Abstract
Progesterone (P4) is essential for female fertility. The objective of this study was to evaluate the functional requirement of the nonclassical P4 receptor (PGR), PGR membrane component 1, in regulating female fertility. To achieve this goal, the Pgrmc1 gene was floxed by insertion of loxP sites on each side of exon 2. Pgrmc1 floxed (Pgrmc1fl/fl) mice were crossed with Pgrcre or Amhr2cre mice to delete Pgrmc1 (Pgrmc1d/d) from the female reproductive tract. A 6-month breeding trial revealed that conditional ablation of Pgrmc1 with Pgrcre/+ mice resulted in a 40% reduction (P = .0002) in the number of pups/litter. Neither the capacity to ovulate in response to gonadotropin treatment nor the expression of PGR and the estrogen receptor was altered in the uteri of Pgrmc1d/d mice compared with Pgrmc1fl/fl control mice. Although conditional ablation of Pgrmc1 from mesenchymal tissue using Amhr2cre/+ mice did not reduce the number of pups/litter, the total number of litters born in the 6-month breeding trial was significantly decreased (P = .041). In addition to subfertility, conditional ablation of Pgrmc1 using either Amhr2cre/+ or Pgrcre/+ mice resulted in the development of endometrial cysts starting around 4 months of age. Interestingly, pregnancy attenuated the formation of these uterine cysts. These new findings demonstrate that PGR membrane component 1 plays an important role in female fertility and uterine tissue homeostasis.
Progesterone (P4) is an essential hormone that exerts its actions at all levels of the reproductive axis. Faulty P4 signaling is linked to a number of reproductive diseases including endometriosis, infertility, breast and endometrial cancer, irregular menstrual bleeding, adenomyosis, miscarriage, preterm labor, and leiomyoma (1–7). In the uterus, P4 attenuates estradiol (E2)-induced epithelial cell proliferation and facilitates cellular differentiation in preparation for the establishment and maintenance of pregnancy (8). P4 is essential for endometrial stromal cell proliferation and decidualization in invasively implanting species (9, 10). In the rodent ovary, P4 plays an essential role in the ovulatory process and these actions are mediated by PGR. P4 also acts directly on granulosa cells to inhibit mitosis and apoptosis despite lacking expression of the classical nuclear P4 receptor (PGR) (11). P4 also promotes the viability and steroidogenic potential of luteal cells and stimulates both its own secretion and cholesterol biosynthesis (12, 13). Many of the actions of P4 within the ovary and uterus are mediated by the PGR. However, not all of these actions can be explained by activation of PGR.
If not PGR, then what other receptors could mediate the actions of P4 in the uterus and ovary? The rapid, nonclassical actions of P4 are well known to regulate ion flux in neurons (14), vascular smooth muscle (15), and epithelial cells (16), as well as in meiotic maturation (17), the acrosome reaction (18, 19) and sexual behavior (20). Further, P4 elicits modulatory actions in immune cells that are completely devoid of PGR expression (21). Although nonclassical P4 signaling is often synonymously referred to as “nongenomic,” several studies have now shown that P4 regulates transcriptional activity in cells and tissues that lack expression of PGR (22, 23), so perhaps the “nonclassical” designation is more appropriate.
Recent in vitro studies revealed that some actions of P4 are mediated by PGR membrane component 1 (PGRMC1). Spectroscopic and mutagenesis studies show human PGRMC1 binds P4 with high affinity (24). Moreover, the presence of PGRMC1 in membrane fractions correlates with an increase in high-affinity P4 binding (25, 26). PGRMC1 mediates the antiapoptotic and antimitotic actions of P4 in granulosa cells (27), as well as in ovarian and endometrial cancer cell lines (28, 29). PGRMC1 is proposed as a nonclassical PGR that, in general terms, localizes at the subcellular level to the endoplasmic reticulum, nucleus, and plasma membrane in most cells that express the protein (30, 31). PGRMC1 is most abundantly expressed in the endometrium during the proliferative phase of the macaque and human menstrual cycles (32–34) and therefore may have a role in the management of endometrial epithelial cell cycle progression. In the macaque, the cellular distribution of PGRMC1 changed in response to steroid hormone supplementation in an artificial model of the menstrual cycle (32). The cellular distribution of PGRMC1 was most abundant during the proliferative phase in both epithelial and stromal cells. By the late secretory phase, PGRMC1 expression was restricted to most stromal cells of the basalis, and scattered stromal cells within the functionalis. Individual cells of the luminal epithelium expressed PGRMC1, particularly at the apical surface. In the mouse, PGRMC1 is weakly expressed in both the stromal and epithelial compartments during estrus, becomes robustly expressed in both tissue types during metestrus, and then declines again during diestrus (35). PGRMC1 expression is also highly regulated at the maternal:fetal interface in human and rodents, as well as during the estrous/menstrual cycle in a number of species (35, 36). The expression pattern for PGRMC1 is quite distinct from PGR. For instance, during the periimplantation period on day 4 of pregnancy, whereas PGR expression is lost in the luminal epithelium, PGRMC1 expression is retained in this tissue, suggesting a distinct uterine role for this P4 mediator (35). Finally, PGRMC1 expression is reduced in fetal membranes among women with preterm premature rupture of the membranes (37), as well as in ectopic and eutopic endometrial stroma of women with endometriosis (38). PGRMC1 was recently identified as being differentially regulated in receptive vs nonreceptive endometria of women undergoing hormone therapy for in vitro fertilization (39). With this collective information from women and model organisms, it is hypothesized that PGRMC1 plays an important role in maintaining normal female reproductive functions. This hypothesis was tested through the development and use of a floxed Pgrmc1 allele (Pgrmc1fl/fl) and evaluation of conditional Pgrmc1 ablation in the female reproductive tract.
Materials and Methods
Floxing the Pgrmc1 allele and genotyping
All animal procedures were approved by Institutional Animal Care and Use Committees at Washington State University or the University of Connecticut Health Center. A Pgrmc1 targeting vector was prepared by recombineering according to Lee et al (40). Briefly, an 11-kb section of the Pgrmc1 genomic sequence containing all 3 exons and 0.5 kb of the 5′-upstream and 3′-downstream sequences was retrieved from the BAC, RP23-75F15, pPL253 by gap repair. A 5′ loxP site was inserted into intron 1 approximately 500 bp upstream of exon 2 followed by insertion of Frt-PGFneo-Frt-LoxP into intron 2 approximately 600 bp downstream of exon 2. The vector, which contained approximately 6 and 3.8 kb of the 5′ and 3′ arms, respectively, was linearized by NotI and electroporated into mouse ES cells derived from an F1(129Sv/C57BL6j) blastocyst. The cells were then cultured in the presence of 150 μg/ml of G418 and 2μM ganciclovir. Drug resistant colonies were selected and screened by nested long range PCR using primers corresponding to sequences outside the arms and specific to the 5′ and 3′ loxP sites to identify targeted ES clones. Targeted ES cells were used to generate chimeric animals by aggregation with CD1 morula. Chimeric male animals were then bred with ROSA26-Flpe mice to remove the PGKneo cassette to generate the Pgrmc1 floxed (Pgrmc1fl/fl) founder mice. Conditional Pgrmc1-ablated (Pgrmc1d/d) mice were produced by crossing Pgrmc1fl/fl mice with Pgrcre/+ (41) or Amhr2cre/+ (42) mice. After DNA isolation from tail snips, PCR was completed to detect the presence of the floxed Pgrmc1 allele and cre recombinase using primer sets shown in Table 1. For simplification, Pgrmc1 conditional knockout mice are referred to as Pgrmc1d/d. Information on PCR-based genotyping conditions is available upon request.
Table 1.
PCR Primers
| Gene Identifier | Primer Sequence |
|---|---|
| Amhr2-cre F | 5′-TCCAATTTACTGACCGTACACCAA-3′ |
| Amhr2-cre R | 5′-CCTGATCCTGGCAATTTCGGCTA-3′ |
| Pgr-cre P1 | 5′-ATGTTTAGCTGGCCCAAATG-3′ |
| Pgr-cre P2 | 5′-TATACCGATCTCCCTGGACG-3′ |
| Pgr-cre P3 | 5′-CCCAAAGAGACACCAGGAAG-3′ |
| Pgrmc1_lox_gt F | 5′-GGCTCAAGCACCCAGAATAG-3′ |
| Pgrmc1_lox_gt R | 5′-GCTTCCTTGCTTTCAACACC-3′ |
| Esr1 F | 5′-CCAAAGCCTCGGGAATG-3′ |
| Esr1 R | 5′-CTTTCTCGTTACTGCTGG-3′ |
| Pgr F | 5′-ATGGTCCTTGGAGGTCGTAA-3′ |
| Pgr R | 5′-CACCATCAGGCTCATCC-3′ |
| bActin F | 5′-GATGACGATATCGCTGCGCTG-3′ |
| bActin R | 5′-GTACGACCAGAGGCATACAGG-3′ |
Animals and treatments
Fertility of Pgrmc1fl/fl and Pgrmc1d/d mice from the Pgrcre/+ and Amhr2cre/+ colonies was assessed by mating 6-week-old female mice with male mice of proven breeding capacity in 6-month breeding trials. The number of litters and number of pups/litter were determined through the duration of the trials. Female reproductive tracts were collected from mice at the end of the breeding trials, as well as from young and aged nulliparous female mice for histological analyses.
Superovulation was used to assess ovarian capacity to ovulate. Female postnatal day 25 Pgrmc1fl/fl and Pgrmc1d/d mice (n = 3, Pgrcre) were treated ip with 5 IU of equine chorionic gonadotropin (Sigma Chemical Co). This was followed by 5 IU of human chorionic gonadotropin (Sigma Chemical Co) 46 hours later. Blood was collected 16 hours later for serum P4 analysis, and mice were then euthanized by carbon dioxide asphyxiation and cervical dislocation. Ovaries and oviducts were collected to assess ovulation. Oviductal ampullas were punctured and oocytes were retrieved and counted in PBS after brief enzymatic digestion with hyaluronidase. Serum P4 was measured by ELISA at the Washington State University Center for Reproductive Biology Radioimmunoassay Core facility. Ovarian tissues were processed and sectioned for histological analysis to confirm ovulation by observation of corpora hemorrhagica.
To compare estrogen receptor (ESR1) and PGR expression in uterine tissues obtained from Pgrmc1fl/fl and Pgrmc1d/d (Pgr-cre) mice under synchronized conditions, sexually mature female mice were first ovariectomized. The mice were then given daily sc injections of E2 (100 ng) diluted in sesame oil for 3 consecutive days. After 2 days, mice were treated with E2 (100 ng) for 2 days as before and uteri were collected 18 hours after the last injection. Uterine tissues were collected and partitioned for RNA isolation and fixation in 4% paraformaldehyde in preparation for paraffin embedding and RT-PCR as described below.
Western blotting validation of uterine Pgrmc1 ablation
To confirm that PGRMC1 was conditionally ablated from uterine tissues, protein was isolated from uterine and liver tissue samples from sexually mature female Pgrmc1fl/fl and Pgrmc1d/d (Pgr-cre) mice. Protein lysates were generated as described elsewhere (43). Briefly, 25 μg of total protein from each sample were separated electrophoretically using the NuPage system (Invitrogen) and transferred (30 V, 1 h) onto polyvinylidene difluoride membranes. Nonspecific binding was blocked with 5% BSA in 0.1% Tween 20 in PBS (PBST) buffer for 1 hour at room temperature. Primary antibody (anti-PGRMC1, 1:1000 dilution; Sigma Chemical Co) was diluted in PBST with 5% BSA and applied to membranes for overnight incubation at 4°C. Membranes were washed (3 × 60 min) in PBST and incubated with secondary antibody (Cell Signaling Technology) for 1 hour at room temperature. After a second series of washes, bound antibodies were detected with enhanced chemiluminescent reagents using the manufacturer's recommendations (Thermo Scientific). Chemiluminescent signals were captured and quantified using the Bio-Rad ChemiDoc MP Imaging System.
Histology and immunohistochemistry
Paraffin-embedded uterine and ovarian tissues were sectioned (5 μm), deparaffinized, and rehydrated in graded ethanol. For general histology, slides were stained with hematoxylin and eosin (Scytek Laboratories, Inc). For sections used in immunohistochemical studies, peroxidase quenching (10 min in 10% hydrogen peroxide) was completed, and antigen retrieval was performed by boiling sections in a 0.1M sodium citrate buffer for 10 minutes. After boiling and reaching room temperature, sections were blocked (0.1% BSA, 0.1% normal goat serum, and 1% Triton X-100 in PBS) for 1 hour at room temperature. Sections were then incubated overnight at 4°C with the primary antibody diluted into blocking solution. After incubation, slides were washed in PBS 3 times 10 minutes each and then incubated with biotinylated secondary antibody (1:500 goat antirabbit IgG-B; Santa Cruz Biotechnology, Inc) for 45 minutes. Slides were washed as before and then incubated with horseradish peroxidase-conjugated streptavidin (Vector Laboratories). Washes were performed and the sections were exposed to 3,3′-diaminobenzidine (BD Biosciences) followed by a 5-minute inactivation in PBS. Sections were then counterstained with hematoxylin. Sections on slides were sequentially rehydrated and mounted after air drying. In all experiments, specificity of the primary antibody was confirmed by omission of this antibody in parallel slides.
RNA isolation and RT-PCR
Total cellular RNA was isolated from uterine tissues obtained from Pgrmc1fl/fl and Pgrmc1d/d (Pgrcre) female mice using Tri Reagent (Sigma Chemical Co). Each sample was first deoxyribonuclease 1 treated to eliminate residual genomic DNA. One microgram of RNA was then converted to cDNA by SuperScript II Reverse Transcriptase using the manufacturer's instructions (Life Technologies). Expression levels of Esr1, Pgr, and β-actin were assessed by semiquantitative RT-PCR using primer sequences shown in Table 1. Expression levels of Esr1 and Pgr are shown as normalized values vs the housekeeping gene β-actin.
Data analyses
Assignment to all treatment groups was made randomly. Data are presented as the mean ± SEM for n = 3–8 for each experiment where individual animals represent a single experimental replicate. Differences between treatment groups were assessed by Student's t test where the mean values of only 2 groups were compared. A two-way ANOVA was used to identify treatment effects in the breeding trials followed by a Bonferroni post hoc test. P ≤ .05 was considered significant. All data were analyzed using GraphPad 5.0 software.
Results
Conditional ablation of the Pgrmc1 allele
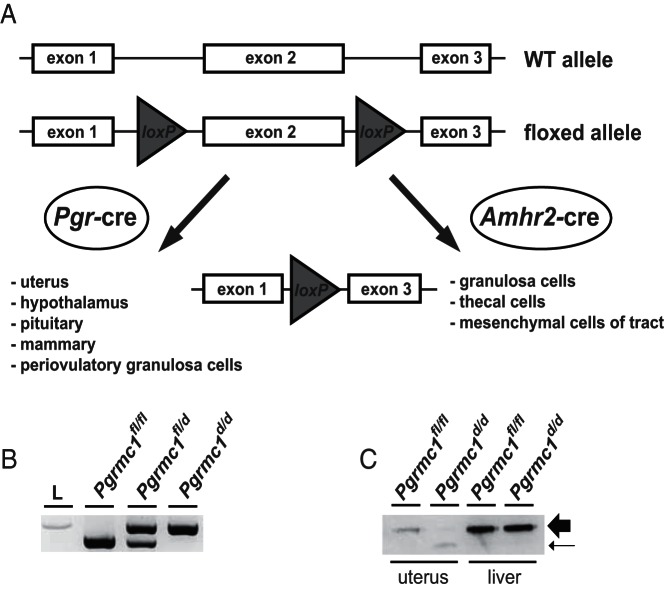
The X-linked Pgrmc1 allele was conditionally ablated by crossing Pgrmc1fl/fl mice with either Amhr2cre/+ or Pgrcre/+ mice. An illustration of the floxing strategy, as well as tissues expressing cre recombinase in each driver is shown in Figure 1A. After crosses between cre driver mice and Pgrmc1fl/fl mice, DNA isolated from tail snip samples was used to confirm mouse genotype. The wild type allele was recognized by PCR as a 459-bp cDNA, and the mutant allele was 532 bp in size (Figure 1B). The detection of both cDNAs identified heterozygous animals. The presence of the cre recombinase transgene was determined by PCR as described elsewhere (41, 42). Western blot analysis confirmed that the approximately 28-kDa PGRMC1 protein was conditionally ablated from the uteri of Pgrmc1d/d mice using the Pgrcre/+ mice but not from the liver (Figure 1C). A lower molecular weight immunoreactive band was detected at reduced levels in Pgrmc1d/d uterine tissue samples, which presumably represents a truncated form of PGRMC1 that lacks exon 2.
Figure 1.
Generation of the floxed Pgrmc1 allele. A, loxP sites were inserted on either side of exon 2. Mice harboring floxed alleles and mice expressing cre recombinase under the direction of Pgr or Amhr2 promoters were mated to generate control (Pgrmc1fl/fl) and conditional knockout (Pgrmc1d/d) animals. B, PCR-based genotyping results showing PCR amplification of Pgrmc1fl/fl (control), Pgrmc1fl/d (heterozygous), and Pgrmc1d/d (conditional knockout) alleles using Pgrcre/+ mice. L, DNA ladder. C, Western blotting showing ablation of PGRMC1 protein (large arrow) in Pgrcre/+;Pgrmc1d/d uterus but not liver (Pgr-cre). A truncated form of PGRMC1 is present in the uterus (small arrow).
Conditional ablation of Pgrmc1 impairs fertility
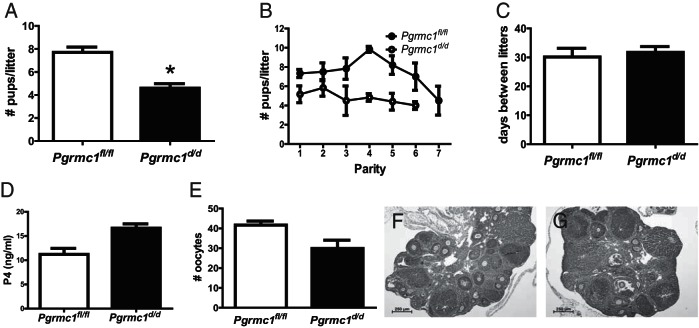
The number of pups/litter was determined in 6-month breeding trials to evaluate fecundity of Pgrmc1d/d mice as compared with Pgmrc1fl/fl control littermates using both Amhr2cre/+ and Pgrcre/+ mice. Using Amhr2cre/+, there was no difference in the number of pups/litter born to Pgrmc1d/d mice compared with Pgrmc1fl/fl control mice (Table 2). However, 50% of the Pgrmc1d/d female mice delivered 4 or fewer litters during the 6-month breeding trial, and this resulted in a significant decrease in fecundity compared with Pgrmc1fl/fl female mice (P = .041) (Table 2). Using Pgrcre/+, Pgrmc1d/d mice experienced impaired fertility with a significant reduction in the number of pups/litter (Pgrmc1fl/fl, 7.68 ± 0.5 vs Pgrmc1d/d, 4.64 ± 0.4; P = .0002) (Figure 2A and Table 3) despite a comparable number of total litters and average number of litters/female (Pgrmc1fl/fl, 6.83 ± 0.6 vs Pgrmc1d/d, 6.00 ± 0.3) over the 6-month trial. The impaired fertility phenotype was observed from the first parity onward (Figure 2B) and likely did not stem from faulty pituitary function given that the time between pregnancies did not differ between Pgrmc1fl/fl and Pgrmc1d/d mice (Figure 2C).
Table 2.
Reduced Fecundity in Amhr2cre/+;Pgrmc1d/d Mice
| Genotype | Females (n) | Litters (n) | Pups (n) | Average Pups/Litter | Average Litters/Female |
|---|---|---|---|---|---|
| Pgrmc1fl/fl | 8 | 47 | 330 | 7.02 ± 0.4 | 5.88 ± 0.1 |
| Pgrmc1d/d | 8 | 36 | 291 | 8.08 ± 0.4 | 4.50 ± 0.6a |
P = .041 vs Pgrmc1fl/fl.
Figure 2.
Impaired fertility in conditional Pgrmc1-deficient mice using Pgrcre/+. A, Pgrmc1fl/fl and Pgrmc1d/d mice were placed with males of proven breeding capacity for 6 months. Conditional ablation of Pgrmc1 resulted a 43% decrease in the number of pups/litter (*, P = .0002). B, The number of pups/litter is plotted against parity. Note the decrease in the number of pups/litter from the first parity onward. C, No difference in the number of days between litters was observed between Pgrmc1fl/fl and Pgrmc1d/d mice. After superovulation of postnatal day (pnd) 25 Pgrmc1fl/fl and Pgrmc1d/d mice, there was no difference in serum P4 (D), the number of retrieved oocytes (E), or ovarian histology (F and G).
Table 3.
Subfertility Phenotype in Pgrcre/+;Pgrmc1d/d Mice
| Genotype | Females (n) | Litters (n) | Pups (n) | Average Pups/Litter | Average Litters/Female |
|---|---|---|---|---|---|
| Pgrmc1fl/fl | 6 | 41 | 315 | 7.68 ± 0.5 | 6.83 ± 0.6 |
| Pgrmc1d/d | 6 | 36 | 167 | 4.64 ± 0.4a | 6.00 ± 0.3 |
P < .0002 vs Pgrmc1fl/fl.
Importantly, conditional ablation of Pgrmc1 in the Pgr-expressing cells of the ovulatory follicle could adversely affect ovulation and luteal function, thereby accounting for the reduction in litter size in the Pgrmc1d/d mice. This possibility was evaluated by subjecting 22 day-old Pgrmc1fl/fl and Pgrmc1d/d mice to a superovulation protocol. This study revealed that luteal function after superovulation was not affected by Pgrmc1 ablation as judged by equitable serum P4 (Figure 2D), a similar number of oocytes collected from the ampulla (Figure 2E), and the formation and morphology of the corpora hemorrhagica in ovaries from both Pgrmc1fl/fl and Pgrmc1d/d female mice (Figure 2, F and G).
Equitable expression of ESR1 and PGR in Pgrmc1fl/fl and Pgrmc1d/d mice
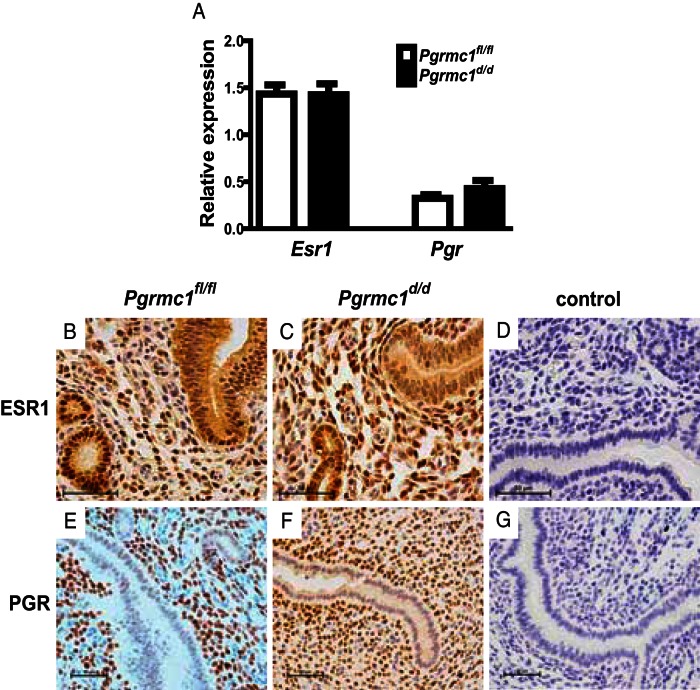
In order to determine whether or not the Pgrmc1fl/fl and Pgrmc1d/d mice maintained similar capacities to respond to steroid hormones, the presence of the ESR1 and PGR were assessed by RT-PCR and immunohistochemistry. After ovariectomy and E2 treatment to synchronize uteri, Esr1 and Pgr mRNAs were found to be expressed at similar levels in uteri of Pgrmc1fl/fl and Pgrmc1d/d mice (Figure 3A). This observation at the mRNA level extended to the protein level where ESR1 and PGR were found to be temporospatially expressed at comparable levels between Pgrmc1fl/fl and Pgrmc1d/d mice (Figure 3B). Specifically, ESR1 was expressed in most cells of the stromal and epithelial compartments, whereas PGR was restricted in its expression to the stromal compartment.
Figure 3.
Uterine expression of ESR1 and PGR. A, After ovariectomy and E2 treatment to synchronize sexually mature Pgrmc1fl/fl and Pgrmc1d/d mice on a Pgrcre background, qPCR analysis indicates similar levels of uterine expression for Esr1 and Pgr. Similarly, the temporospatial uterine expression of ESR1 (B and C) and PGR (E and F) were not different between Pgrmc1fl/fl and Pgrmc1d/d mice based on IHC analysis. No primary antibody control panels are shown in D and G. Representative images from n = 3 individual experiments.
Development of endometrial cysts in Pgrmc1fl/d and Pgrmc1d/d mice
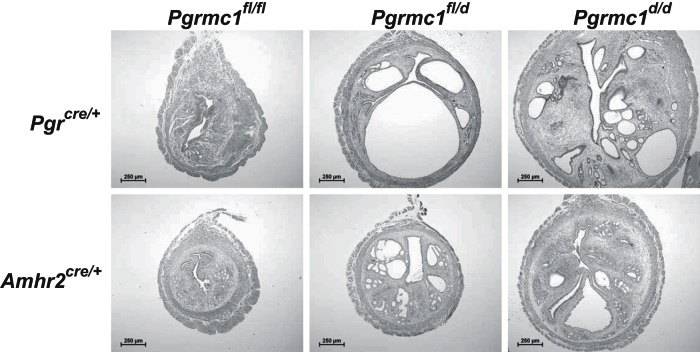
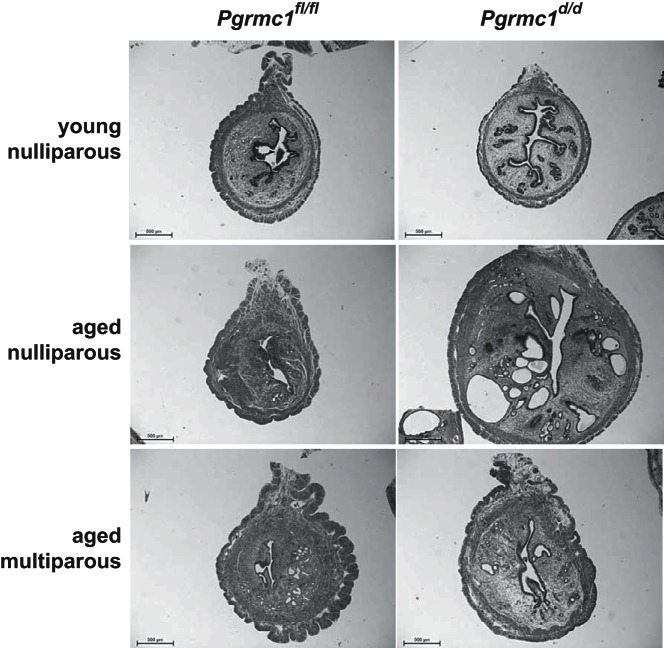
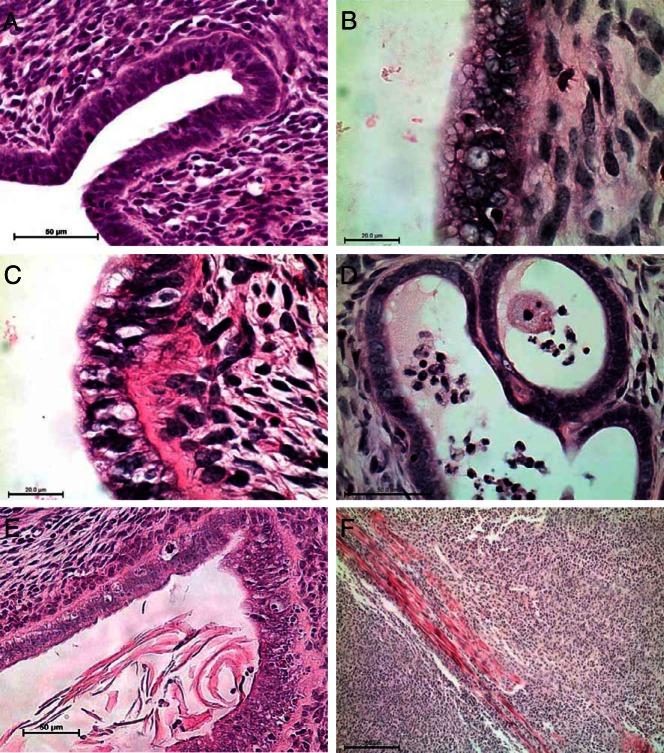
In addition to impaired fertility, conditional ablation of Pgrmc1 using either Amhr2cre/+ or Pgrcre/+ mice resulted in development of endometrial cysts starting around 4 months of age (Figure 4). A more profound phenotype was observed in PGRMC1-deficient mice using Pgrcre/+ which resulted in a 2- to 4-fold increase in uterine volume due to formation of larger cystic glands. Ablation of Pgrmc1 using Amhr2cre/+ mice also resulted in cyst formation. Interestingly, heterozygous female mice harboring one intact Pgrmc1 allele also displayed cysts. Development of the cystic phenotype was absent from young nulliparous animals and was dependent on age (Figure 5). Of note, Pgrmc1d/d multiparous mice on the Pgrcre/+ background experienced fewer and smaller cysts than their nulliparous counterparts (Figure 5). Development of endometrial cysts was accompanied by the formation of excessive epithelial intracellular secretory vesicles, disruption and thickening of the basement membrane, accumulation of immune cells and some stratification of the epithelium (Figure 6, B–E). About 5% of the Pgrmc1d/d female mice developed tumors as shown in Figure 6F in which the carcinoma migrated through the myometrium into the overlying perimetrium.
Figure 4.
Ablation of Pgrmc1 results in development of endometrial cysts. Conditional ablation of one (Pgrmc1fl/d) or both (Pgrmc1d/d) Pgrmc1 alleles using either Pgrcre or Amhr2cre mice results in development of endometrial cysts at 10 months of age. Representative images of n = 8–25 individual replicates.
Figure 5.
Parity reduces endometrial cyst formation in Pgrmc1d/d mice. Conditional ablation of Pgrmc1 from uterine tissue with Pgrcre caused formation in endometrial cysts in aged (10 mo), but not young (2 mo), nulliparous Pgrmc1d/d mice. Pregnancy attenuated cyst formation as evidenced by a lack of endometrial cysts in multiparous female mice at 10 months of age. Representative images of n = 6–25 independent experiments.
Figure 6.
Endometrial histological abnormalities in Pgrmc1d/d mice. Compared with uterine tissue from sexually mature female control mice (A), uterine tissue from Pgrmc1d/d displayed a number of abnormalities including presences of a heavily vacuolated epithelium (B), increased thickness of the basement membrane (C), presence of immune cells with a thinning glandular epithelium (D), sloughing of glandular tissue into the lumen and high incidence of apoptosis (E), and, at least in 1 case, presence of endometrial cancer (F); n = 25.
Discussion
An unequivocal role for PGR in mediating the actions of P4 has been established through functional studies in Pgr null female mice and through pharmacological studies where PGR antagonists such as RU486 have been employed. The Pgr null mutation results in complete infertility and development of hyperplasia (44). However, PGR is not the sole receptor mechanism for mediating P4 actions, because cells that lack expression of PGR still respond to P4 and synthetic nonmetabolizable progestins such as R5020 (11, 22, 25, 27, 36, 45–47). To date, 2 families of nonclassical PGRs have been identified including the Progestin and AdipoQ receptor (PAQR) and PGRMC families (30, 44, 48–50). Members of the PAQR family belong to the G protein-coupled receptor superfamily and purported P4 mediators include Paqr5, Paqr7, and Paqr8, all of which have been cloned and partially characterized in mammals (51). Although the activity of these receptors has been established in in vitro studies (52), others have challenged the validity of the PAQRs as bona fide progestin receptors in vivo (53, 54). The second family of nonclassical progestin receptors is the PGRMC family, which include PGRMC1, PGRMC2, neudecin, and neuferricin. PGRMC1 and PGRMC2 were originally cloned as heme-1 domain proteins referred to as HPR6.6 and Dg6, respectively (55–57). The actions of PGRMC1 and PGRMC2 have been demonstrated in a number of in vitro culture systems. However, in vivo functional studies have not been completed for members of the PGRMC family with regard to fertility, although PGRMC1 was recently shown to be necessary for altering cyclic regulation of sensory perception by P4 (58). Interestingly, the actions of members of the PGRMC and PAQR families may be linked, as a physical interaction between PGRMC1 and PAQR7 in cultured cells has been demonstrated (59, 60).
The present study demonstrates for the first time that Pgrmc1 is necessary for reproductive function in the female where conditional ablation of this gene results in impaired fertility and contributes to the development of endometrial cysts consistent with premature aging. The Pgrmc1 floxed mouse was generated by inserting loxP sites on either side of exon 2. Exon 2 is significant because it contains the cytochrome b5-like heme/steroid-binding domain, as well as several predicted phosphorylation sites, kinase binding sites, and Src homology 2 and Src homology 3 targeting domains. This region of PGRMC1 has been shown to be essential for binding P4 and for mediating the antiapoptotic actions of P4 (61).
Using Pgrcre mice to ablate Pgrmc1, the present studies reveal a greater than 40% reduction in the number of pups/litter throughout the duration of the 6-month breeding trial. The observed reduction in the number of pups/litter is evident from the first pregnancy at 6 weeks of age onward. In these mice, cre recombinase is expressed in all cells of the female reproductive tract, granulosa cells of periovulatory follicles, mammary glands, gonadotrophs, and select hypothalamic neurons. Although more extensive analyses are required, the subfertility phenotype observed when using the Pgrcre driver mouse likely does not stem from faulty hypothalamic or pituitary function, which would result in abnormal estrous cycles, because there was no difference in the number of days between litters when comparing Pgrmc1fl/fl and Pgrmc1d/d mice. Similarly, the subfertility phenotype does not stem from luteal insufficiency or failure of the ovary to respond to gonadotropins given that superovulation resulted in an equivalent number of retrieved oocytes and serum P4 levels in Pgrmc1fl/fl and Pgrmc1d/d mice 16 hours after induction of ovulation. Histological evaluation of ovaries obtained from superovulated female mice showed no structural differences in ovarian tissue from Pgrmc1fl/fl and Pgrmc1d/d mice. These findings suggest that subfertility arises through disrupted uterine function.
Analysis of ESR1 and PGR suggest that uteri from Pgrmc1d/d mice retain the capacity to respond to E2 and P4 through classical routes, because the expression of these classical steroid receptors was similar to that observed in Pgrmc1fl/fl mice. This observation suggests that ablating Pgrmc1 from uterine cells dramatically alters uterine responses to ovarian steroids without altering the expression of either of the classical ESR1 or PGR. The mechanism by which conditional ablation of Pgrmc1 from uterine cells alters uterine function and impairs fertility remains to be determined.
It is interesting that the number of pups/litter did not decrease when Pgrmc1 was ablated using Amhr2cre driver mice but was dramatically reduced using Pgrcre driver mice. Cre recombinase is expressed in mesenchymal tissue of the female reproductive tract in Amhr2cre mice but not epithelial cells of the endometrium. It is possible that uterine epithelial cell expression of PGRMC1 is absolutely essential for normal fertility. Expression of cre recombinase in mesenchymal tissue of Amhr2cre mice is incompletely penetrant, particularly in the mesometrial region of the endometrium. As such, there may be sufficient uterine PGRMC1 expression retained in Amhr2cre;Pgrmc1d/d mice to yield a normal phenotype within parity. However, variability in penetrance could account for 50% of female Amhr2cre;Pgrmc1d/d mice only having 4 or fewer pregnancies during the 6- month breeding trial. The mechanism by which selective ablation of PGRMC1 from mesometrial or epithelial cells of the uterus awaits a more complete analysis.
An unexpected consequence of conditionally ablating Pgrmc1 was the development of endometrial cysts starting around 4 months of age. The cysts were accompanied by a vacuolated epithelium, an increase in the thickness of the basement membrane, the presence of immune cells within the glandular compartment, a sloughing of glandular tissue into the luminal space and high incidence of apoptotic epithelial cells. This phenotype was observed not only in Pgrmc1d/d mice but also Pgrmc1fl/d mice, and this is likely due to the presence of the Pgrmc1 gene on the X chromosome, where 1 allele is likely inactivated. It was also interesting to observe formation of endometrial glandular cysts when Pgrmc1 was selectively deleted from mesenchymal tissue using Amhr2cre mice. This finding suggests that mesenchymal ablation of Pgrmc1 results in faulty communication between the stromal and epithelial compartments. Endometrial cysts are common in the endometrium of aged mice and women. That Pgrmc1d/d mice develop endometrial cysts earlier than control mice suggests that PGRMC1 deficiency accelerates the aging process of this tissue. Finally, the age onset development of cysts likely did not contribute to the subfertility phenotype given that the reduction in the number of pups/litter was observed from the time of sexual maturity at 6 weeks of age, and this remained consistent through the duration of the breeding trial even after cyst formation. It therefore seems that parity realigned the Pgrmc1d/d endometrial histoarchitecture or slowed the aging process so that the functional capacity of the uterus was not diminished below the observed 4.6 pups/litter. This is supported by the dramatic decline in cyst formation in endometrial tissue of aged multiparous Pgrmc1d/d mice as shown in Figure 5. The cysts that form are not mitotic and therefore are more consistent with inclusion cysts. Pregnancy likely minimizes cyst formation by decreasing the total number of estrous cycles throughout reproductive life and by eliminating disrupted endometrial tissue that may accumulated during estrous cyclicity.
The exact function(s) of PGRMC1 in the gravid uterus and its regulation by P4 remain to be determined. However, several recent reports from in vitro studies provide a framework with which to begin to evaluate how PGRMC1 functions in the uterus. In vitro studies using A549 lung cancer cells demonstrate that PGRMC1 forms a physical interaction with the epidermal growth factor receptor (EGFR) and this interaction is necessary for EGFR translocation from cytoplasmic vesicles to the cell surface for interactions with its cognate ligand. Depletion of PGRMC1 in these cells also made them increasingly sensitivity to the EGFR inhibitor, erlotinib (62, 63). If a similar EGFR vesicular transport function for PGRMC1 exists in the endometrial stromal compartment, it is possible that conditional ablation of Pgrmc1 results in a diminished presence of EGFR at the cell surface. Such a scenario could account for the postimplantation phenotype in the Pgrmc1d/d mouse given that EGFR was recently shown to be essential for decidualization and normal fertility (64). We previously reported on the expression of PGRMC1 in gravid human and murine uteri (35). Importantly, during the periimplantation period, PGRMC1 is expressed in the luminal epithelium surrounding the implanting embryo, as well as in subluminal stromal cells undergoing the decidualization program. This is in contrast to expression of the classical PGR, which is known to be absent from the luminal epithelium at the time of embryo implantation in all mammalian species studies to date regardless of the type of implantation. The interesting nuclear staining of PGRMC1 in decidualizing stromal cells during early gestation suggests a function for this protein in the decidualization process.
Alternative actions for PGRMC1 include its roles in regulating cell cycle progression and apoptosis. In spontaneously immortalized rat granulosa cells and human granulosa/lutein cells PGRMC1 is necessary for mediating the antimitotic actions of P4 in these cells (60, 65). During early pregnancy, stromal cells undergo several rounds of proliferation before terminal differentiation into decidual cells. This proliferative event is stimulated by P4, as is the terminal differentiation of these cells. Previous expression analysis of PGRMC1 at the maternal-embryo interface in mice revealed that PGRMC1 is abundantly expressed in the nucleus of cells that are transitioning out of the cell cycle and toward a differentiated state (35). It will be interesting to determine whether PGRMC1 mediates the antimitotic actions of P4 in this context. Likewise, PGRMC1 and PGRMC2 have an antiapoptotic function in spontaneously immortalized rat granulosa cells (65, 66). Similarly, it has now been established in endometrial, ovarian, and breast cancer cell lines, all of which are deficient in PGR expression, that PGRMC1 mediates the antiapoptotic actions of P4 (28, 29, 67). Depletion of PGRMC1 from each of these cancer cell lines makes them more sensitive to the killing actions of chemotherapeutic agents such as cisplatin and doxorubicin. One explanation for the postimplantation decidualization defect in Pgrmc1d/d mice is that the absence of PGRMC1 could make decidual cells more sensitive to apoptotic stimuli. Further evaluation of this concept is needed.
Acknowledgments
This work was supported by National Institutes of Health Grants RR030264 and OD016564.
Disclosure Summary: J.J.P. was awarded a patent on the nongenomic regulators of P4 action. All other authors have nothing to disclose.
Appendix
Antibody Table
Table 4.
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| PGRMC1 | Anti-PGRMC1 | Sigma, HPA002877 | Rabbit; polyclonal | 1:1000 | |
| ERα | MC-20 | Santa Cruz Biotechnology, Inc, SC-542 | Rabbit; polyclonal | 1:300 | |
| PGR | SP2 | Thermo Scientific, RM-9102 | Rabbit; monoclonal | 1:200 | |
| Secondary | Antirabbit IgG | Cell Signaling, 7074 | Goat; polyclonal | 1:2000 | |
| Secondary | Antirabbit IgG | Santa Cruz Biotechnology, Inc, SC-2040 | Goat; polyclonal | 1:500 |
Footnotes
- E2
- estradiol
- EGFR
- epidermal growth factor receptor
- ESR1
- estrogen receptor
- P4
- progesterone
- PAQR
- progestin and AdipoQ receptor
- PBST
- 0.1% Tween 20 in PBS
- PGR
- P4 receptor
- PGRMC1
- PGR membrane component 1.
References
- 1. Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. Progesterone as an immunomodulatory molecule. Int Immunopharmacol. 2001;1:1037–1048. [DOI] [PubMed] [Google Scholar]
- 2. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- 3. Boruban MC, Altundag K, Kilic GS, Blankstein J. From endometrial hyperplasia to endometrial cancer: insight into the biology and possible medical preventive measures. Eur J Cancer Prev. 2008;17:133–138. [DOI] [PubMed] [Google Scholar]
- 4. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1:106–111. [DOI] [PubMed] [Google Scholar]
- 5. Salazar EL, Calzada L. The role of progesterone in endometrial estradiol- and progesterone-receptor synthesis in women with menstrual disorders and habitual abortion. Gynecol Endocrinol. 2007;23:222–225. [DOI] [PubMed] [Google Scholar]
- 6. Ismail PM, Amato P, Soyal SM, et al. Progesterone involvement in breast development and tumorigenesis–as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids. 2003;68:779–787. [DOI] [PubMed] [Google Scholar]
- 7. Ehn NL, Cooper ME, Orr K, et al. Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr Res. 2007;62:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci USA. 2006;103:14021–14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vasquez YM, DeMayo FJ. Role of nuclear receptors in blastocyst implantation. Semin Cell Dev Biol. 2013;24:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peluso JJ, Pru JK. Non-canonical progesterone signaling in granulosa cell function. Reproduction. 2014;147:R169–R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothchild I. The corpus luteum revisited: are the paradoxical effects of RU486 a clue to how progesterone stimulates its own secretion? Biol Reprod. 1996;55:1–4. [DOI] [PubMed] [Google Scholar]
- 13. Stouffer RL, Bishop CV, Bogan RL, Xu F, Hennebold JD. Endocrine and local control of the primate corpus luteum. Reprod Biol. 2013;13:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viéro C, Méchaly I, Aptel H. Rapid inhibition of Ca2+ influx by neurosteroids in murine embryonic sensory neurones. Cell Calcium. 2006;40:383–391. [DOI] [PubMed] [Google Scholar]
- 15. Barbagallo M, Dominguez LJ, Licata G, et al. Vascular effects of progesterone: role of cellular calcium regulation. Hypertension. 2001;37:142–147. [DOI] [PubMed] [Google Scholar]
- 16. Head GM, Downing JE, Brucker C, Mentlein R, Kendall MD. Rapid progesterone actions on thymulin-secreting epithelial cells cultured from rat thymus. Neuroimmunomodulation. 1999;6:31–38. [DOI] [PubMed] [Google Scholar]
- 17. Finidori-Lepicard J, Schorderet-Slatkine S, Hanoune J, Baulieu EE. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981;292:255–257. [DOI] [PubMed] [Google Scholar]
- 18. Foresta C, Rossato M, Di Virgilio F. Ion fluxes through the progesterone-activated channel of the sperm plasma membrane. Biochem J. 1993;294:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lösel R, Dorn-Beineke A, Falkenstein E, Wehling M, Feuring M. Porcine spermatozoa contain more than one membrane progesterone receptor. Int J Biochem Cell Biol. 2004;36:1532–1541. [DOI] [PubMed] [Google Scholar]
- 20. Frye CA, Sumida K, Lydon JP, O'Malley BW, Pfaff DW. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3α,5α-THP-facilitated lordosis. Psychopharmacology. 2006;185:423–432. [DOI] [PubMed] [Google Scholar]
- 21. Ehring GR, Kerschbaum HH, Eder C, et al. A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J Exp Med. 1998;188:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peluso JJ, DeCerbo J, Lodde V. Evidence for a genomic mechanism of action for progesterone receptor membrane component-1. Steroids. 2012;77:1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peluso JJ, Lodde V, Liu X. Progesterone regulation of progesterone receptor membrane component 1 (PGRMC1) sumoylation and transcriptional activity in spontaneously immortalized granulosa cells. Endocrinology. 2012;153:3929–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaluka D, Batabyal D, Chiang BY, Poulos TL, Yeh SR. Spectroscopic and mutagenesis studies of human PGRMC1. Biochemistry. 2015;54:1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab. 2009;94:2644–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peluso JJ. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids. 2011;76:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peluso JJ. Progesterone receptor membrane component 1 and its role in ovarian follicle growth. Front Neurosci. 2013;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peluso JJ, Gawkowska A, Liu X, Shioda T, Pru JK. Progesterone receptor membrane component-1 regulates the development and Cisplatin sensitivity of human ovarian tumors in athymic nude mice. Endocrinology. 2009;150:4846–4854. [DOI] [PubMed] [Google Scholar]
- 29. Friel AM, Zhang L, Pru CA, et al. Progesterone receptor membrane component 1 deficiency attenuates growth while promoting chemosensitivity of human endometrial xenograft tumors. Cancer Lett. 2015;356:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. [DOI] [PubMed] [Google Scholar]
- 31. Lösel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1–many tasks for a versatile protein. Steroids. 2008;73:929–934. [DOI] [PubMed] [Google Scholar]
- 32. Keator CS, Mah K, Slayden OD. Alterations in progesterone receptor membrane component 2 (PGRMC2) in the endometrium of macaques afflicted with advanced endometriosis. Mol Hum Reprod. 2012;18:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen JI, Hannan NJ, Mak Y, et al. Proteomic characterization of midproliferative and midsecretory human endometrium. J Proteome Res. 2009;8:2032–2044. [DOI] [PubMed] [Google Scholar]
- 34. Ace CI, Okulicz WC. Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase. Reprod Biol Endocrionol. 2004;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Kanda Y, Roberts DJ, et al. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol. 2008;287:81–89. [DOI] [PubMed] [Google Scholar]
- 36. Pru JK, Clark NC. PGRMC1 and PGRMC2 in uterine physiology and disease. Front Neurosci. 2013;19:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feng L, Antczak BC, Lan L, et al. Progesterone receptor membrane component 1 (PGRMC1) expression in fetal membranes among women with preterm premature rupture of the membranes (PPROM). Placenta. 2014;35:331–333. [DOI] [PubMed] [Google Scholar]
- 38. Bunch K, Tinnemore D, Huff S, Hoffer ZS, Burney RO, Stallings JD. Expression patterns of progesterone receptor membrane components 1 and 2 in endometria from women with and without endometriosis. Reprod Sci. 2014;21:191–197. [DOI] [PubMed] [Google Scholar]
- 39. Garrido-Gómez T, Quiñonero A, Antúnez O, et al. Deciphering the proteomic signature of human endometrial receptivity. Hum Reprod. 2014;29:1957–1967. [DOI] [PubMed] [Google Scholar]
- 40. Lee EC, Yu D, Martinez de Velasco J, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. [DOI] [PubMed] [Google Scholar]
- 41. Soyal SM, Mukherjee A, Lee KY, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. [DOI] [PubMed] [Google Scholar]
- 42. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. [DOI] [PubMed] [Google Scholar]
- 43. Kashiwagi A, DiGirolamo CM, Kanda Y, et al. The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology. 2007;148:4173–4184. [DOI] [PubMed] [Google Scholar]
- 44. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. [DOI] [PubMed] [Google Scholar]
- 45. Peluso JJ. Multiplicity of progesterone's actions and receptors in the mammalian ovary. Biol Reprod. 2006;75:2–8. [DOI] [PubMed] [Google Scholar]
- 46. Peluso JJ. Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod. 2007;25:198–207. [DOI] [PubMed] [Google Scholar]
- 47. Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology. 2006;147:3133–3140. [DOI] [PubMed] [Google Scholar]
- 48. Tang YT, Hu T, Arterburn M, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. [DOI] [PubMed] [Google Scholar]
- 49. Thomas P. Characteristics of membrane progestin receptor α (mPRα) and progesterone membrane receptor component 1 (PGRMC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thomas P, Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012;96:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karteris E, Zervou S, Pang Y, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. [DOI] [PubMed] [Google Scholar]
- 53. Krietsch T, Fernandes MS, Kero J, et al. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRα, β, γ) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006;20:2146–3164. [DOI] [PubMed] [Google Scholar]
- 54. Fernandes MS, Brosens JJ, Gellersen B. Honey, we need to talk about the membrane progestin receptors. Steroids. 2008;73:942–952. [DOI] [PubMed] [Google Scholar]
- 55. Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full-length cDNA sequence of progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;229:86–89. [DOI] [PubMed] [Google Scholar]
- 56. Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem. 1996;239:726–731. [DOI] [PubMed] [Google Scholar]
- 57. Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379:907–911. [DOI] [PubMed] [Google Scholar]
- 58. Dey S, Chamero P, Pru JK, et al. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell. 2015;161:1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thomas P, Pang Y, Dong J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology. 2014;155:1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sueldo C, Liu X, Peluso JJ. Progestin and AdipoQ receptor 7, progesterone membrane receptor component 1 (PGRMC1), and PGRMC2 and their role in regulating progesterone's ability to suppress human granulosa/luteal cells from entering into the cell cycle. Biol Reprod. 2015;93:1–11. [DOI] [PubMed] [Google Scholar]
- 61. Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hampton KK, Craven RJ. Pathways driving the endocytosis of mutant and wild-type EGFR in cancer. Oncoscience. 2014;1:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J Biol Chem. 2010;285:24775–24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Large MJ, Wetendorf M, Lanz RB, et al. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014;10:e1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peluso JJ, Griffin D, Liu X, Horne M. Progesterone receptor membrane component-1 (PGRMC1) and PGRMC2 interact to suppress entry into the cell cycle in spontaneously immortalized rat granulosa cells. Biol Reprod. 2014;91:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Griffin D, Liu X, Pru C, Pru JK, Peluso JJ. Expression of progesterone receptor membrane component-2 within the immature rate ovary and its role in regulating mitosis and apoptosis of spontaneously immortalized granulosa cells. Biol Reprod. 2014;91:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Clark NC, Friel AM, Pru CA, et al. Progesterone receptor membrane component 1 promotes survival of human breast cancer cells and the growth of xenograft tumors. Cancer Biol Ther. 2016;17:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]