Abstract
Lymphangioleiomyomatosis (LAM) is a devastating rare lung disease affecting primarily childbearing age women in which tumors consisting of abnormal smooth-muscle-like cells grow within the lungs and progressively lead to loss of pulmonary function. LAM cells metastasize to the lungs, predominantly through the lymphatics; however, the source of the LAM cell is still unknown. LAM cells contain inactivating mutations in genes encoding tuberous sclerosis 1 or 2, proteins that normally limit cell growth through suppression of mammalian target of rapamycin complex 1. As of today, sirolimus (an mammalian target of rapamycin complex 1 inhibitor) is the only treatment, available for LAM patients that is approved by the Food and Drug Administration; however, this drug and others in its class provide stabilization but not remission of LAM. One of the biggest problems in treating LAM is that both the origin of the LAM cells and the mechanism of the sexual dimorphism in LAM are still not understood. LAM cells express estrogen and progesterone receptors, and lung function declines during periods of high circulating estrogen levels. Moreover, numerous basic research studies find that estrogen is a key driving force in LAM cell proliferation, migration, and metastasis. In this review, we highlight recent insights regarding the role of steroid hormones in LAM and discuss possible explanations for the profound female sexual dimorphism of LAM.
When thinking of steroid-responsive cancers, prostate cancer in men and breast cancer in women are usually the first two that come mind. Here, we will discuss an additional tumor that has features of steroid-sensitivity: lymphangioleiomyomatosis (LAM). LAM is a rare progressive smooth muscle cell-like tumor of the lungs that often leads to progressive lung failure due to the formation of large cystic lesions that occupy normal functioning air space. In fact, lung function can be so severely compromised that some patients require lung transplantation (1, 2). Interestingly, LAM affects almost exclusively women, with only a handful of cases reported in men (3, 4). It is most often diagnosed during reproductive years, seems to progress more rapidly during pregnancy or with patients taking exogenous estrogens, and often progresses more slowly after menopause (5–8). Together, these features suggest that LAM may be responsive to the female sex steroids estrogen and progesterone. Here, we will introduce LAM and it many fascinating and unusual features, with a focus on the potential roles of estrogen and progesterone in LAM cell biology.
The Genetic Features of LAM
Researchers have discovered that LAM tumor cells contain a very specific genetic defect: an inactivating mutation in tumor suppressor gene tuberous sclerosis (TSC)1 or TSC2 (9, 10). These proteins normally complex with Tre2-Bub2-Cdc16 1 domain family, member 7 (TBC1D7) (11) to inhibit Ras homolog-enriched in brain, an activator of mammalian target of rapamycin complex 1 (mTORC1) (12, 13). Thus, in LAM, the TSC1/TSC2/TBC1D7 complex is nonfunctional and mTORC1 is activated, leading to increased cell growth due to enhanced protein translation (see figure 2 below) (14, 15). LAM can occur in 2 different settings: 1) genetically, in about 40% of female patients with the autosomal dominant disease called TSC (16), which is caused by inactivating mutations in either TSC1 or TSC2 genes; or 2) sporadically, primarily as a result of acquired mutations, usually in the TSC2 gene, in patients with no evidence of a genetic disease (17, 18). Common extrapulmonary manifestations of LAM include lymph node infiltrations in the pelvic area (in 100% of patients) (19, 20), uterine microscopic LAM lesions (perhaps in 90% of patients) (20), and renal angiomyolipomas (AMLs) (in up to 60% of patients) (21–24). Renal AMLs are benign tumors, usually carrying mutations in the TSC2 gene, that are composed of blood vessels, adipose cells, and smooth muscle cells (25). Given the enhanced mTORC1 activity in LAM cells, rapamycin and its analogues (eg, sirolimus) are used to treat LAM and are currently the only treatment for LAM approved by the Food and Drug Administration. In fact, sirolimus effectively slows LAM progression in most patients; however, the drug is only cytostatic and does not eliminate LAM tumors; thus patients can never stop the medication. In addition many patients cannot tolerate sirolimus for various reasons; thus, alternative treatments are in needed.
The Metastatic Manifestations of LAM
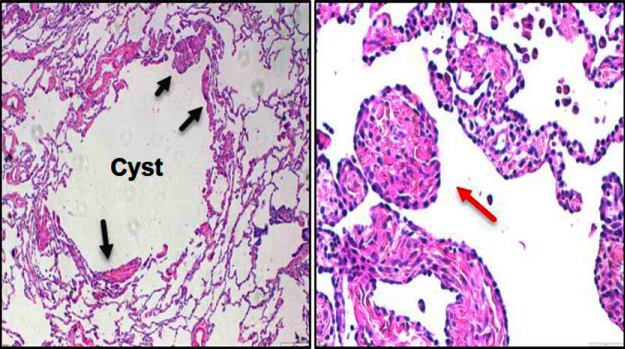
LAM cells are morphologically heterogeneous and usually identified by their spindle-shaped or epithelioid morphology and large amount of eosinophilic cytoplasm. Tumor cells are usually found as a thin layer in cyst walls or aggregate into small nodules in the lungs (Figure 1) (26). A great mystery in LAM concerns the origin of the lung tumor cell. Evidence suggests that LAM cells, which are histologically benign, populate the lung in a metastatic fashion (27). For example, women with sporadic LAM who have required lung transplantation developed recurrent LAM lesions in their new lungs that are genetically identical to those in the original lungs (28, 29), suggesting that the new lung LAM cells must have come from elsewhere in the body. Further proof came from genetic analysis of sporadic lung LAM lesions from a LAM patient. TSC2 mutations in the lung LAM cells were identical to those found in AML tumors, but not normal kidney cells, of the same patient, again suggesting a metastatic nature of LAM (30). Furthermore, LAM cells were found in the blood and other body fluids of patients with sporadic LAM (31), supporting metastatic manifestation and ability to leave primary lesions and implant into secondary sites.
Figure 1.
Lung LAM cell histology. Hematoxylin and eosin staining of lung tissue with thin walled cyst filled with LAM cells (arrow heads, ×4 magnification, left panel) and of a lung LAM nodule (×10 magnification, right panel).
Matrix metalloproteinases (MMPs) expression
MMPs are a large group of proteases responsible for tissue remodeling and degradation of the extracellular matrix (32). MMPs are suggested to play a role in regulating tumor migration, invasion, and metastasis (33). Elevated MMP-2 levels were identified in LAM cells compared with the surrounding lung tissue in LAM patients by immunohistochemistry (34–36), and MMP-2 activation increased invasiveness of primary LAM cells in culture (37). In addition, MMP-9 levels were significantly elevated in the serum and urine of LAM patients (38, 39), and MMP-9, through Src kinase activation, was suggested to promote invasiveness of Tsc2-null embryonic fibroblasts in vitro (40). Together, these data suggest that excessive production of MMPs by LAM cells may contribute to lung destruction and cyst formation.
Doxycycline is an antibiotic capable of inhibiting the activity of several MMPs, including MMP-2 and MMP-9 (41). Doxycycline decreased MMP-2 levels and cell metabolic activity (42), and also reduced RhoA-GTPase activity, focal adhesion kinase phosphorylation, and migration capability of Tsc2 deficient cells in vitro (43). A case report described that 3 months of doxycycline treatment in a LAM patient improved lung function and decreased urine MMP-2 and MMP-9 to undetectable levels (44). A clinical trial in Brazil, which included 31 LAM patients treated with doxycycline for 12 months, resulted in an effective urinary MMP-9 and serum MMP-2 blockade; however, improved or stabled lung function by doxycycline was reported only in patients with mild symptoms (45). Two years of a placebo-controlled doxycycline trial in LAM patients in the United Kingdom suggested that, despite a reduction in urine MMP-9 levels after treatment, no change in serum MMP-9 was detected, and no significant improvement in lung function was reported (46). Together, these observations raise a few questions regarding the efficacy of doxycycline in LAM patients. Further investigations are needed to determine if doxycycline is simply not potent or specific enough to inhibit MMP activity in LAM patients, or perhaps if, in advanced stages of the disease, when extensive lung destruction is already present, an antimigratory drug on its own is not sufficient to control LAM.
Cathepsin-K and neutrophil elastase (NE) in LAM
Apart from MMPs, other proteases such cathepsin-K and NE are suggested to promote migration, invasion and metastasis of several cancers (47, 48). Immuonohistochemical study in LAM patients found cathepsin-K to be restricted and highly expressed in LAM cells in the lungs of patients, suggesting cathepsin-K may contribute to the lung destruction seen in LAM (49).
NE is known to mediate lung damage in acute injury, asthma or emphysema (50, 51) and a recent study suggests that NE-mediated degradation of the antitumorigenic factor thrombospondin-1 in the mouse lung plays a role in cancer metastasis to the lungs (52). In a uterine-specific Tsc2-null mouse model that spontaneously develop myometrial tumors after puberty that recapitulate most LAM cell characteristics in women, NE expression was induced by estradiol and found to be highly active in LAM-like myometrial tumors compared with normal myometrium (53). More studies are needed to determine whether NE is up-regulated and active in LAM cells in women, and if so, to investigate how NE and cathepsins may play roles in the destructive and metastatic behavior of LAM cells.
Sexual Dimorphism and Hormonal Effects in LAM
A second mystery in LAM concerns the aforementioned overwhelming female prevalence of LAM. LAM displays an amazing sexual dimorphism, as it is diagnosed almost exclusively in childbearing age women (54). In fact, only a handful of male TSC complex patients were identified with cystic LAM in their lungs, and their phenotype appears to be much less severe (3, 4). To date, there is only a single reported case of sporadic LAM in a man; however, TSC mutations were not identified in the lung tissue (55). The gender-specific prevalence of LAM in reproductive-age women suggests that the hormonal milieu may contribute to LAM development and progression. In accordance with that, LAM cells express estrogen receptors (ERs) and progesterone receptors (PRs) (56, 57), suggesting that hormonal factors may modulate LAM cell proliferation. The expression of both receptor types was observed in the nuclei of large epithelioid and spindle-shaped LAM cells (58).
Estrogen
Several clinical observations suggest that LAM development and progression depend on estrogen signaling. LAM progression seems to increase when estrogen levels are high, for example, during pregnancy (5, 59) or in women taking exogenous estrogen via hormone replacement therapy (6, 7), and slows when estrogen levels decline, such as after menopause (8). In addition, in vitro and in vivo studies support a role for estrogen in LAM progression. First, as mentioned earlier, isolated LAM cells from the lungs of LAM patients express high levels of ERα (56, 57), which is known to be a potent mediator of estrogen-induced proliferation in the uterus (60, 61) and in breast cancer (62, 63). Second, observation of the proliferative effects of estradiol in Tsc2-null cells in vitro supported a positive effect of estrogen in these cells. Estradiol stimulation of isolated primary human TSC2-null AML cells promoted significant proliferation and activated both genomic (c-myc transcription) and nongenomic (ERK activation) pathways (64). Rat Tsc2-null leiomyoma-derived ELT3 and primary human lung LAM cells displayed up-regulated MMP-2 protein and activity as well as increased invasiveness by estradiol (37, 65). Estradiol stimulation in LAM patient-derived TSC2-null 621–101 AML cells activated ERK (66) and increased proliferation, migration, and invasion in vitro (67). Knockdown experiments in 621–101 cells determined that ERK2, rather than ERK1, was the main contributor in this estradiol-induced increased migration and invasion (67). Observations of the proliferative effects of estradiol and kinase activation using rat ELT3 cells in vitro were similar to 621–101 cells (68). Estradiol also reduced apoptosis in cultured ELT3 cells, possibly through ERK-mediated decreased accumulation of the proapoptotic protein BCL-2-interacting mediator of cell death (BIM) (68). In addition, a recent study suggested that estrogen promotes a metabolic programming in Tsc2-null cells in a similar way to cancer cells of the breast, uterus and ovary (69). Using ELT3 and 621–101 cells, investigators determined that estrogen promoted glucose metabolism via the pentose phosphate pathway in an Protein kinase B(AKT)-dependent, but MEK-independent, mechanism. Third, studies using Tsc2-null cell xenografts demonstrated increased tumor growth and lung metastasis by estrogen. Estradiol promoted metastasis and the survival of ELT3 cells in the lung as well as in the circulation that have been sc injected into mice. MEK inhibition prevented these prometastatic effects of estradiol on ELT3 cells (68). Similarly, estradiol treatment of animals with immortalized AML xenografts resulted in enhanced tumor growth, AKT activation and increased gene transcription, such as the angiogenic growth factor platelet-derived growth factor C (70). Lastly, using oophorectomy and aromatase inhibition, estrogen was shown to be necessary for the development (71) and maintenance (53) of LAM-like tumors in uterine-specific Tsc2-null mice. Antiestrogen therapy in these uterine-specific Tsc2-null mice decreased MMP-2 activity as well as MMP-9 and melanocyte differentiation markers expression (53).
Notably, in the uterine-specific Tsc2 null mouse tumors, estrogen also appeared to regulate melanocytic-associated markers, which are associated with LAM tumors (72). For example, LAM cells express gp100, a premelanosomal protein also called PMEL. The antibody HMB-45 recognizes PMEL, and immunohistochemical positivity for HMB-45 staining is a useful tool for LAM diagnosis (73). An additional melanocytic marker identified in LAM is microphthalmia-associated transcription factor (MITF) (74), an oncogene in melanoma (75). MITF transcriptionally regulates a set of melanocytic-differentiation genes (76, 77) often up-regulated in LAM that includes gp100/PMEL (74), the protein melan-A (MLANA), also known as melanoma antigen recognized by T cells-1 (MART-1) (78), and the melanin synthesis enzymes tyrosinase-related protein 1 and 2 (TYRP1 and TYRP2), the latter also known as dopachrome tautomerase (DCT) (79). Although the significance or the origin of melanocytic markers in LAM is poorly understood, one melanocytic-associated marker called glycoprotein NMB (GPNMB) (80) was found to be highly expressed in LAM-like myometrial and metastasizing lung cells in the uterine-specific Tsc2-null mouse model as well as in LAM lesions in the lungs of women (53). Thus, GPNMB may serve as an additional marker to identify LAM clinically. Interestingly, GPNMB is expressed on the surface of cancers such as melanoma and triple negative breast cancer, where it is thought to regulate migration and invasion. As such, GPNMB is being used as a potential cell surface target for these tumors and may similarly be used in LAM (81–83).
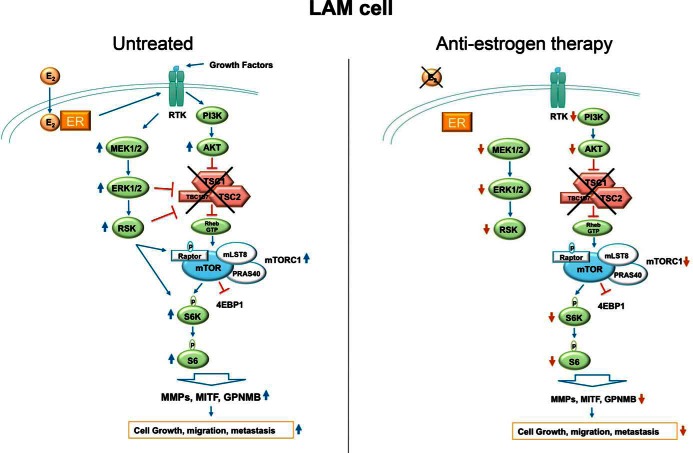
Surprisingly, in addition to reducing tumor size, antiestrogen therapy also markedly reduced mTORC1/S6 kinase (S6K) activity in Tsc2-null myometrium, and estradiol significantly enhanced mTORC1/S6K activity in wild-type and Tsc2-null uteri (71), suggesting that loss of TSC-mediated inhibition alone is not enough to promote mTORC1/S6K activity, a positive signal from estradiol may also be required (Figure 2). Together, these data indicate that estrogen (most likely through ERK, as well as AKT, activation) is an important promoter of LAM progression and that antiestrogen therapy might prove to be an effective approach in treating women with LAM.
Figure 2.
LAM cell signaling. In LAM tumor cells, the TSC1/TSC2/TBC1D7 complex is nonfunctional, which allows mTORC1 to be activated and leads to up-regulation of S6K activity, translational machinery, melanocytic differentiation markers, and proteases such as MMPs. This ultimately results in increased cell growth, migration, and metastasis. Importantly, loss of TSC-mediated suppression is not sufficient to fully activate mTOR. In addition, a positive signal must be present, such as estrogen or growth factors, which, through EGF receptor-mediated ERK activation, promotes mTOR and S6K activities. Estrogen may be a major positive signal in LAM; thus, antiestrogen therapy would reduce mTOR and S6K signaling, as well as the downstream processes of proliferation, migration, and invasion.
Accordingly, several case reports and a small number of uncontrolled studies have documented a beneficial effect of antiestrogen therapies such as oophorectomy and GnRH agonist usage in LAM (84–87) but to date, there is no conclusive indication that antiestrogen treatments are effective in LAM. In fact, a new placebo-controlled trial called Trial of Aromatase Inhibition in Lymphangioleiomyomatosis will examine for the first time the potential use of aromatase inhibitors in the treatment of LAM.
Progesterone
Argument against the theory of estrogen being the leading cause for LAM is that although estradiol levels are higher in women than in men at around ovulation and pregnancy, males still produce significant amounts of estrogens (88). Another steroid that may contribute to the gender specificity seen in LAM is progesterone. Although progesterone production in men is constant and low, in women progesterone levels are high during the luteal phase of the menstrual cycle (10–14 d a month), during pregnancy, and with oral contraceptives, consistent with situations associated with LAM progression. As mentioned earlier, LAM cells express PRs (56) and recent immunohistochemical study found that LAM cells have a uniquely high PR to ER ratio, which is in contrast to other female hormone-mediated tumors (89). Very few studies examined the effect of progesterone on Tsc2-null cells growth in culture and the reports are somewhat contradictory. In one study using ELT3 cells, progesterone alone and synergistically with estradiol activated AKT and ERK pathways and increased proliferation in vitro and lung metastasis and invasiveness in vivo (90). In contrast, two different studies using ELT3 cells showed that progesterone suppressed the estrogen-induced gene expression and inhibited estrogen-induced cell proliferation (91, 92). Furthermore, in Tsc2-null mouse uteri, estradiol was required for myometrial tumor growth, whereas progesterone alone had no effect on proliferation, nor did it modulate estradiol-induced growth (71).
With regard to human disease, high dose progestins have been tested in patients with LAM, with some reports suggesting stabilization or improvement but others showing no difference or possibly deterioration of the disease (93–95). More basic research and controlled studies are required to determine progesterone role in LAM pathology.
Selective ER Modulators in LAM
Apart from estrogen, different endogenous and exogenous ligands, also called selective ER modulators, can signal through the ER (96, 97). These ligands can mimic estrogen function (agonist), oppose it (antagonist), or have a mixed function. For reasons not fully understood, the same ligand can have different function on the ER in a cell and tissue-dependent manner (98, 99). For example, tamoxifen has been reported to be an antagonist in the breast (100, 101) and an agonist in the bone (102, 103) and uterine endometrium (104, 105). In vitro studies using tamoxifen supported a partial agonist/antagonist effect in Tsc2-null cells. In patient-derived AML cells (621–101), tamoxifen stimulation, similar to estradiol, resulted in increased cell growth, phosphorylation of ERK and expression of c-myc (64). In contrast, in rat Tsc2-null myometrial cells (ELT3), tamoxifen exposure had no effect (96) or decreased (103) cell proliferation in vitro. In one study, tamoxifen was not able to induce transcriptional activation function of the ER or PR expression in vitro (96), whereas in another study, tamoxifen induced PR expression up-regulation in vitro, but reduced tumor size of xenografted ELT3 cells (106). Overall, these studies suggest that tamoxifen has agonist effects on TSC2-null kidney AML cells, and mostly antagonist effects on Tsc2-null rat leiomyoma cells. In LAM patients, tamoxifen therapy resulted in no improvement and possible exacerbation of the disease (107, 108), suggesting that tamoxifen has partial agonist effects on LAM cells in the lungs, and therefore may not be the best choice as an antiestrogen treatment in LAM.
The Uterus, Leiomyoma, and LAM
Another potential explanation for the sexual dimorphism of LAM is the unique presence of a uterus in women. Interestingly, tumors of the uterine myometrium share many characteristics with LAM lesions in the lung, suggesting these two tumors may be linked, and that perhaps LAM smooth muscle cells tumors might originate from the uterus.
Uterine leiomyomas
Myometrial cells are smooth muscle cells that form the outer portion of the uterus. Myometrial cells express ERs and PRs and during pregnancy the myometrium undergoes hormonally induced proliferation and enlargement (109). Leiomyomas are benign common adenomas of the myometrium (found in up to 70% of reproductive age women), are the leading cause of abnormal bleeding and uterine pain in women, and are the top reason for hysterectomy (110, 111). Unlike normal myometrium, leiomyomas are very sensitive to estrogen and progesterone (61). Both LAM and uterine leiomyomas are pathologically characterized by abnormal smooth muscle cell proliferation, with similar cell arrangement and appearance. Like LAM lesions, leiomyomas are rarely seen before puberty, have their highest prevalence during reproductive years, can worsen with hormone replacement, and decrease in severity after menopause (112). If the uterus is indeed the source of the LAM cell, this would explain the female sexual dimorphism, the estrogen sensitivity of tumors, the metastatic nature of LAM, and the observed histology of lung LAM tumors (smooth cells that are ER/PR positive). In fact, myometrial cells are known to be capable of traveling from the uterus to the lungs, in a rare condition called benign metastasizing leiomyoma (BML).
Benign metastatic leiomyoma
Very rarely, leiomyomas have been found in the lungs of young women (113). These BML tumors consist of smooth muscle cells that are ER and PR positive. Some BML tumors regress with antiestrogens or menopause (114), and all women patients with BML have a history of uterine leiomyomas. Diagnosis of BML is based on common histologic and immunohistochemical characterization of the primary uterine leiomyomas and the metastatic lesions (115). However, like in LAM, a few men have been reported to have pulmonary smooth muscle neoplasms in their lungs, similar in appearance to BML (116), suggesting that on rare circumstances, smooth muscle tumor cells can be formed in the lungs or migrate from other organs in the body. Unlike LAM, BML is usually asymptomatic; lesions have low mitotic index, minimal atypia, and no invasion. Notably, no genetic mutations (eg, TSC1 or TSC2) have been linked to leiomyomas or BML. Furthermore, leiomyomas and BML do not express melanocyte differentiation markers commonly seen in LAM. Thus, the overall similarities between LAM tumors and leiomyomas (117) suggest a potential theory that LAM is a more aggressive form of BML in the setting of a TSC1 or TSC2 mutations.
Although one study showed a leiomyoma rate of only 30% in LAM patients, this was retrospective and used insensitive screening computed tomography scans, which could not detect small leiomyomas or microscopic LAM lesions (118). In contrast, a recent study looked carefully at surgically removed uteri of 10 LAM patients, in which 100% of uteri contained leiomyomas and 90% contained microscopic uterine LAM lesions, whereas women without pulmonary LAM had no detectable LAM lesions in their uterus (20). It is important to note that those microscopic LAM lesions in the uterus of LAM patients were grossly nondetectable and discovered only during microscopic examination of specimens removed after total or partial hysterectomy surgery (20, 119). This recent study suggests that the uterus has high potential to be the primary site of origin of LAM.
Two rodent models with uterine smooth muscle LAM-like cells exists. First is the Eker rat, which carries a global heterozygous mutation that lead to tumors at multiple tissue locations including the uterus myometrium. Tsc2-null leiomyomas in the Eker rat share many characteristics with LAM, and ELT leiomyoma-derived cell lines from these rats (mainly ELT3) are widely used in culture and in xenograft models to study cellular mechanisms affecting proliferation, growth and lung metastasis of LAM-like cells. However, in the intact rat, tumors appear at a late stage (∼15 mo of age) and affect different cell types throughout the animal body (120); therefore, the ability to evaluate for spontaneous metastasis or cell of origin is impossible.
A second model, the uterine-specific Tsc2-null mouse was recently developed to test the likelihood of LAM to be originating from the uterus (71). Uterine-specific Tsc2-null mice develop LAM-like myometrial tumors, and approximately 50% of animals developed metastatic myometrial lung tumors that share most of the characteristics of LAM tumor cells in women. These LAM characteristics include mTORC1/S6K activation, smooth muscle actin, ER, PR, and melanocytic markers expression as well as MMP-2 and MMP-9 up-regulation and increased protease activity, suggesting that LAM might indeed originate from the uterine myometrium. Further studies in women with LAM, including genetic and phenotypic analysis of lung and uterine lesions in individual patients, will be necessary to formally determine whether lung LAM tumors in humans are in fact originating from the uterus or from other sources of smooth muscle cells.
Conclusions
In summary, LAM is a complex disease that progressively leads to loss of pulmonary function in young women. The difficulty in developing useful therapeutic options is that the true source of the LAM cell is unknown and the mechanisms that contribute to LAM cell proliferation, metastasis and migration are not fully understood. Apart from the loss of function of TSC gene product, estrogen seems to be critical for LAM progression, and numerous studies support an essential role for estrogen in the development, growth and spread of LAM cells. In addition, the relationship between LAM and leiomyomas should be further investigated. Because the epidemiology of LAM, as well as nearly every in vitro and in vivo model of LAM, suggest a critical role for estrogen in LAM tumor progression, antiestrogen therapy may be an important modality for treating LAM that has not yet been formally investigated. In fact, given that nearly every phenotype of LAM tumors, including activation of mTORC1 itself, seems to require estrogen in Tsc2 null uterine tumors, antiestrogen therapy might either mitigate the need for rapamycin or at least reduce to doses necessary to control LAM progression. Finally, new estrogen-dependent proteins such as GPNMB might serve as novel markers or targets for LAM and therefore merit further investigation.
Acknowledgments
This work was supported by the LAM Foundation, the Department of Defense Grant TS110032, and the National Institutes of Health Grant R01CA193583-01 (to H.P. and S.R.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT
- Protein kinase B
- AML
- angiomyolipoma
- BML
- benign metastasizing leiomyoma
- ER
- estrogen receptor
- GPNMB
- glycoprotein NMB
- LAM
- lymphangioleiomyomatosis
- MMP
- matrix metalloproteinase
- mTORC1
- mammalian target of rapamycin complex 1
- NE
- neutrophil elastase
- PMEL
- premelanosomal protein
- PR
- progesterone receptor
- S6K
- S6 Kinase
- TBC1D7
- Tre2-Bub2-Cdc16 1 domain family, member 7
- TSC
- tuberous sclerosis.
References
- 1. Abbott GF, Rosado-de-Christenson ML, Frazier AA, Franks TJ, Pugatch RD, Galvin JR. From the archives of the AFIP: lymphangioleiomyomatosis: radiologic-pathologic correlation. Radiographics. 2005;25:803–828. [DOI] [PubMed] [Google Scholar]
- 2. Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151:527–533. [DOI] [PubMed] [Google Scholar]
- 3. Aubry MC, Myers JL, Ryu JH, et al. Pulmonary lymphangioleiomyomatosis in a man. Am J Respir Crit Care Med. 2000;162:749–752. [DOI] [PubMed] [Google Scholar]
- 4. Miyake M, Tateishi U, Maeda T, et al. Pulmonary lymphangioleiomyomatosis in a male patient with tuberous sclerosis complex. Radiat Med. 2005;23:525–527. [PubMed] [Google Scholar]
- 5. Brunelli A, Catalini G, Fianchini A. Pregnancy exacerbating unsuspected mediastinal lymphangioleiomyomatosis and chylothorax. Int J Gynaecol Obstet. 1996;52:289–290. [DOI] [PubMed] [Google Scholar]
- 6. Shen A, Iseman MD, Waldron JA, King TE. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous estrogens. Chest. 1987;91:782–785. [DOI] [PubMed] [Google Scholar]
- 7. Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax. 2002;57:1085–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999;160:628–633. [DOI] [PubMed] [Google Scholar]
- 9. Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dibble CC, Elis W, Menon S, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marygold SJ, Leevers SJ. Growth signaling: TSC takes its place. Curr Biol. 2002;12:R785–R787. [DOI] [PubMed] [Google Scholar]
- 13. McManus EJ, Alessi DR. TSC1-TSC2: a complex tale of PKB-mediated S6K regulation. Nat Cell Biol. 2002;4:E214–E216. [DOI] [PubMed] [Google Scholar]
- 14. Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292:E1647–E1655. [DOI] [PubMed] [Google Scholar]
- 15. Goncharova EA, Goncharov DA, Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem. 2002;277:30958–30967. [DOI] [PubMed] [Google Scholar]
- 16. Cudzilo CJ, Szczesniak RD, Brody AS, et al. Lymphangioleiomyomatosis screening in women with tuberous sclerosis. Chest. 2013;144:578–585. [DOI] [PubMed] [Google Scholar]
- 17. Xu KF, Lo BH. Lymphangioleiomyomatosis: differential diagnosis and optimal management. Ther Clin Risk Manag. 2014;10:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Astrinidis A, Khare L, Carsillo T, et al. Mutational analysis of the tuberous sclerosis gene TSC2 in patients with pulmonary lymphangioleiomyomatosis. J Med Genet. 2000;37:55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta R, Kitaichi M, Inoue Y, Kotloff R, McCormack FX. Lymphatic manifestations of lymphangioleiomyomatosis. Lymphology. 2014;47:106–117. [PubMed] [Google Scholar]
- 20. Hayashi T, Kumasaka T, Mitani K, et al. Prevalence of uterine and adnexal involvement in pulmonary lymphangioleiomyomatosis: a clinicopathologic study of 10 patients. Am J Surg Pathol. 2011;35:1776–1785. [DOI] [PubMed] [Google Scholar]
- 21. Matsui K, Tatsuguchi A, Valencia J, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum Pathol. 2000;31:1242–1248. [DOI] [PubMed] [Google Scholar]
- 22. Torres VE, Björnsson J, King BF, et al. Extrapulmonary lymphangioleiomyomatosis and lymphangiomatous cysts in tuberous sclerosis complex. Mayo Clin Proc. 1995;70:641–648. [DOI] [PubMed] [Google Scholar]
- 23. Kerr LA, Blute ML, Ryu JH, Swensen SJ, Malek RS. Renal angiomyolipoma in association with pulmonary lymphangioleiomyomatosis: forme fruste of tuberous sclerosis? Urology. 1993;41:440–444. [DOI] [PubMed] [Google Scholar]
- 24. De Pauw RA, Boelaert JR, Haenebalcke CW, Matthys EG, Schurgers MS, De Vriese AS. Renal angiomyolipoma in association with pulmonary lymphangioleiomyomatosis. Am J Kidney Dis. 2003;41:877–883. [DOI] [PubMed] [Google Scholar]
- 25. Qin W, Bajaj V, Malinowska I, et al. Angiomyolipoma have common mutations in TSC2 but no other common genetic events. PLoS One. 2011;6:e24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrans VJ, Yu ZX, Nelson WK, et al. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nihon Med Sch. 2000;67:311–329. [DOI] [PubMed] [Google Scholar]
- 27. Yu J, Henske EP. mTOR activation, lymphangiogenesis, and estrogen-mediated cell survival: the “perfect storm” of pro-metastatic factors in LAM pathogenesis. Lymphat Res Biol. 2010;8:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karbowniczek M, Astrinidis A, Balsara BR, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. [DOI] [PubMed] [Google Scholar]
- 29. Bittmann I, Rolf B, Amann G, Löhrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol. 2003;34:95–98. [DOI] [PubMed] [Google Scholar]
- 30. Steagall WK, Zhang L, Cai X, Pacheco-Rodriguez G, Moss J. Genetic heterogeneity of circulating cells from patients with lymphangioleiomyomatosis with and without lung transplantation. Am J Respir Crit Care Med. 2015;191:854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crooks DM, Pacheco-Rodriguez G, DeCastro RM, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2004;101:17462–17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 33. Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–2268. [DOI] [PubMed] [Google Scholar]
- 34. Chang WY, Cane JL, Blakey JD, Kumaran M, Pointon KS, Johnson SR. Clinical utility of diagnostic guidelines and putative biomarkers in lymphangioleiomyomatosis. Respir Res. 2012;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000;124:267–275. [DOI] [PubMed] [Google Scholar]
- 36. Hayashi T, Fleming MV, Stetler-Stevenson WG, et al. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM). Hum Pathol. 1997;28:1071–1078. [DOI] [PubMed] [Google Scholar]
- 37. Glassberg MK, Elliot SJ, Fritz J, et al. Activation of the estrogen receptor contributes to the progression of pulmonary lymphangioleiomyomatosis via matrix metalloproteinase-induced cell invasiveness. J Clin Endocrinol Metab. 2008;93:1625–1633. [DOI] [PubMed] [Google Scholar]
- 38. Odajima N, Betsuyaku T, Nasuhara Y, Inoue H, Seyama K, Nishimura M. Matrix metalloproteinases in blood from patients with LAM. Respir Med. 2009;103:124–129. [DOI] [PubMed] [Google Scholar]
- 39. Pimenta SP, Baldi BG, Acencio MM, Kairalla RA, Carvalho CR. Doxycycline use in patients with lymphangioleiomyomatosis: safety and efficacy in metalloproteinase blockade. J Bras Pneumol. 2011;37:424–430. [DOI] [PubMed] [Google Scholar]
- 40. Tyryshkin A, Bhattacharya A, Eissa NT. SRC kinase is a novel therapeutic target in lymphangioleiomyomatosis. Cancer Res. 2014;74:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. [DOI] [PubMed] [Google Scholar]
- 42. Moir LM, Ng HY, Poniris MH, et al. Doxycycline inhibits matrix metalloproteinase-2 secretion from TSC2-null mouse embryonic fibroblasts and lymphangioleiomyomatosis cells. Br J Pharmacol. 2011;164:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ng HY, Oliver BG, Burgess JK, Krymskaya VP, Black JL, Moir LM. Doxycycline reduces the migration of tuberous sclerosis complex-2 null cells - effects on RhoA-GTPase and focal adhesion kinase. J Cell Mol Med. 2015;19:2633–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006;354:2621–2622. [DOI] [PubMed] [Google Scholar]
- 45. Pimenta SP, Baldi BG, Kairalla RA, Carvalho CR. Doxycycline use in patients with lymphangioleiomyomatosis: biomarkers and pulmonary function response. J Bras Pneumol. 2013;39:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chang WY, Cane JL, Kumaran M, Lewis S, Tattersfield AE, Johnson SR. A 2-year randomised placebo-controlled trial of doxycycline for lymphangioleiomyomatosis. Eur Respir J. 2014;43:1114–1123. [DOI] [PubMed] [Google Scholar]
- 47. Brubaker KD, Vessella RL, True LD, Thomas R, Corey E. Cathepsin K mRNA and protein expression in prostate cancer progression. J Bone Miner Res. 2003;18:222–230. [DOI] [PubMed] [Google Scholar]
- 48. Sato T, Takahashi S, Mizumoto T, et al. Neutrophil elastase and cancer. Surg Oncol. 2006;15:217–222. [DOI] [PubMed] [Google Scholar]
- 49. Chilosi M, Pea M, Martignoni G, et al. Cathepsin-k expression in pulmonary lymphangioleiomyomatosis. Mod Pathol. 2009;22:161–166. [DOI] [PubMed] [Google Scholar]
- 50. Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol. 2006;13:21–27. [DOI] [PubMed] [Google Scholar]
- 51. Chughtai B, O'Riordan TG. Potential role of inhibitors of neutrophil elastase in treating diseases of the airway. J Aerosol Med. 2004;17:289–298. [DOI] [PubMed] [Google Scholar]
- 52. El Rayes T, Catena R, Lee S, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA. 2015;112:16000–16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prizant H, Taya M, Lerman I, et al. Estrogen maintains myometrial tumors in a lymphangioleiomyomatosis model. Endocr Relat Cancer. 2016;23:265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schiavina M, Fabiani A, Cornia B, et al. Lymphangioleiomyomatosis: clinical course. Monaldi Arch Chest Dis. 1994;49:6–14. [PubMed] [Google Scholar]
- 55. Schiavina M, Di Scioscio V, Contini P, et al. Pulmonary lymphangioleiomyomatosis in a karyotypically normal man without tuberous sclerosis complex. Am J Respir Crit Care Med. 2007;176:96–98. [DOI] [PubMed] [Google Scholar]
- 56. Berger U, Khaghani A, Pomerance A, Yacoub MH, Coombes RC. Pulmonary lymphangioleiomyomatosis and steroid receptors. An immunocytochemical study. Am J Clin Pathol. 1990;93:609–614. [DOI] [PubMed] [Google Scholar]
- 57. Kinoshita M, Yokoyama T, Higuchi E, et al. Hormone receptors in pulmonary lymphangiomyomatosis. Kurume Med J. 1995;42:141–144. [DOI] [PubMed] [Google Scholar]
- 58. Grzegorek I, Drozdz K, Podhorska-Okolow M, Szuba A, Dziegiel P. LAM cells biology and lymphangioleiomyomatosis. Folia Histochem Cytobiol. 2013;51:1–10. [DOI] [PubMed] [Google Scholar]
- 59. Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d'Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P). Medicine (Baltimore). 1999;78:321–337. [DOI] [PubMed] [Google Scholar]
- 60. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. [DOI] [PubMed] [Google Scholar]
- 61. Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology. 1999;57 Suppl 2:49–58. [DOI] [PubMed] [Google Scholar]
- 62. Battaglia F, Polizzi G, Scambia G, et al. Receptors for epidermal growth factor and steroid hormones in human breast cancer. Oncology. 1988;45:424–427. [DOI] [PubMed] [Google Scholar]
- 63. Dubik D, Dembinski TC, Shiu RP. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987;47:6517–6521. [PubMed] [Google Scholar]
- 64. Yu J, Astrinidis A, Howard S, Henske EP. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2004;286:L694–L700. [DOI] [PubMed] [Google Scholar]
- 65. Li C, Zhou X, Sun Y, et al. Faslodex inhibits estradiol-induced extracellular matrix dynamics and lung metastasis in a model of lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2013;49:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li C, Lee PS, Sun Y, et al. Estradiol and mTORC2 cooperate to enhance prostaglandin biosynthesis and tumorigenesis in TSC2-deficient LAM cells. J Exp Med. 2014;211:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gu X, Yu JJ, Ilter D, Blenis N, Henske EP, Blenis J. Integration of mTOR and estrogen-ERK2 signaling in lymphangioleiomyomatosis pathogenesis. Proc Natl Acad Sci USA. 2013;110:14960–14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu JJ, Robb VA, Morrison TA, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci USA. 2009;106:2635–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun Y, Gu X, Zhang E, et al. Estradiol promotes pentose phosphate pathway addiction and cell survival via reactivation of Akt in mTORC1 hyperactive cells. Cell Death Dis. 2014;5:e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clements D, Asprey SL, McCulloch TA, Morris TA, Watson SA, Johnson SR. Analysis of the oestrogen response in an angiomyolipoma derived xenograft model. Endocr Relat Cancer. 2009;16:59–72. [DOI] [PubMed] [Google Scholar]
- 71. Prizant H, Sen A, Light A, et al. Uterine-specific loss of Tsc2 leads to myometrial tumors in both the uterus and lungs. Mol Endocrinol. 2013;27:1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhe X, Schuger L. Combined smooth muscle and melanocytic differentiation in lymphangioleiomyomatosis. J Histochem Cytochem. 2004;52:1537–1542. [DOI] [PubMed] [Google Scholar]
- 73. Tanaka H, Imada A, Morikawa T, et al. Diagnosis of pulmonary lymphangioleiomyomatosis by HMB45 in surgically treated spontaneous pneumothorax. Eur Respir J. 1995;8:1879–1882. [DOI] [PubMed] [Google Scholar]
- 74. Martignoni G, Pea M, Reghellin D, et al. Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch Pathol Lab Med. 2010;134:33–40. [DOI] [PubMed] [Google Scholar]
- 75. Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. [DOI] [PubMed] [Google Scholar]
- 76. Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, Fisher DE. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol. 2003;163:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. [DOI] [PubMed] [Google Scholar]
- 78. Fetsch PA, Fetsch JF, Marincola FM, Travis W, Batts KP, Abati A. Comparison of melanoma antigen recognized by T cells (MART-1) to HMB-45: additional evidence to support a common lineage for angiomyolipoma, lymphangiomyomatosis, and clear cell sugar tumor. Mod Pathol. 1998;11:699–703. [PubMed] [Google Scholar]
- 79. Klarquist J, Barfuss A, Kandala S, et al. Melanoma-associated antigen expression in lymphangioleiomyomatosis renders tumor cells susceptible to cytotoxic T cells. Am J Pathol. 2009;175:2463–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Loftus SK, Antonellis A, Matera I, et al. Gpnmb is a melanoblast-expressed, MITF-dependent gene. Pigment Cell Melanoma Res. 2009;22:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rose AA, Annis MG, Dong Z, et al. ADAM10 releases a soluble form of the GPNMB/osteoactivin extracellular domain with angiogenic properties. PLoS One. 2010;5:e12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tse KF, Jeffers M, Pollack VA, et al. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12:1373–1382. [DOI] [PubMed] [Google Scholar]
- 83. Fiorentini C, Bodei S, Bedussi F, et al. GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp Cell Res. 2014;323:100–111. [DOI] [PubMed] [Google Scholar]
- 84. Banner AS, Carrington CB, Emory WB, et al. Efficacy of oophorectomy in lymphangioleiomyomatosis and benign metastasizing leiomyoma. N Engl J Med. 1981;305:204–209. [DOI] [PubMed] [Google Scholar]
- 85. Schiavina M, Contini P, Fabiani A, et al. Efficacy of hormonal manipulation in lymphangioleiomyomatosis. A 20-year-experience in 36 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:39–50. [DOI] [PubMed] [Google Scholar]
- 86. Rossi GA, Balbi B, Oddera S, Lantero S, Ravazzoni C. Response to treatment with an analog of the luteinizing-hormone-releasing hormone in a patient with pulmonary lymphangioleiomyomatosis. Am Rev Respir Dis. 1991;143:174–176. [DOI] [PubMed] [Google Scholar]
- 87. van Milligen de Wit AW, Meilof-Planteydt MN. Successful treatment of pulmonary lymphangioleiomyomatosis with oophorectomy and medroxyprogesterone-acetate: report of a case and brief review of the literature. Neth J Med. 1990;36:246–251. [PubMed] [Google Scholar]
- 88. Mathur S, Mathur RS, Goust JM, Williamson HO, Fudenberg HH. Sex steroid hormones and circulating IgE levels. Clin Exp Immunol. 1977;30:403–407. [PMC free article] [PubMed] [Google Scholar]
- 89. Gao L, Yue MM, Davis J, Hyjek E, Schuger L. In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor. Virchows Arch. 2014;464:495–503. [DOI] [PubMed] [Google Scholar]
- 90. Sun Y, Zhang E, Lao T, et al. Progesterone and estradiol synergistically promote the lung metastasis of tuberin-deficient cells in a preclinical model of lymphangioleiomyomatosis. Horm Cancer. 2014;5:284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Glace L, Grygielko ET, Boyle R, et al. Estrogen-induced stromal cell-derived factor-1 (SDF-1/Cxcl12) expression is repressed by progesterone and by selective estrogen receptor modulators via estrogen receptor α in rat uterine cells and tissues. Steroids. 2009;74:1015–1024. [DOI] [PubMed] [Google Scholar]
- 92. Hodges LC, Houston KD, Hunter DS, et al. Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands in uterine leiomyoma cells. Mol Cell Endocrinol. 2002;196:11–20. [DOI] [PubMed] [Google Scholar]
- 93. Sawicka EH, Morris AJ. A report of two long-surviving cases of pulmonary lymphangioleiomyomatosis and the response to progesterone therapy. Br J Dis Chest. 1985;79:400–406. [DOI] [PubMed] [Google Scholar]
- 94. Poh SC, Wang YT. Lymphangioleiomyomatosis–treatment with progesterone. Singapore Med J. 1991;32:258–261. [PubMed] [Google Scholar]
- 95. Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–1874. [DOI] [PubMed] [Google Scholar]
- 96. Hunter DS, Hodges LC, Vonier PM, Fuchs-Young R, Gottardis MM, Walker CL. Estrogen receptor activation via activation function 2 predicts agonism of xenoestrogens in normal and neoplastic cells of the uterine myometrium. Cancer Res. 1999;59:3090–3099. [PubMed] [Google Scholar]
- 97. Feng Q, O'Malley BW. Nuclear receptor modulation–role of coregulators in selective estrogen receptor modulator (SERM) actions. Steroids. 2014;90:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Haskell SG. Selective estrogen receptor modulators. South Med J. 2003;96:469–476. [DOI] [PubMed] [Google Scholar]
- 99. Shanle EK, Xu W. Selectively targeting estrogen receptors for cancer treatment. Adv Drug Deliv Rev. 2010;62:1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee WL, Cheng MH, Chao HT, Wang PH. The role of selective estrogen receptor modulators on breast cancer: from tamoxifen to raloxifene. Taiwan J Obstet Gynecol. 2008;47:24–31. [DOI] [PubMed] [Google Scholar]
- 101. Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 102. Fitts JM, Klein RM, Powers CA. Tamoxifen regulation of bone growth and endocrine function in the ovariectomized rat: discrimination of responses involving estrogen receptor α/estrogen receptor β, G protein-coupled estrogen receptor, or estrogen-related receptor γ using fulvestrant (ICI 182780). J Pharmacol Exp Ther. 2011;338:246–254. [DOI] [PubMed] [Google Scholar]
- 103. Perry MJ, Gujra S, Whitworth T, Tobias JH. Tamoxifen stimulates cancellous bone formation in long bones of female mice. Endocrinology. 2005;146:1060–1065. [DOI] [PubMed] [Google Scholar]
- 104. Gielen SC, Santegoets LA, Hanifi-Moghaddam P, Burger CW, Blok LJ. Signaling by estrogens and tamoxifen in the human endometrium. J Steroid Biochem Mol Biol. 2008;109:219–223. [DOI] [PubMed] [Google Scholar]
- 105. Hu R, Hilakivi-Clarke L, Clarke R. Molecular mechanisms of tamoxifen-associated endometrial cancer (review). Oncol Lett. 2015;9:1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hodges LC, Hunter DS, Bergerson JS, Fuchs-Young R, Walker CL. An in vivo/in vitro model to assess endocrine disrupting activity of xenoestrogens in uterine leiomyoma. Ann NY Acad Sci. 2001;948:100–111. [DOI] [PubMed] [Google Scholar]
- 107. Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med. 1990;323:1254–1260. [DOI] [PubMed] [Google Scholar]
- 108. Svendsen TL, Viskum K, Hansborg N, Thorpe SM, Nielsen NC. Pulmonary lymphangioleiomyomatosis: a case of progesterone receptor positive lymphangioleiomyomatosis treated with medroxyprogesterone, oophorectomy and tamoxifen. Br J Dis Chest. 1984;78:264–271. [PubMed] [Google Scholar]
- 109. Hertelendy F, Zakar T. Regulation of myometrial smooth muscle functions. Curr Pharm Des. 2004;10:2499–2517. [DOI] [PubMed] [Google Scholar]
- 110. Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10:207–220. [DOI] [PubMed] [Google Scholar]
- 111. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. [DOI] [PubMed] [Google Scholar]
- 112. Stovall DW. Clinical symptomatology of uterine leiomyomas. Clin Obstet Gynecol. 2001;44:364–371. [DOI] [PubMed] [Google Scholar]
- 113. Feng J, Ye B, Yang Y, et al. [Pulmonary benign metastasizing leiomyoma: a clinicopathological study of 5 cases]. Zhongguo Fei Ai Za Zhi. 2014;17:550–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nasu K, Tsuno A, Takai N, Narahara H. A case of benign metastasizing leiomyoma treated by surgical castration followed by an aromatase inhibitor, anastrozole. Arch Gynecol Obstet. 2009;279:255–257. [DOI] [PubMed] [Google Scholar]
- 115. Esteban JM, Allen WM, Schaerf RH. Benign metastasizing leiomyoma of the uterus: histologic and immunohistochemical characterization of primary and metastatic lesions. Arch Pathol Lab Med. 1999;123:960–962. [DOI] [PubMed] [Google Scholar]
- 116. Wolff M, Silva F, Kaye G. Pulmonary metastases (with admixed epithelial elements) from smooth muscle neoplasms. Report of nine cases, including three males. Am J Surg Pathol. 1979;3:325–342. [DOI] [PubMed] [Google Scholar]
- 117. Pitts S, Oberstein EM, Glassberg MK. Benign metastasizing leiomyoma and lymphangioleiomyomatosis: sex-specific diseases? Clin Chest Med. 2004;25:343–360. [DOI] [PubMed] [Google Scholar]
- 118. Taveira-Dasilva AM, Rabel A, Gochuico BR, Avila NA, Moss J. Prevalence of uterine leiomyomas in lymphangioleiomyomatosis. Fertil Steril. 2011;96:711–714.e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gyure KA, Hart WR, Kennedy AW. Lymphangiomyomatosis of the uterus associated with tuberous sclerosis and malignant neoplasia of the female genital tract: a report of two cases. Int J Gynecol Pathol. 1995;14:344–351. [DOI] [PubMed] [Google Scholar]
- 120. Walker CL, Hunter D, Everitt JI. Uterine leiomyoma in the Eker rat: a unique model for important diseases of women. Genes Chromosomes Cancer. 2003;38:349–356. [DOI] [PubMed] [Google Scholar]