Abstract
Islet amyloid deposition in human type 2 diabetes results in β-cell loss. These amyloid deposits contain the unique amyloidogenic peptide human islet amyloid polypeptide (hIAPP), which is also a known substrate of the protease insulin-degrading enzyme (IDE). Whereas IDE inhibition has recently been demonstrated to improve glucose metabolism in mice, inhibiting it has also been shown to increase cell death when synthetic hIAPP is applied exogenously to a β-cell line. Thus, we wanted to determine whether a similar deleterious effect is observed when hIAPP is endogenously produced and secreted from islets. To address this issue, we cultured hIAPP transgenic mouse islets that have the propensity to form amyloid for 48 and 144 hours in 16.7 mM glucose in the presence and absence of the IDE inhibitor 1. At neither time interval did IDE inhibition increase amyloid formation or β-cell loss. Thus, the inhibition of IDE may represent an approach to improve glucose metabolism in human type 2 diabetes, without inducing amyloid deposition and its deleterious effects.
A major pathophysiological component of type 2 diabetes is islet β-cell failure, which is linked to both decreased β-cell mass and reduced β-cell secretory function, resulting in decreased insulin release (1). As such, a major focus for treating type 2 diabetes is to increase circulating insulin levels and thereby reduce glucose levels. Aside from increasing insulin release by using β-cell secretagogues, another approach is to inhibit insulin degradation. The degradation of insulin occurs mostly through the ubiquitously expressed protease, insulin-degrading enzyme (IDE), and inhibition of IDE has been suggested as a strategy to improve glucose metabolism in diabetes by prolonging insulin's half-life and its action (2).
Islet amyloid deposition is a pathological hallmark of type 2 diabetes, occurring in the vast majority of patients with the disease (3), and is associated with increased apoptosis of β-cells (4). In humans, islet amyloid polypeptide (IAPP), also known as amylin, is the 37-amino acid peptide constituent of these islet amyloid deposits (5). It is a normal secretory product of the β-cell that is cosecreted with insulin (6), and its secretion into the extracellular space is essential for islet amyloid deposition (7–10) and subsequent induction of β-cell apoptosis. IAPP is also a substrate of IDE, and inhibiting IDE's activity results in increased fibril formation by synthetic IAPP (11, 12). Together these data suggest that inhibition of IDE, although preventing catabolism of insulin, may also increase islet amyloid deposition and amyloid-induced cytotoxicity.
A recent study by Maianti et al (13), using a novel small molecule inhibitor of IDE, demonstrated that inhibiting IDE in high-fat-fed mice improved glucose metabolism. Their findings also suggested that this improvement in glucose metabolism occurred in part by inhibition of IDE's ability to degrade insulin, glucagon, and IAPP. However, these experiments were completed in mouse models of diabetes, and because the IAPP sequence in rodents does not contain the critical amyloidogenic sequence, mouse IAPP does not form amyloid fibrils (14) and is not toxic. Thus, it is feasible that the negative effect of IDE inhibition on islet amyloid formation by human IAPP (hIAPP) would not have been observed. We therefore examined the effect of IDE inhibition on islet amyloid deposition and its toxic effects in isolated islets from a mouse model that expresses the human form of IAPP in β-cells.
Materials and Methods
Mice
Transgenic mice hemizygous for the expression of hIAPP under a rat insulin promoter on a F1 C57BL/6 × DBA/2 background as well as nontransgenic littermate controls were used for islet isolation (15–17). The Institutional Animal Care and Use Committee at Veterans Affairs Puget Sound Health Care System approved the use of animals for these studies.
Islet isolation, culture, and insulin content
Pancreatic islets were isolated from 10- to 12-week-old male and female mice by collagenase digestion, purified by gradient separation, and handpicked. Islets were recovered overnight in RPMI 1640 medium (Life Technologies) with 10% fetal bovine serum, 1% sodium pyruvate, 1% penicillin/streptomycin, and 11.1 mM glucose. Equal numbers of islets were then cultured for 48–144 hours in media containing 16.7 mM glucose to induce amyloid deposition (16, 18) in the presence or absence of 30 μM of the IDE-specific inhibitor 1 (Ii1) (2), dissolved in sterile dimethylsulfoxide (DMSO). Media containing Ii1 or DMSO were changed every 48 hours.
At the end of the period of incubation, triplicate samples of five islets per condition were extracted using acid ethanol for subsequent measurement of insulin content using an insulin ELISA (ALPCO).
Histological measurements
Islets were fixed in 10% neutral buffered formalin for 30 minutes, resuspended in agar, refixed in neutral buffered formalin for 3 hours, embedded in paraffin, and processed for histology. Ten-micrometer sections, 100 μm apart from throughout the agar pellet, were mounted on slides. β-Cells were identified by staining with an antiserum against insulin (1:2000; clone K36AC10, batch 014M4750; Sigma-Aldrich) for 18 hours, followed by a 1-hour incubation with secondary antisera (Jackson ImmunoResearch) conjugated with indocarbocyanine (Cy3; 1:250). Amyloid and nuclei were visualized by counterstaining with thioflavin S (0.5% g/vol; Sigma-Aldrich) and Hoechst 33258 (2 μg/ml; Sigma-Aldrich), respectively (16, 17). Islet area and thioflavin S-positive area were determined using Nikon Elements AR 4.30.02 software, and β-cell area (Σ insulin area/Σ islet area × 100), amyloid severity (Σ thioflavin S area/Σ islet area × 100), and amyloid prevalence (proportion of islets with amyloid) were then calculated as we have done before (16, 17).
To quantify β-cell apoptosis, sections were stained with propidium iodide (4 μg/mL; Invitrogen) for 20 minutes to visualize condensed and fragmented apoptotic nuclei and an insulin antibody as above to visualize β-cells (19). The percentage of β-cells that were apoptotic was determined by manual counting of condensed nuclei in insulin-positive cells relative to total nuclei.
An average of 27 islets per condition per experiment was analyzed, with the observer blinded to the genotype and culture conditions of the islets (17). Seven studies with hIAPP transgenic islets and three with nontransgenic littermates were performed.
IDE inhibition assay
Quantification of IDE activity was performed using a well-characterized Aβ degradation assay, based on a synthetic Aβ (1–40) peptide derivatized with fluorescein isothiocyanate and biotin at the N and C termini, respectively (20). Assays were conducted using the avidin-agarose precipitation method, as previously described (20). Briefly, conditioned media samples (100 μL) or lysates (10 μL) were incubated at 37°C with the derivatized Aβ peptide (100 nM) in a final volume of 500 μL in PBS. Samples (100 μL) were collected at various time points and the reactions terminated with excess 1,10-phenanthroline (1 M). Intact substrate was separated from cleaved products by incubation with neutravidin-agarose beads (5 μL; Pierce/Thermo Fisher Scientific) followed by centrifugation at 10 000 × g for 10 minutes, after which the levels of cleaved, fluoresceinated N-terminal fragments were quantified using a fluorescence plate reader (SpectraMAX M2; Molecular Devices; λex = 488 nm, λem = 525 nm). Incubation times were adjusted to obtain progress curves within the linear range (eg, not in excess of 30% of maximum hydrolysis, as obtained with excess recombinant IDE). Linear regression was performed through a minimum of five data points per reaction to derive reaction rates, which were subsequently normalized to the average obtained for controls.
Statistical methods
Data are presented as mean ± SEM for the number of experiments indicated. Statistical significance was determined using the Mann-Whitney U nonparametric t test or unpaired two-tailed Student's t test. A value of P ≤ .05 was considered statistically significant.
Results
Islet amyloid deposition
Culture of hIAPP transgenic islets in 16.7 mM glucose was associated with islet amyloid deposition, while, as expected, no amyloid was observed in nontransgenic islets (Figure 1). Amyloid deposition was present at 48 hours and, consistent with its time-dependent deposition, was further increased at 144 hours of culture (1.0% ± 0.20% to 3.4% ± 0.56%, P < .01, Figure 1, C and D).
Figure 1.
Islet amyloid deposition in nontransgenic (NT) and transgenic (TG) islets cultured at 16.7 mM glucose in the absence or presence of the IDE-specific inhibitor Ii1 (30 μM). Islet amyloid prevalence at 48 hours (A) and 144 hours (B) is shown. Islet amyloid severity at 48 hours (C) and 144 hours (D) is also shown (n = 3 for nontransgenic islets; n = 7 for transgenic islets).
Forty-eight-hour incubation of hIAPP transgenic islets with the IDE inhibitor Ii1 did not increase amyloid prevalence (40.6% ± 12.20% vs 52.0% ± 6.7%; Figure 1A) or amyloid severity (1.1% ± 0.35% vs 1.0% ± 0.20%; Figure 1C) compared with treatment with the vehicle, DMSO. Similarly, IDE inhibition for 144 hours did not change amyloid prevalence (70.4% ± 8.17% vs 62.2% ± 7.06%; Figure 1B) or severity (4.4% ± 0.76% vs 3.4% ± 0.56%; Figure 1D).
β-Cell area and insulin content
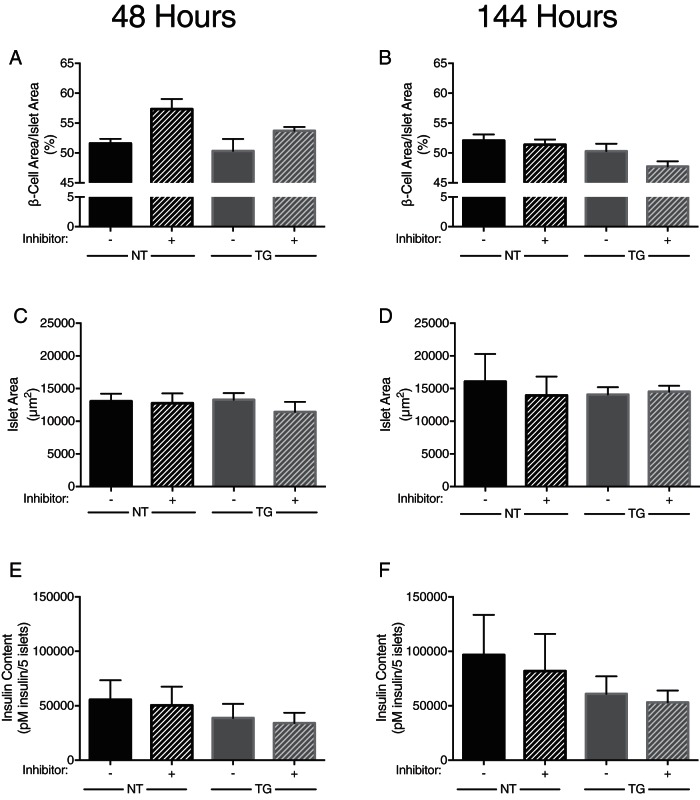
After 48 hours, nontransgenic islets, which do not have the propensity to form amyloid, had similar β-cell area when cocultured with Ii1 or with DMSO (57.4% ± 1.68% vs 51.6% ± 0.77%; Figure 2A). Similarly, and in line with the lack of change of amyloid deposition with IDE inhibition, amyloid-prone hIAPP transgenic islets did not exhibit a difference in β-cell area when they were cultured in the presence or absence of Ii1 (53.7% ± 0.64% vs 50.3% ± 2.01%; Figure 2A). Furthermore, after 144 hours of culture in 16.7 mM glucose, the β-cell area did not differ with IDE inhibition in nontransgenic islets (51.4% ± 1.68% vs 52.1% ± 1.01%) or hIAPP transgenic islets (50.3% ± 1.24% vs 47.7% ± 0.88%; Figure 2B).
Figure 2.
β-Cell area and insulin content of nontransgenic (NT) and transgenic (TG) islets cultured at 16.7 mM glucose in the absence or presence of the IDE-specific inhibitor Ii1 (30 μM). β-Cell area for islets at 48 hours (A) and 144 hours (B) is shown. Islet size for islets after 48 hours (C) and 144 hours (D) is also shown. Insulin content of islets cultured for 48 hours (E) and 144 hours (F) is also shown (n = 3 for nontransgenic islets; n = 7 for transgenic islets, n = 3 for content measurements).
Islet area did not differ between treatment groups at either 48 or 144 hours in transgenic or nontransgenic islets (Figure 2, C and D). Therefore, this variable did not confound the ability to detect changes in β-cell area. Insulin content was also not different between treatment groups at either 48 or 144 hours (Figure 2, E and F).
β-Cell apoptosis
After 48 or 144 hours of culture in 16.7 mM glucose, rates of β-cell apoptosis were not different in hIAPP transgenic islets cultured with DMSO (0.23% ± 0.044% vs 0.44% ± 0.110%; Figure 3, A and B). Furthermore, the addition of the IDE inhibitor did not increase the rates of β-cell apoptosis after either 48 (0.30% ± 0.083% vs 0.23% ± 0.044%; Figure 3A) or 144 hours (0.40% ± 0.091% vs 0.44% ± 0.110%; Figure 3B).
Figure 3.

β-Cell apoptosis rates of transgenic (TG) islets cultured at 16.7 mM glucose in the absence or presence of the IDE-specific inhibitor Ii1 (30 μM). Proportion of cells double positive for propidium iodide and insulin after 48 hours (A) and 144 hours (B) is shown (n = 7).
IDE activity
Given the lack of effect of IDE inhibition on islet amyloid deposition, we verified that the dose of Ii1 used in these studies was effective in inhibiting IDE activity in hIAPP transgenic islets. IDE activity was quantified in conditioned media and lysates from transgenic islets treated with Ii1 (30 μM) or vehicle (DMSO) for 48 hours using a well-established amyloid β-protein (Aβ) degradation assay (20). Aβ degradation in the conditioned media from islets treated for 48 hours with Ii1 was reduced by greater than 95% compared with that observed in the conditioned medium from islets treated for 48 hours with DMSO (Figure 4A). The addition of fresh Ii1 to conditioned media from DMSO-treated islets immediately prior to the determination of IDE activity resulted in comparable reductions in Aβ degradation with that of islets treated for 48 hours with Ii1, indicating that no loss of inhibitor activity occurred over the 48-hour treatment period, which, because the media were changed every 48 hours, meant that even islets treated with Ii1 for a total of 144 hours were exposed to full bioactivity of the inhibitor throughout that period. Lysates from islets pretreated with Ii1 demonstrated 44% less IDE activity compared with lysates from islets treated with DMSO (Figure 4B). The residual 56% activity was not due to non IDE-mediated cleavage of the fluorescent Aβ peptide substrate, as demonstrated by the 87% inhibition of IDE activity achieved by the addition of excess Ii1 to control islet lysates immediately prior to the assay.
Figure 4.

IDE activity assays in conditioned medium and lysates from islets. IDE activity in conditioned medium from hIAPP transgenic islets treated for 48 hours with the IDE inhibitor Ii1 or DMSO or the latter supplemented with Ii1 (30 μM) just prior to performance of the IDE activity assay (Added Ii1) is shown. Nonconditioned medium (Medium) and buffer were also tested as controls (A). Islet lysates from hIAPP transgenic islets treated for 48 hours with the IDE inhibitor Ii1 or DMSO or the latter supplemented with excess Ii1 just prior to the performance of the IDE activity assay (Added Ii1) are shown. Buffer was also tested as a control (B) (n = 4). *, P < .05; ** P < .01.
Discussion
In this study, we sought to determine whether inhibition of the protease IDE would increase amyloid deposition and β-cell loss in our in vitro model of islet amyloid formation. Previous work by others has demonstrated that IAPP is a substrate of IDE in cell-free systems and in immortalized cell lines when exogenous hIAPP was applied to a tumor-derived β-cell line (11, 12, 21). In the latter, the effect of inhibiting IDE was to increase β-cell apoptosis (13). However, this previous work was not performed in a system in which amyloid deposition occurs due to production and release of endogenous hIAPP from β-cells, therefore limiting its pathophysiological interpretation to what may occur in islets. We therefore examined the effect of inhibiting IDE in islets predisposed to form amyloid, hypothesizing that this would result in an increase in amyloid deposition and thus exacerbate β-cell loss. However, rather than confirming our hypothesis, we found that short- and longer-term (48 or 144 h) inhibition of IDE in islets results in no change in islet amyloid deposition or loss of β-cells.
This lack of effect of IDE inhibition to increase islet amyloid deposition contrasts with our previous studies in which the inhibition of two other islet proteases increased islet amyloid formation. Specifically, inhibition of neprilysin and matrix metalloproteinase-9 (MMP-9) increased amyloid deposition by 50% and 120%, respectively, and correspondingly resulted in β-cell loss (22, 23). This lack of an increase in amyloid with the inhibition of IDE by Ii1 is not due to lack of efficacy of the inhibitor because we confirmed that Ii1 inhibits IDE activity at this dose both in conditioned media and islet lysates. The IDE inhibition observed in the islet lysates by Ii1 is in keeping with the previous observation that Ii1 is cell penetrant and effective at inhibiting cytosolic IDE (24).
The differences observed in amyloid deposition with the inhibition of neprilysin and MMP-9 vs that of IDE are more likely related to the primary location of each of these proteases and the site of IAPP aggregation. After synthesis by the β-cell, IAPP resides in the secretory granule along with insulin and is released into the extracellular space upon β-cell stimulation by a variety of secretagogues (1). We and others have observed that islet amyloid deposits extracellularly in a number of species, including humans (25–27). Consistent with this extracellular deposition, we found that IAPP release is required for amyloid formation to occur (6, 28). Thus, amyloid formation is reduced when IAPP release is decreased in vitro with somatostatin and diazoxide (10) and when its secretion is reduced in vivo by either decreased glucokinase expression (7) or when β-cell demand is diminished by rosiglitazone or metformin treatment (8).
Given that IDE is predominantly localized to the cytoplasm (21) and despite the fact that we were able to demonstrate an approximate 50% inhibition of IDE activity in cell lysates of islets treated with Ii1, there is no change in amyloid deposition. This would indicate that within the β-cell, IDE does not have access to IAPP, which is most likely protected from IDE degradation by being localized in the secretory granule. Furthermore, despite IDE being present in culture medium in control conditions, suggesting it is released in part from the cell, and its activity being nearly completely inhibited with Ii1, it would appear that IDE was not able to prevent IAPP undergoing aggregation into amyloid in the extracellular space. In contrast, neprilysin is a transmembrane protein, with the active site being contained in its extracellular domain (29), whereas MMP-9 is located in the extracellular matrix (30); both are therefore likely better located to degrade secreted IAPP and thereby limit islet amyloid formation. Thus, the cytoplasmic localization of IDE probably explains in large part the lack of an effect on the amyloid formation we observed when inhibiting this protease.
While we focused on the in vitro effects of IDE inhibition on amyloid deposition, it is important to consider our observations in light of the recent literature linking IDE inhibition to improved glucose metabolism in mice (13), a species that is not prone to developing islet amyloid (14). Although we found IDE inhibition not to increase β-cell loss due to amyloid deposition in cultured islets in vitro, it is possible that the findings could differ in vivo as IDE circulates and thus could possibly still interact with IAPP near the β-cell. If that were the case, it is possible that β-cell dysfunction could still materialize, resulting in reduced insulin secretion and a less favorable effect in humans. Furthermore, because IDE is present in plasma and inhibiting its activity increases circulating IAPP levels in mice (13), it would also be important to ensure that IDE inhibition does not have deleterious effects if it reduces degradation of circulating IAPP in humans (31). Were either of these scenarios to occur, IDE inhibition as a therapeutic approach may not be optimal for humans.
In summary, we have observed that unlike in systems using synthetic hIAPP applied exogenously in vitro, the inhibition of IDE does not increase amyloid deposition when hIAPP is endogenously produced and released as occurs in vivo. Whether it could be safely administered for prolonged periods of time to humans with type 2 diabetes as a therapeutic approach to enhance insulin action needs to be determined.
Acknowledgments
We thank Breanne Barrow, Michael Peters, Joshua Willard, Phillip Bergquist, Daryl Hackney, Atiqur Rahman, Jessica Wilkins-Gutierrez, and Chantruyen Ho for their excellent technical support.
Author contributions include the following: M.F.H. contributed to the study design, performed the research, analyzed the data, and wrote the manuscript. D.T.M. contributed to the study design, performed the research, analyzed the data, and reviewed/edited the manuscript. A.T.T. and M.M. helped interpret the data and reviewed/edited the manuscript. S.Z., R.L.H., and S.E.K. contributed to the study design, helped interpret the data, and reviewed/edited the manuscript. M.A.L. provided the IDE inhibitor Ii1, performed the IDE activity assays, and reviewed/edited the manuscript. M.F.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the work as a whole.
This work was supported, in whole or in part, by Department of Veterans Affairs Grant BX001060; Veterans Affairs Puget Sound Health Care System (Seattle, WA) and National Institutes of Health Grants P30DK017047 (to the University of Washington Diabetes Research Center), Grant 5T32HL007028 (to M.F.H.), and T32DK007247 (to A.T.T.); Swiss National Foundation Fellowship (to D.T.M.); and Dick and Julia McAbee Endowed Fellowship (to D.T.M.).
Disclosure Summary: The authors have nothing disclose.
Appendix
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| Insulin | Purified immunoglobulin | Anti-insulin antibody, mouse monoclonal | Sigma-Aldrich, SAB4200691 SIGMA, clone K36AC10, batch 014M4750 | Mouse, monoclonal | 1:2000 |
Footnotes
- DMSO
- dimethylsulfoxide
- hIAPP
- human IAPP
- IAPP
- islet amyloid polypeptide
- IDE
- insulin-degrading enzyme
- Ii1
- IDE inhibitor 1
- MMP-9
- matrix metalloproteinase-9.
References
- 1. Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. [DOI] [PubMed] [Google Scholar]
- 2. Leissring MA, Malito E, Hedouin S, et al. Designed inhibitors of insulin-degrading enzyme regulate the catabolism and activity of insulin. PLoS One. 2010;5(5):e10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Westermark P. Quantitative studies of amyloid in the islets of Langerhans. Ups J Med Sci. 1972;77(2):91–94. [DOI] [PubMed] [Google Scholar]
- 4. Jurgens CA, Toukatly MN, Fligner CL, et al. β-Cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178(6):2632–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89(8):3629–3643. [DOI] [PubMed] [Google Scholar]
- 6. Kahn SE, D'Alessio DA, Schwartz MW, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes. 1990;39(5):634–638. [DOI] [PubMed] [Google Scholar]
- 7. Andrikopoulos S, Verchere CB, Terauchi Y, Kadowaki T, Kahn SE. β-Cell glucokinase deficiency and hyperglycemia are associated with reduced islet amyloid deposition in a mouse model of type 2 diabetes. Diabetes. 2000;49:2056–2062. [DOI] [PubMed] [Google Scholar]
- 8. Hull RL, Shen Z-P, Watts MR, et al. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54(7):2235–2244. [DOI] [PubMed] [Google Scholar]
- 9. Aston-Mourney K, Subramanian SL, Zraika S, et al. One year of sitagliptin treatment protects against islet amyloid-associated β-cell loss and does not induce pancreatitis or pancreatic neoplasia in mice. Am J Physiol Endocrinol Metab. 2013;305(4):E475–E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aston-Mourney K, Hull RL, Zraika S, Udayasankar J, Subramanian SL, Kahn SE. Exendin-4 increases islet amyloid deposition but offsets the resultant β cell toxicity in human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2011;54(7):1756–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000;275(47):36621–36625. [DOI] [PubMed] [Google Scholar]
- 12. Bennett RG, Hamel FG, Duckworth WC. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes. 2003;52(9):2315–2320. [DOI] [PubMed] [Google Scholar]
- 13. Maianti JP, McFedries A, Foda ZH, et al. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature. 2014;511(7507):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87(13):5036–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Alessio DA, Verchere CB, Kahn SE, et al. Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes. 1994;43(12):1457–1461. [DOI] [PubMed] [Google Scholar]
- 16. Zraika S, Hull RL, Udayasankar J, et al. Glucose- and time-dependence of islet amyloid formation in vitro. Biochem Biophys Res Commun. 2007;354(1):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meier DT, Entrup L, Templin AT, et al. Determination of optimal sample size for quantification of β-cell area, amyloid area and β-cell apoptosis in isolated islets. J Histochem Cytochem. 2015;63(8):663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verchere CB, D'Alessio DA, Palmiter RD, Kahn SE. Transgenic mice overproducing islet amyloid polypeptide have increased insulin storage and secretion in vitro. Diabetologia. 1994;37(7):725–728. [DOI] [PubMed] [Google Scholar]
- 19. Zraika S, Hull RL, Udayasankar J, et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced β cell apoptosis. Diabetologia. 2009;52(4):626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leissring MA, Lu A, Condron MM, et al. Kinetics of amyloid β-protein degradation determined by novel fluorescence- and fluorescence polarization-based assays. J Biol Chem. 2003;278(39):37314–37320. [DOI] [PubMed] [Google Scholar]
- 21. Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19(5):608–624. [DOI] [PubMed] [Google Scholar]
- 22. Zraika S, Aston-Mourney K, Marek P, et al. Neprilysin impedes islet amyloid formation by inhibition of fibril formation rather than peptide degradation. J Biol Chem. 2010;285(24):18177–18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aston-Mourney K, Zraika S, Udayasankar J, et al. Matrix metalloproteinase-9 reduces islet amyloid formation by degrading islet amyloid polypeptide. J Biol Chem. 2013;288(5):3553–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdul-Hay SO, Bannister TD, Wang H, et al. Selective targeting of extracellular insulin-degrading enzyme by quasi-irreversible thiol-modifying inhibitors. ACS Chem Biol. 2015;10(12):2716–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Koning EJ, Morris ER, Hofhuis FM, et al. Intra- and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1994;91(18):8467–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verchere CB, D'Alessio DA, Palmiter RD, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic β cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1996;93(8):3492–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaikaran ET, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta. 2001;1537(3):179–203. [DOI] [PubMed] [Google Scholar]
- 28. MacArthur DL, de Koning EJ, Verbeek JS, Morris JF, Clark A. Amyloid fibril formation is progressive and correlates with β-cell secretion in transgenic mouse isolated islets. Diabetologia. 1999;42(10):1219–1227. [DOI] [PubMed] [Google Scholar]
- 29. Terashima H, Okamoto A, Menozzi D, Goetzl EJ, Bunnett NW. Identification of neuropeptide-degrading enzymes in the pancreas. Peptides. 1992;13(4):741–748. [DOI] [PubMed] [Google Scholar]
- 30. Tomita T, Iwata K. Gelatinases and inhibitors of gelatinases in pancreatic islets and islet cell tumors. Mod. Pathol. 1997;10(1):47–54. [PubMed] [Google Scholar]
- 31. Kahn SE, Verchere CB, Andrikopoulos S, et al. Reduced amylin release is a characteristic of impaired glucose tolerance and type 2 diabetes in Japanese Americans. Diabetes. 1998;47(4):640–645. [DOI] [PubMed] [Google Scholar]