8):743–751. doi: 10.1038/sj.bmt.1703207. [DOI] [PubMed] [Google Scholar]
- 37.McCune JS, Batchelder A, Guthrie KA, et al. Personalized dosing of cyclophosphamide in the total body irradiation-cyclophosphamide conditioning regimen: a phase II trial in patients with hematologic malignancy. Clin Pharmacol Ther. 2009;85(6):615–622. doi: 10.1038/clpt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neely M, Jelliffe R. Practical, individualized dosing: 21st century therapeutics and the clinical pharmacometrician. J Clin Pharmacol. 2010;50(7):842–847. doi: 10.1177/0091270009356572. [DOI] [PubMed] [Google Scholar]
- 39.Barrett JS. Improving pharmacotherapy decision-making. International Innovation. 2011;1 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.