Abstract
Determining how an autoimmune response is initiated is essential to understanding the mechanisms of autoimmunity. Self-reactive T cells, self-protein, and a failure of tolerance to that self-protein are all involved in the pathogenesis of autoimmune disease; yet it is not clear how self-reactive T cells find the target self-protein to initiate an autoimmune response. Although a variety of self-proteins have been shown to be presented on both class I and class II major histocompatibility complex (MHC) molecules, the relationship of these self-proteins to autoimmune disease has not been established. To explore this further, we generated a T-cell hybridoma that recognizes mouse cardiac myosin, the self-protein that induces murine autoimmune myocarditis. Using this hybridoma as a probe to detect myosin-class II MHC complexes, we isolated a class II MHC+/CD45+ residential antigen-presenting cell (APC) population directly from the hearts of normal mice and looked for evidence of endogenous processing of cardiac myosin by these APC. In this report we show that myosin-class II MHC complexes are found on residential APC in the normal mouse heart. Induction of autoimmune myocarditis increased the expression of myosin-class II MHC in the heart and enhanced their APC functions. This result is a direct demonstration that epitopes of a self-antigen involved in initiating an autoimmune disease are endogenously processed and presented within the target organ.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Bikoff E. K., Yu H., Eckhardt L. A. T cell recognition of endogenous IgG2a expressed in B lymphoma cells. Eur J Immunol. 1988 Mar;18(3):341–348. doi: 10.1002/eji.1830180304. [DOI] [PubMed] [Google Scholar]
- Bill J., Kanagawa O., Woodland D. L., Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J Exp Med. 1989 Apr 1;169(4):1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet J. P., Couderc J., Bouthillier Y., Franc B., Decreusefond C., Mouton D. Collagen-induced arthritis in Biozzi mice. Joint involvement is not correlated with collagen II IgG2a autoantibodies nor restricted to only H-2q and H-2r. J Immunol. 1989 Sep 1;143(5):1537–1542. [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Flavell R. A. Tolerance in transgenic mice expressing major histocompatibility molecules extrathymically on pancreatic cells. Science. 1990 Jun 15;248(4961):1364–1368. doi: 10.1126/science.1694042. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K., Portas M., Bergman B., Lafferty K., Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner H., Odermatt B., Schneider R., Schreyer M., Wälle G., MacDonald H. R., Zinkernagel R. M. Deletion of self-reactive T cells before entry into the thymus medulla. Nature. 1988 Nov 24;336(6197):388–390. doi: 10.1038/336388a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz A., Ahmed-Ansari A., Neumann D. A., Beschorner W. E., Rose N. R., Soule L. M., Burek C. L., Sell K. W., Baughman K. L. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990 Mar 1;15(3):624–632. doi: 10.1016/0735-1097(90)90637-5. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Larsen C. P., Morris P. J., Austyn J. M. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. G., Allen P. M. Direct evidence for functional self-protein/Ia-molecule complexes in vivo. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5220–5223. doi: 10.1073/pnas.85.14.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. G., Blum J. S., Allen P. M. Constitutive competition by self proteins for antigen presentation can be overcome by receptor-enhanced uptake. J Immunol. 1990 Mar 1;144(5):1600–1606. [PubMed] [Google Scholar]
- Murphy K. M., Heimberger A. B., Loh D. Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Weaver C. T., Elish M., Allen P. M., Loh D. Y. Peripheral tolerance to allogeneic class II histocompatibility antigens expressed in transgenic mice: evidence against a clonal-deletion mechanism. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10034–10038. doi: 10.1073/pnas.86.24.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Rose M. L., Page C., Hengstenberg C., Yacoub M. H. Identification of antigen presenting cells in normal and transplanted human heart: importance of endothelial cells. Hum Immunol. 1990 Jun;28(2):179–185. doi: 10.1016/0198-8859(90)90017-j. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Rath S., Preston-Hurlburt P., Murphy D. B., Janeway C. A., Jr On the complexity of self. Nature. 1991 Oct 17;353(6345):660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiverick K. T., Thomas L. L., Alpert N. R. Purification of cardiac myosin. Application to hypertrophied myocardium. Biochim Biophys Acta. 1975 May 30;393(1):124–133. doi: 10.1016/0005-2795(75)90222-6. [DOI] [PubMed] [Google Scholar]
- Singh V. K., Yamaki K., Donoso L. A., Shinohara T. Molecular mimicry. Yeast histone H3-induced experimental autoimmune uveitis. J Immunol. 1989 Mar 1;142(5):1512–1517. [PubMed] [Google Scholar]
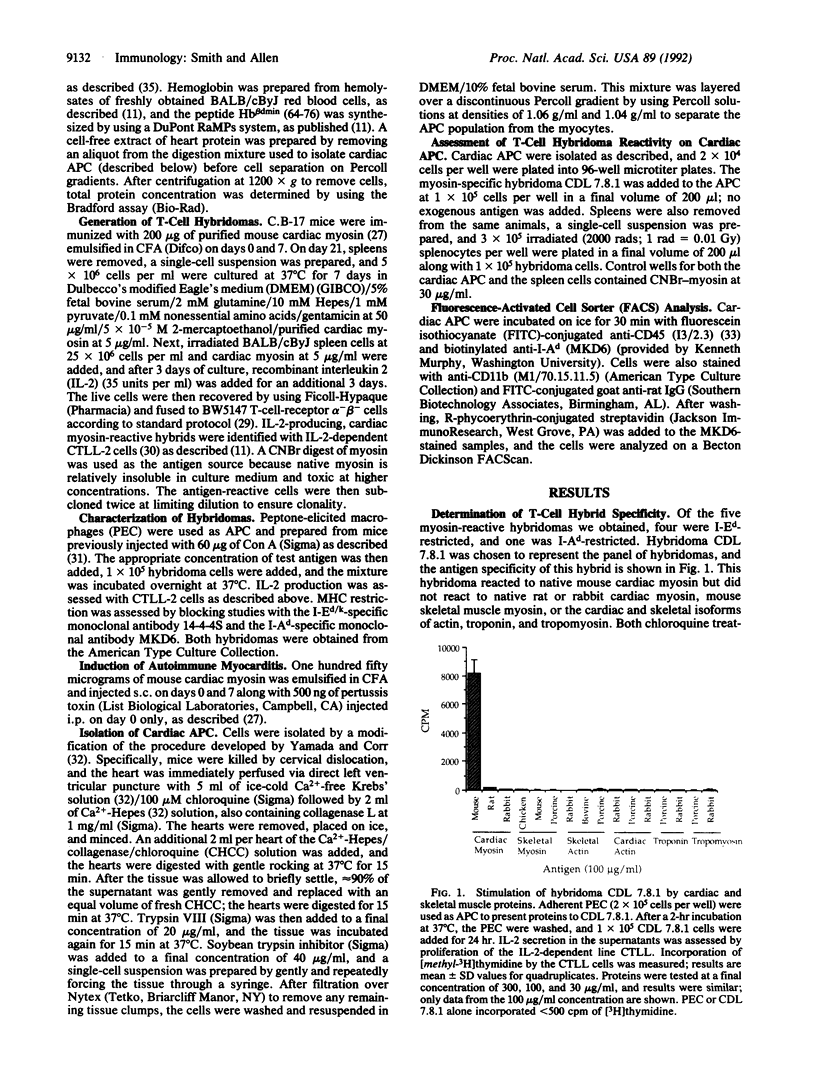
- Smith S. C., Allen P. M. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991 Oct 1;147(7):2141–2147. [PubMed] [Google Scholar]
- Smith S. C., Allen P. M. Neutralization of endogenous tumor necrosis factor ameliorates the severity of myosin-induced myocarditis. Circ Res. 1992 Apr;70(4):856–863. doi: 10.1161/01.res.70.4.856. [DOI] [PubMed] [Google Scholar]
- Spencer S. C., Fabre J. W. Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J Exp Med. 1990 Jun 1;171(6):1841–1851. doi: 10.1084/jem.171.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- Stockinger B., Hausmann B. Induction of an immune response to a self antigen. Eur J Immunol. 1988 Feb;18(2):249–253. doi: 10.1002/eji.1830180211. [DOI] [PubMed] [Google Scholar]
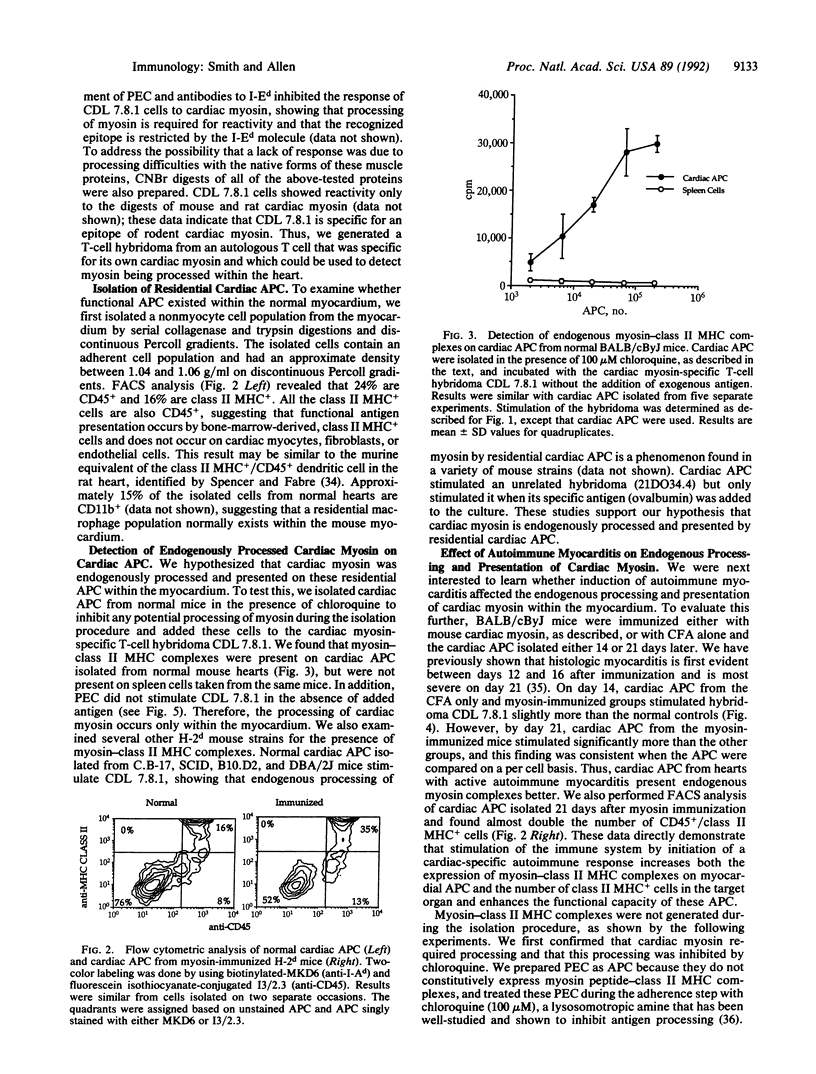
- Trowbridge I. S. Interspecies spleen-myeloma hybrid producing monoclonal antibodies against mouse lymphocyte surface glycoprotein, T200. J Exp Med. 1978 Jul 1;148(1):313–323. doi: 10.1084/jem.148.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITEBSKY E., ROSE N. R., TERPLAN K., PAINE J. R., EGAN R. W. Chronic thyroiditis and autoimmunization. J Am Med Assoc. 1957 Jul 27;164(13):1439–1447. doi: 10.1001/jama.1957.02980130015004. [DOI] [PubMed] [Google Scholar]
- Weiss S., Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Schwab M., Linington C., Meyermann R. Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol. 1986 Dec;16(12):1551–1557. doi: 10.1002/eji.1830161214. [DOI] [PubMed] [Google Scholar]
- Winchester G., Sunshine G. H., Nardi N., Mitchison N. A. Antigen-presenting cells do not discriminate between self and nonself. Immunogenetics. 1984;19(6):487–491. doi: 10.1007/BF00403439. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. 1985 Sep 26-Oct 2Nature. 317(6035):355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]