Abstract
In a recent genome-wide association study, hexokinase domain-containing protein 1, or HKDC1, was found to be associated with gestational glucose levels during 2-hour glucose tolerance tests at 28 weeks of pregnancy. Because our understanding of the mediators of gestational glucose homeostasis is incomplete, we have generated the first transgenic mouse model to begin to understand the role of HKDC1 in whole-body glucose homeostasis. Interestingly, deletion of both HKDC1 alleles results in in utero embryonic lethality. Thus, in this study, we report the in vivo role of HKDC1 in whole-body glucose homeostasis using a heterozygous-deleted HKDC1 mouse model (HKDC1+/−) as compared with matched wild-type mice. First, we observed no weight, fasting or random glucose, or fasting insulin abnormalities with aging in male and female HKDC1+/− mice. However, during glucose tolerance tests, glucose levels were impaired in both female and male HKDC1+/− mice at 15, 30, and 120 minutes at a later age (28 wk of age). These glucose tolerance differences also existed in the female HKDC1+/− mice at earlier ages but only during pregnancy. And finally, the impaired glucose tolerance in HKDC1+/− mice was likely due to diminished whole-body glucose use, as indicated by the decreased hepatic energy storage and reduced peripheral tissue uptake of glucose in HKDC1+/− mice. Collectively, these data highlight that HKDC1 is needed to maintain whole-body glucose homeostasis during pregnancy but also with aging, possibly through its role in glucose use.
Pregnancy induces a number of metabolic adaptations to ensure that the nutrient requirements of the mother and fetus are met (1). These pregnancy-induced adaptions include lower fasting glucose and higher rates of hepatic gluconeogenesis with increased glucose-stimulated insulin secretion in the setting of peripheral insulin resistance (1, 2). Understanding gestational glucose metabolism is important as elevated gestational glucose levels lead to higher morbidity risk for both the mother and newborn, and can have lasting effects on the metabolic health of the newborn (3).
Recently, in a genome-wide association study (GWAS) exploring gestational glucose homeostasis in human pregnancy, we reported the genome-wide association of variants in HKDC1 with 2-hour blood glucose levels during a glucose tolerance test in pregnant mothers at 24–28 weeks' gestation (4). Further investigation demonstrated that genetic variants regulate the expression of HKDC1, with lower HKDC1 expression being associated with higher 2-hour gestational glucose levels (4, 5). Interestingly, prior type 2 diabetes and related GWAS examining glucose-related metabolic parameters failed to demonstrate a genome-wide significance with HKDC1, suggesting that the role of hexokinase domain-containing protein 1 (HKDC1) in glucose homeostasis may be specific to pregnancy (2, 6) and that it may represent a novel gene specifically involved in gestational glucose metabolism.
In our original GWAS report (4), we discussed that HKDC1 may be a novel hexokinase based on a phylogenetic analysis by Irwin and Tan (7). Their analysis suggested this possibility due to HKDC1 sharing approximately 70% sequence homology with HK1 at both the nucleotide and amino acid level. Hexokinases are an evolutionarily conserved family of kinases responsible for the phosphorylation of glucose upon entry into the cell, thereby trapping glucose and introducing it into cellular metabolism (8). Four family members in humans have previously been described and characterized, with each possessing unique expression patterns, substrate affinities, and subcellular localization. For instance, hexokinases 1–3 have a high enzymatic activity in phosphorylating glucose (represented by their lower Michaelis constant [Km] values) and are responsible for glucose metabolism and energy production. Hexokinase (HK)-4 (or glucokinase [GCK]), on the other hand, has traditionally been termed the glucose sensor of the body due to its restricted expression to tissues that participate in whole-body glucose sensing (such as pancreatic β-cells and the liver) and because the Km value of GCK is higher and closer to the physiological levels of glucose observed in the blood (9). Of importance, for the past 50 years, these four HKs have been thought to be the only HKs in humans mediating this pivotal step of glucose metabolism (8). Following up on our genetic findings and the prediction that HKDC1 may be a HK, we subsequently observed that HKDC1 encodes a novel fifth hexokinase, through purified protein studies. In that report, we supported those observations with cell-based assays in which HKDC1 was either overexpressed or knocked down (5). Collectively these data confirmed that HKDC1 is a fifth, previously unrecognized, HK.
Considering the importance of gestational glucose metabolism to the metabolic health of mother and offspring (3, 10), we sought to better define the in vivo function of HKDC1. To do this, we have developed the first mouse model using a gene trap system to globally ablate HKDC1 expression through stem cells from the Knockout Mouse Repository (11). Because homozygous transgenic mice are embryonic lethal, in this report, we provide the first in vivo characterization of HKDC1 using heterozygous mice (HKDC1+/−). Collectively these data have begun to reveal the in vivo role of HKDC1 in whole-body glucose homeostasis under normal and gestational conditions.
Materials and Methods
Immunohistochemistry
Paraffin-embedded human colon, liver, and kidney were obtained from the Northwestern Pathology core (Chicago, IL) or the Loyola University Pathology core (Maywood, Illinois) and deparaffinized followed by antigen retrieval performed for 30 minutes at 95°C in retrieval buffer (10 mM sodium citrate; 0.05% Tween-20, pH 6.0). Sections were incubated overnight with an anti-HKDC1 antibody (Sigma) at 1:250 dilution. Diaminobenzidine staining (brown) was done using the Vectastain avidin biotin complex antirabbit kit (Vector Labs) and hematoxylin (blue) used as a nuclear counterstain.
Animals
The transgenic mouse strain was created from embryonic stem cell clone (EPD0846_5_B10) obtained from the Knockout Mouse Repository (www.komp.org) and generated by the Wellcome Trust Sanger Institute (11). In brief, the gene encoding HKDC1 contains 18 exons. An IRES-LacZ-Neo cassette was inserted before exon 3, producing a truncated gene product. Mice were genotyped using the following primer sets: 5′-TCATGGAAATCCTACAGCAGGGACC-3′ and 5′-GTCATGCTGGAGAGAATGAGCAAGC-3′ for the wild-type allele generating a 764-bp product; 5′-GAGATGGCGCAACGCAATTAATG-3′ and 5′-AGCTAAGTCCAAGCCCACAAACTCC-3′ for the transgenic allele generating a 322-bp product. All mice were maintained in a colony on a 12-hour light, 12-hour dark cycle with ad libitum access to normal chow (Envigo). All animal experiments were approved by the Institutional Animal Care and Use Committee at Northwestern University.
Reverse transcription-polymerase chain reaction
Tissues were harvested from C57BL/6J mice (Taconic) and RNA isolated using the RNEasy minikit (QIAGEN). cDNA was synthesized as previously described (12) and 50 ng used in each PCR with the following primers: HKDC1 (forward, 5′-GGTGAACCGCCTGGCCACTG-3′, reverse, 5′-CTGCTTCCCGGGGTTGAGCG-3′); HK1 (forward, 5′-TGC CAT GCG GCT CTC TGA TG-3′, reverse, 5′-CTT GAC GGA GGC CGT TGG GTT-3′); GCK (forward, 5′-GAG GTC GGC ATG ATT GTG GGC A-3′, reverse, 5′-ACA CAC ATG CGC CCC TCA TCG CC-3′). All samples were run in triplicate.
Intraperitoneal and oral glucose tolerance test
After an overnight 12-hour fast, animals were given 2 g glucose per kilogram of body weight by either oral gavage or ip injection, as before (13). Blood was obtained from the tail vein and glucose levels monitored using an OneTouch UltraMini glucometer (LifeScan, Inc) at 0, 15, 30, 60, and 120 minutes. Additionally, blood was collected at multiple points between 0 and 30 minutes in heparinized capillary tubes and centrifuged at 5000 rpm for 15 minutes and the serum collected. Insulin levels in these samples were determined by an ELISA (ALPCO).
Insulin tolerance test
Mice were fasted for 6 hours and then injected ip with 0.75 U/kg body weight of Humalog insulin (Eli Lily & Co). Blood glucose levels were determined similar to the aforementioned glucose tolerance tests.
Sodium pyruvate challenge
After a 12-hour fast, animals were given 2 mg/g body weight of sodium pyruvate dissolved in PBS. Blood glucose levels were determined similar to the aforementioned glucose tolerance tests.
Metabolic cages
Male mice 12–16 weeks of age were individually house in automated metabolic cages (TSE Systems) for 5 days. Mice were acclimated for 48 hours prior to data collection. Metabolic activity was then recorded for 72 hours by TSE Lab-Master software in 30-minute intervals. Airflow through the cages was held at 0.25–0.35 L/min, and 12-hour light, 12-hour dark cycles were maintained with ad libitum access to food and water. Resting energy expenditure was plotted by averaging data in 3-hour blocks beginning at the start of the light cycle.
Twenty-four-hour fast
Starting at 6:00 am, animals were fasted and blood glucose monitored at 0, 2, 4, 6, 12, and 24 hours. Animals were refed the following morning.
Total liver triglyceride and glycogen measurement
Animals were fasted for 4 hours prior to being anesthetized. Harvested livers were immediately flash frozen in liquid nitrogen. Livers were homogenized on ice in a glass douncer with 1 mL 1× PBS. Homogenates were cleared by centrifugation at 13 000 rpm, and the supernatant was collected. Total protein for each sample was determined using the Bradford assay. Liver triglyceride levels were determined using the Infinity triglyceride solution (ThermoFisher Scientific). Flash-frozen liver samples were analyzed by 13C-nuclear magnetic resonance for glycogen levels at the Yale Mouse Metabolic Phenotyping Center (Yale University, New Haven, CT).
Isotopomer glucose uptake
Twenty-eight-week-old male mice were given a U-13C6 glucose bolus at 2 mg/g body weight. Animals were anesthetized by ip injection of ketamine/xylazine solution, 90 mg/kg and 10 mg/kg body weight, respectively. Tissue was collected at 120 minutes and flash frozen until used for analyses. Derivatization was performed as previously described and analyzed via gas chromatography/mass spectrometry on select tissues that express higher levels of HKDC1 (14).
Protein expression and Western blotting
Rosetta 2 (DE3) (EMD Millipore) cells were transformed with pReceiver-B01 plasmid containing either human HKDC1 or HK1. One hundred milliliters of tryptone phosphate-buffered media containing 50 μg/mL ampicillin and 34 μg/mL chloramphenicol were inoculated with a single colony and grown overnight at 37ºC. The pregrowth culture was added to 500 mL of the same media and grown to an OD of approximately 0.6. One milliliter of preinduction culture was centrifuged at 13 000 rpm and flash frozen. Protein expression was induced at room temperature by adding isopropyl-1-thio-β-D-galactopyranoside to a final concentration of 1 mM and grown for an additional 4 hours. Cultures were spun down at 13 000 rpm and lysed in 100 μL of radioimmunoprecipitation assay buffer supplemented with Halt protease inhibitor (ThermoFisher Scientific). Samples were boiled at 95°C for 20 minutes and the lysates clarified by centrifugation. Ten microliters of clarified supernatant were run on a 8% SDS-PAGE. Blots were incubated overnight with anti-HKDC1 (1:1000 dilution; Abcam).
Statistical analysis
Data are presented as mean ± SEM. Glucose tolerance tests were analyzed by a two-way ANOVA with Bonferroni correction. All other data were compared by a two-tailed unpaired Student's t test for statistical significance.
Results
HKDC1 expression pattern in human and mouse tissues
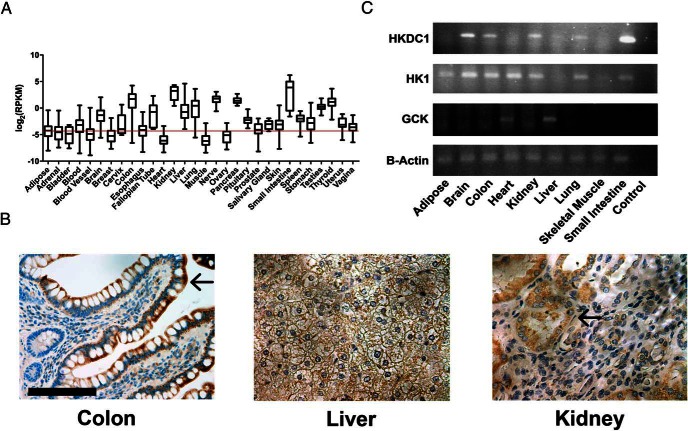
We previously reported that HKDC1 is broadly expressed, although notably absent from both muscle and adipose tissue (4). Using data from the Genotype-Tissue Expression (15, 16), a human tissue-specific RNA expression database, we reconfirmed this expression pattern in human tissue (Figure 1A) with appreciable expression in liver, colon, and kidney. Expanding on these data, we next explored expression and localization of HKDC1 in key human tissues by immunohistochemistry (see Supplemental Figure 1 for the specificity of this antibody for HKDC1 relative to HK1 by Western blot). In the colon, which is one of the highest-expressing HKDC1 tissues, HKDC1 is strongly expressed but is restricted to the epithelial cells of the villi (Figure 1B). In the human liver, HKDC1 is diffusely expressed in hepatocytes at low levels relative to other HKDC1-positive tissues (Figure 1B). In the kidney, HKDC1 is restricted to the renal tubule and excluded from the glomeruli (Figure 1B).
Figure 1.
Human and murine HKDC1 expression. A, Plot of RNA-sequencing reads for HKDC1 in human tissue samples from the Genotype-Tissue Expression Consortium. Data are represented as reads per kilobase. Background threshold is indicated by red line. B, Immunohistochemical staining for HKDC1 in normal human colon, liver, and kidney. Arrows mark villi in colon and glomerulus in kidney. Nuclei are blue, and HKDC1 is brown. Scale bar, 50 μm. C, Semiquantitative RT-PCR for HKDC1, HK1, and GCK in different identified tissues. β-Actin was used as a loading control. *, P < .05.
Because we plan to use a mouse model to study the in vivo role of HKDC1 in metabolism, we next explored the expression of HKDC1 RNA in mouse tissues to assess whether the pattern of expression is similar to that in human tissues. Mouse HKDC1 expression parallels human HKDC1 expression (Figure 1C), eg, in brain, colon, kidney, and small intestines (Figure 1C, lanes 2, 3, 5, and 9, respectively). Together these data do the following: 1) confirm the previously reported pattern of HKDC1 expression in humans (5), 2) provide a more detailed inspection of human HKDC1 protein expression in select human tissues, and 3) establish that the pattern of HKDC1 expression is similar between mouse and human.
HKDC1 transgenic model characterization
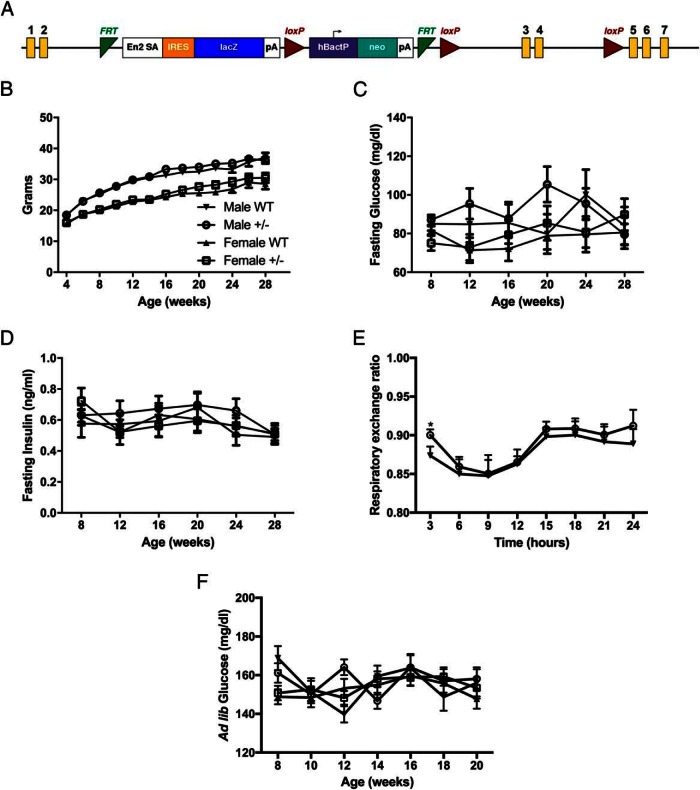
To begin to explore the in vivo role of HKDC1 in glucose metabolism, we developed a transgenic mouse line, as shown in Figure 2A. A LacZ-Neo cassette was inserted into HKDC1 following exon 2, producing a truncated nonfunctional gene product. Of particular importance, since generating this line, no homozygous mice (HKDC1−/−) have been generated from HKDC1+/− × HKDC1+/− breeding approach despite hundreds of offspring. The expected Mendelian ratio of heterozygous to wild-type mice offspring (2:1) was observed. The lack of HKDC1−/− mice suggests that complete ablation of HKDC1 in animals is embryonic lethal at an undefined point in utero. Similar embryonic lethality or perinatal death has been reported with other hexokinase transgenic animals, including HK2 and GCK (see references 17 and 18). HK1 knockout animals have yet to be generated and characterized in the literature. Thus, all studies presented here were carried out using heterozygous (HKDC1+/−) and wild-type (WT) littermates. In Supplemental Figure 2, we show data that in the HKDC1+/− mice, the expression of full-length HKDC1 is reduced approximately 50% relative to WT mice in tissues with the highest level of expression (eg, colon, kidney, and liver).
Figure 2.
Characterization of the HKDC1 transgenic mouse model. A, Diagram of the HKDC1 transgenic mouse model locus. Yellow rectangles indicate the exons of HKDC1. Image is adapted from the Knockout Mouse Project. B, Animal weights in grams from 4 to 28 weeks of age (n = 12–18 per group). Blood glucose (C) and insulin levels (D) after a 12-hour fast (n = 12–18 per group) are shown. E, Average respiratory exchange ratio of animals maintained in a metabolic cage over a 24-hour period (n = 4 per group). F, Ad libitum glucose levels (n = 12–18 per group). *, P < .05.
First, the body weights of male and female mice were assessed from 4 to 28 weeks of age, and no statistical difference between WT and HKDC1+/− mice maintained on a normal chow diet was observed (Figure 2B). Blood glucose and insulin levels were assessed after a 12-hour fast at multiple time points from 4 to 28 weeks of age and were not significantly different between male and female WT and HKDC1+/− mice at all ages (Figure 2, C and D). Using metabolic cages, a small but significant shift in resting energy expenditure was observed at the 3-hour time point in male HKDC1+/− mice at 12–16 weeks of age compared with the WT mice (Figure 2E). Additionally, ad libitum blood glucose levels were not different from 8 to 20 weeks of age in male and female WT and HKDC1+/− mice (Figure 2F). Taken together, these results demonstrate that HKDC1+/− mice on a normal chow diet, as compared with the WT mice, develop no overt metabolic phenotype related to glucose or insulin homeostasis.
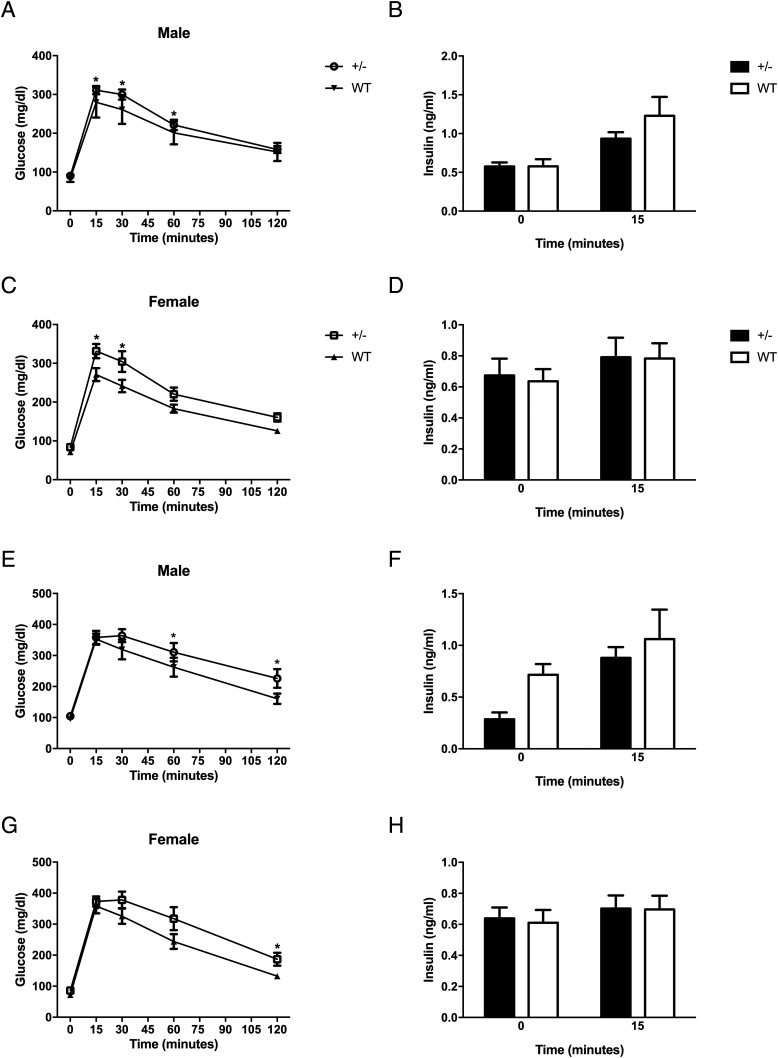
To more rigorously examine glucose homeostasis in these mice on normal chow diet, we performed glucose tolerance tests at 28 weeks of age. We first assessed glucose tolerance by an oral glucose test (OGTT), and as seen in Figure 3, A and C, glucose levels at multiple time points were elevated in both female and male HKDC1+/− mice as compared with the matched WT mice. Next, because HKDC1 is highly expressed in the intestinal epithelium, we also investigated glucose tolerance by intraperitoneal glucose injection because any difference would indicate a possible role of HKDC1 in incretin secretion. Interestingly, both male and female HKDC1+/− mice demonstrated marked differences in glucose tolerance at multiple time points after an IP glucose tolerance test (Figure 3, E and G), suggesting the impairment in glucose tolerance is not a direct effect of glucose on the gut. In addition to the indicated statistically significant time points, the 30-minute time point in HKDC1+/− males (Figure 3E) and 60-minute time point in HKDC1+/− females (Figure 3G) were both trending toward significant (P < .01). Importantly, insulin levels during both glucose tolerance tests were found to be similar at baseline and at 15 minutes after administration (Figure 3, B, D, F, and H). Insulin secretion was further interrogated in male mice at 0, 2, 5, 10, 15, and 30 minutes after oral gavage, and no significant differences were seen (Supplemental Figure 3). Thus, insulin secretion is not dramatically affected in HKDC1+/− mice.
Figure 3.
Impaired glucose tolerance in aged HKDC1 transgenic animals. Blood glucose values for male (A) and female (C) oral glucose tolerance tests and respective insulin values (B and D) are shown. Male (E) and female (G) ip glucose tolerance tests and corresponding insulin values (F and H) (n = 12–15 per group) are shown. *, P < .05.
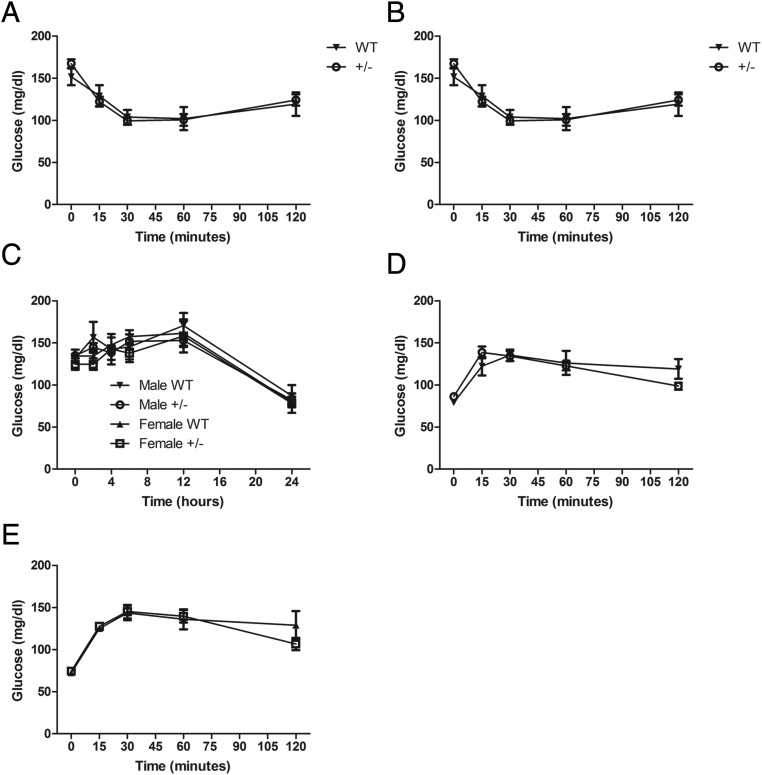
Other possibilities leading to altered glucose tolerance during the ip glucose tolerance test and OGTT include changes in insulin sensitivity and gluconeogenesis or peripheral glucose clearance/use. Insulin tolerance tests were similar in male and female WT and HKDC1+/− mice, suggesting similar insulin sensitivity in the two groups (Figure 4, A and B). To examine gluconeogenesis, 24- to 28-week-old mice were fasted, and blood glucose levels were monitored for 24 hours. No differences in blood glucose levels were recorded at any time point between male and female WT and HKDC1+/− mice (Figure 4C). Furthermore, sodium pyruvate challenges, an additional measure of gluconeogenesis, showed no difference over the course of the experiment between the male and female WT and HKDC1+/− mice (Figure 4, D and E). The above findings demonstrate a role for HKDC1 in nongravid glucose homeostasis but with no observed differences in insulin sensitivity and gluconeogenesis, which possibly indicates that glucose clearance/use is altered in HKDC1+/− mice.
Figure 4.
Assessment of insulin tolerance and gluconeogenesis in aged HKDC1 transgenic animals. A and B, Blood glucose values during an insulin tolerance test from male and female mice, respectively (n = 12–18 per group). C, Blood glucose levels during a 24-hour fast of male and female WT littermates and HKDC1+/− mice (n = 4–10 per group). D and E, Blood glucose levels during a pyruvate challenge for male and female WT littermates and HKDC1+/− mice (n = 12–18 per group).
Impaired glucose tolerance during pregnancy
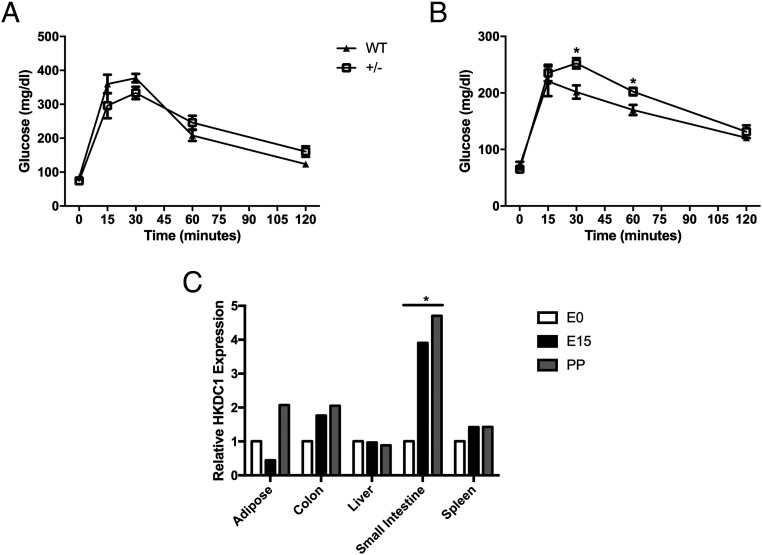
As reported in humans, there is a highly significant association between genetic variants in HKDC1 and 2-hour glucose levels after an OGTT at approximately 28 weeks' gestation (4). Therefore, we assessed whether glucose homeostasis was altered in these mice during pregnancy. We first assessed glucose tolerance in nonpregnant WT and HKDC1+/− female mice between 8 and 12 weeks of age. There was no evidence for impaired glucose tolerance in female HKDC1+/− vs WT mice (Figure 5A). Next, at gestational day 15, which is the height of insulin resistance during pregnancy in mice (19), HKDC1+/− mice demonstrated change in glucose levels after an OGTT (Figure 5B). Because our prior studies in humans suggested that single-nucleotide polymorphisms in HKDC1 associated with maternal 2-hour glucose levels affected HKDC1 expression with lower HKDC1 expression associated with higher maternal 2-hour glucose levels (4, 5), we next examined the expression of HKDC1 throughout pregnancy in mice. We observed that pregnancy increases the expression of HKDC1 in select tissues, in particular in colon and small intestine, but not liver (Figure 5C).
Figure 5.
Impaired glucose tolerance in pregnant HKDC1+/− transgenic mice. A, Blood glucose levels in female WT littermates and HKDC1+/− mice after an oral glucose challenge at 8–12 weeks of age (n = 5 per group). B, Blood glucose values in female WT littermates and HKDC1+/− mice after an oral glucose challenge at gestational day 15 (n = 5–6 per group). C, Expression of HKDC1 RNA in white adipose tissue, colon, liver, small intestine, and spleen before pregnancy (E0), at gestational day 15 (E15), and at 10 days postpartum (PP) in WT mice (n = 3 per time point). *, P < .05.
HKDC1 expression contributes to impaired energy storage and whole-body glucose use
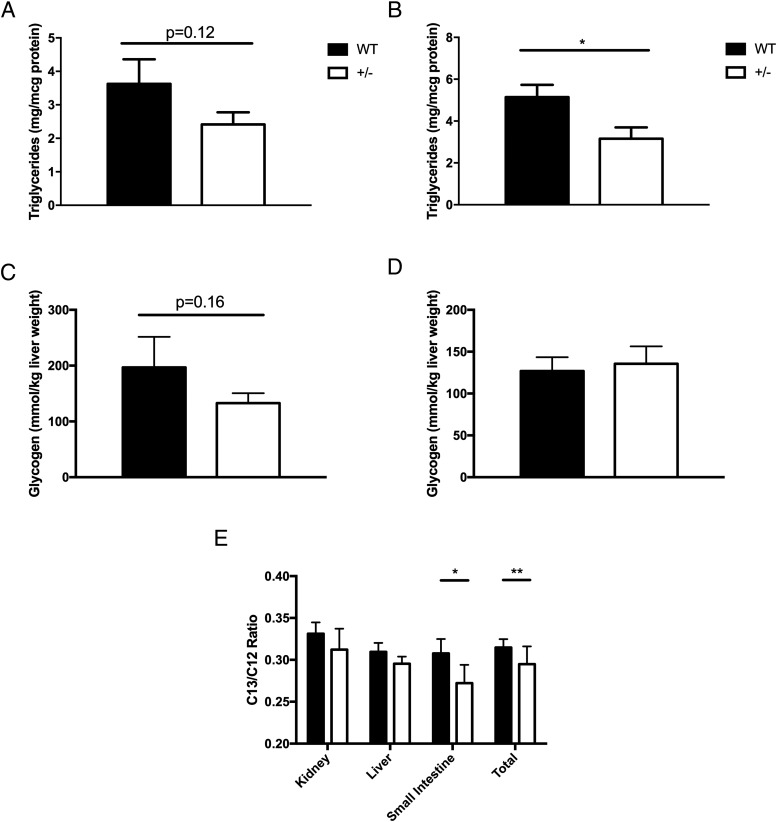
During pregnancy and aging, deletion of one HKDC1 allele leads to impaired glucose tolerance, possibly due to diminished tissue use or clearance of glucose. Because HKs are involved in glucose metabolism, diminished clearance/use would lead to reduced energy storage. Because HKDC1 is expressed in the liver and is a key tissue to store energy, hepatic triglyceride and glycogen levels were evaluated (Figure 6, A–D). Interestingly, we observed that HKDC1+/− female mice compared with WT female mice had lower triglyceride levels (Figure 6B), and a trend existed toward lower levels in HKDC1+/− male mice compared with WT male mice. For glycogen, levels trended lower for HKDC1+/− male mice compared with WT male mice (Figure 6, C and D) but not with female. To more directly assess whole-body glucose use during a glucose challenge, male mice were given a U-13C6 glucose bolus and tissues with substantial expression of HKDC1, such as liver, kidney, and small intestine were collected and analyzed for label enrichment after 120 minutes. The overall quantity of 13C6 glucose was significantly lower in tissue from HKDC1+/− mice, as compared with WT mice (Figure 6E), especially in the small intestine, with a trend toward lower levels in the other tissues.
Figure 6.
Liver triglyceride and glucose uptake and storage. Total liver triglycerides (micrograms per milligram total protein) of male (A) and female (B) mice are shown. The liver glycogen content measured (millimoles per kilogram) liver tissue in male (C) and female (D) mice is shown. For panels A–D, n = 5–7 per group. E, Ratio of C13-labeled glucose to endogenous C12-glucose in kidney, liver, and small intestine (n = 15). *, P < .05.
Discussion
Gestational diabetes complicates approximately 7% of pregnancies in the United States, posing a major health concern (10, 20). Women who develop gestational diabetes are at a 6-fold increased risk of developing type 2 diabetes later in life, whereas 3%–5% remain diabetic, even after pregnancy (10, 20). Left untreated, gestational diabetes can lead to health risks for both the mother and offspring including macrosomia, jaundice, and increased probability of cesarean section (3). The development of gestational diabetes can further lead to an increased risk of gestational diabetes in subsequent pregnancies and poor metabolic outcomes for the offspring later in their life (3). Despite its prevalence, the molecular mechanisms underlying the development and subsequent progression of gestational diabetes remain poorly understood (2). Until now, known risk alleles for impaired glucose homeostasis during pregnancy have overlapped with known type 2 diabetes genes. HKDC1 represents one of the first pregnancy-specific glucose metabolism genes to be identified and characterized (2).
Here we have demonstrated that HKDC1 is broadly expressed across multiple cell types and conserved between human and mouse. Additionally, as mentioned above, HKDC1 is most similar to HK1 (7, 8) based on sequence homology, and these two genes are in close genetic proximity in humans. Despite this, these two genes have distinct expression patterns from each another. For example, HK1 is expressed more diffusely in tissues including muscle and adipose, tissues in which HKDC1 is not expressed. Due to its ubiquitous and constitutive expression and low Km, HK1 is crucial in these tissues for basal glucose metabolism. Overall, HKDC1 has a more limited tissue expression pattern than HK1. Also, the expression pattern of HKDC1 appears conserved between human and mouse. At the protein level, HKDC1 in high HKDC1-expressing tissues such as kidney and intestine shows a cell-specific pattern of expression, further differentiating it from the other HKs. Overall, these data indicate HKDC1 to be a distinct HK from HK1–4 based on its pattern of expression.
To probe the role of HKDC1 in vivo, we generated a traditional gene trap mouse model, which produces a truncated nonfunctional HKDC1 transcript. Similar to some of the other HKs (17, 18), complete loss of HKDC1 during development is embryonic lethal. However, unlike GCK in which the pups die soon after delivery, no pups have been found that have the HKDC1−/ − genotype, suggesting that deletion of both alleles is lethal in utero. This lethality led us to perform our in vivo studies in heterozygous mice, HKDC1+/−. Whereas HKDC1+/− mice maintain normal glucose homeostasis early on in life, aged animals display a mild impairment in glucose tolerance without impaired insulin secretion in both male and female mice, an unexpected finding, given that HKDC1 has been associated with glucose homeostasis only during pregnancy.
Additionally, our mice did not demonstrate a difference in insulin sensitivity or gluconeogenesis, strongly suggesting glucose clearance/use is altered in HKDC1+/− mice. It is unclear why this is, but it may be due to age-dependent metabolic demands and increasing insulin resistance with age. These results are consistent with our previous observation that genetic variants in HKDC1 are associated with maternal 2-hour glucose levels after an oral glucose challenge at 28 weeks' gestation (4) because HKDC1+/− mice also demonstrated glucose intolerance during pregnancy but not impaired glucose tolerance at this particular age. In this case, young mice (8–12 wk of age) did not experience the metabolic stresses conferred by pregnancy or aging, and thus, HKDC1+/− and WT mice demonstrate similar glucose tolerance. It is only under metabolic stress, either from pregnancy or aging, that loss of HKDC1 leads to glucose intolerance. Therefore, we suspect that pregnancy may exacerbate the phenotype due to pregnancy-induced insulin resistance and that this effect also occurs in mice with aging secondary to the age-related increase in insulin resistance.
As we begin to understand how HKDC1 contributes to whole-body glucose homeostasis, and in particular during pregnancy, it is important to relate our findings back to the observation in humans and the negative association of HKDC1 expression with 2-hour gestational glucose. We speculate that elevated HKDC1 expression during pregnancy may be contributing to the gestational nutrient balance that occurs between mother and fetus. Whereas HKDC1 does not appear to have a role in insulin tolerance, gluconeogenesis, or insulin secretion, in the HKDC1+/− model used here, HKDC1 appears to contribute to peripheral tissue glucose use, which may be leading to diminished energy stores in the liver, consistent with this diminished glucose use. Because this mouse model, HKDC1+/−, is limited by only 50% loss of full-length HKDC1 expression, new models are needed to better explore HKDC1, such as a HKDC1fl/fl model, allowing tissue and temporal dissection of HKDC1 function.
Here we have extensively characterized the properties of HKDC1 in vivo. These data together suggest that this novel hexokinase is involved in global glucose use, which is the probable mechanism leading to impaired glucose tolerance during pregnancy in our mouse model and with aging in both female and male mice. Moreover, because HKs contribute to energy stores in certain tissues, it is not surprising that the deletion of HKDC1 appears to decrease hepatic energy stores (ie, triglycerides and glycogen). Taken together, in this report, we provide our first insights into the in vivo role of HKDC1, in which future directions will benefit from additional mouse models.
Acknowledgments
B.T.L. and T.E.R. are supported by the National Institutes of Health under Award R01DK104927-01A1. B.T.L. is supported by The University of Chicago Diabetes Research and Training Center (Grant P30DK020595) and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Career Development (Grant 1IK2BX001587–01). A.E.L. is supported by National Institutes of Health Grant T32 DK007169. M.P. is supported by American Heart Association Postdoctoral Fellowship Grant 15POST22410016. C.G. is supported by National Institutes of Health Grant F31 HD085666.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- GCK
- glucokinase
- GWAS
- genome-wide association study
- HK
- hexokinase
- HKDC1
- hexokinase domain-containing protein 1
- Km
- Michaelis constant
- OGTT
- oral glucose test
- WT
- wild type.
References
- 1. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayes MG, Urbanek M, Hivert MF, et al. Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes. 2013;62:3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo C, Ludvik AE, Arlotto ME, et al. Coordinated regulatory variation associated with gestational hyperglycaemia regulates expression of the novel hexokinase HKDC1. Nat Commun. 2015;6:6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe RM, Black MH, Xiang AH, et al. Genetics of gestational diabetes mellitus and type 2 diabetes. Diabetes Care. 2007;30(suppl 2):S134–S140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irwin DM, Tan H. Molecular evolution of the vertebrate hexokinase gene family: identification of a conserved fifth vertebrate hexokinase gene. Comp Biochem Physiol Part D Genomics Proteomics. 2008;3:96–107. [DOI] [PubMed] [Google Scholar]
- 8. Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. [DOI] [PubMed] [Google Scholar]
- 9. Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5:171–176. [DOI] [PubMed] [Google Scholar]
- 10. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 11. Skarnes WC, Rosen B, West AP, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krug MS, Berger SL. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. [DOI] [PubMed] [Google Scholar]
- 13. Priyadarshini M, Villa SR, Fuller M, et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol Endocrinol. 2015;29:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Paul Lee WN, et al. Determination of a glucose-dependent futile recycling rate constant from an intraperitoneal glucose tolerance test. Anal Biochem. 2003;315:238–246. [DOI] [PubMed] [Google Scholar]
- 15. Carithers LJ, Moore HM. The Genotype-Tissue Expression (GTEx) project. Biopreserv Biobank. 2015;13:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heikkinen S, Pietila M, Halmekyto M, et al. Hexokinase II-deficient mice. Prenatal death of homozygotes without disturbances in glucose tolerance in heterozygotes. J Biol Chem. 1999;274:22517–22523. [DOI] [PubMed] [Google Scholar]
- 18. Postic C, Shiota M, Niswender KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. [DOI] [PubMed] [Google Scholar]
- 19. Fuller M, Priyadarshini M, Gibbons SM, et al. The short chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am J Physiol Endocrinol Metab. 2015;309(10):E840–E851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Metzger BE, Gabbe SG, Persson B, et al. The diagnosis of gestational diabetes mellitus: new paradigms or status quo? J Matern Fetal Neonatal Med. 2012;25:2564–2569. [DOI] [PubMed] [Google Scholar]