Abstract
Burn injury is a cause of significant mortality and morbidity worldwide and is frequently associated with severe and long-lasting pain that remains difficult to manage throughout recovery. We characterised a mouse model of burn-induced pain using pharmacological and transcriptomic approaches. Mechanical allodynia elicited by burn injury was partially reversed by meloxicam (5 mg/kg), gabapentin (100 mg/kg) and oxycodone (3 and 10 mg/kg), while thermal allodynia and gait abnormalities were only significantly improved by amitriptyline (3 mg/kg) and oxycodone (10 mg/kg). The need for relatively high opioid doses to elicit analgesia suggested a degree of opioid resistance, similar to that shown clinically in burn patients. We thus assessed the gene expression changes in dorsal root ganglion neurons and pathophysiological mechanisms underpinning burn injury-induced pain using a transcriptomic approach. Burn injury was associated with significantly increased expression of genes associated with axon guidance, neuropeptide signalling, behavioural defence response and extracellular signalling, confirming a mixed neuropathic and inflammatory aetiology. Notably, among the pain-related genes that were upregulated post-injury was the cholecystokinin 2 receptor (Cckbr), a G protein-coupled receptor known as a pain target involved in reducing opioid effectiveness. Indeed, the clinically used cholecystokinin receptor antagonist proglumide (30 mg/kg) was effective at reversing mechanical allodynia, with additional analgesia evident in combination with low-dose oxycodone (1 mg/kg), including significant reversal of thermal allodynia. These findings highlight the complex pathophysiological mechanisms underpinning burn injury-induced pain and suggest that cholecystokinin-2 receptor antagonists may be useful clinically as adjuvants to decrease opioid requirements and improve analgesic management.
Keywords: Burn-induced pain, transcriptome, dorsal root ganglion neurons, animal models, proglumide
Introduction
Burn injury is one of the most common injuries around the world, causing an estimated 265,000 deaths annually worldwide and necessitating medical treatment of nearly 11 million people in 2004.1 Out of the three potential causes of a thermal burn – scald, flame and contact burns – burn injury from a flame is consistently responsible for approximately 40% of all cases across patients of all ages with the exception of young children under five, who primarily suffer from contact burns.2 Electric and chemical burns, two non-thermal causes of burn injuries, only make up less than 10% of all burn incidences when combined. Pain is present in most patients following a burn injury, particularly as the patient recovers and scar tissues develop. Burn pain presents as a complex symptom consisting of multiple components, including spontaneous or non-evoked pain as well as hypersensitivity to mechanical and thermal stimuli that can transition from an acute, self-limiting nociceptive response to chronic pain with neuropathic components.3
Procedural pain, which is pain associated with surgical interventions including debridement, grafting and dressing changes, is short lasting but severe in comparison to background pain 4 and often requires additional intensive analgesic treatment in addition to the patient’s existing regimen. Pain intensity can also fluctuate unpredictably over time and requires constant dose monitoring. The degree of pain appears to be independent of existing analgesic dose and burn severity.5 Moreover, pain can last long after the burn injury had healed, and evidence of chronic pain with neuropathic features have been observed in patients irrespective of burn intensity,6 making pain a difficult-to-manage and frequently undertreated consequence of burn injury.
While opioids remain a central approach for analgesia especially in the acute phase,7 concerns remain regarding opioid dependence, withdrawal and side effects in burn patients. In addition, the fluid and unpredictable nature of burn-induced pain results in frequent dose adjustments and pain control that is often unsatisfactory. Accordingly, adjuvant analgesics such as antidepressants, anticonvulsants and non-steroidal anti-inflammatory drugs are used extensively in burn pain,8 but often with conflicting results.9,10 Thus, a real need exists for the identification of novel therapeutic targets and the development of improved analgesics based on the molecular mechanisms underlying burn pain.
The aim of this study was to behaviourally characterise a mouse model of burn-induced pain suitable for rapid in vivo analgesic efficacy profiling of novel analgesics and to identify novel analgesic targets through transcriptomic analysis of gene expression changes in whole dorsal root ganglia (DRGs) following burn injury. Using this approach, we identified the cholecystokinin (CCK) receptor 2 as a novel analgesic target in burn injury, with the clinically used CCK receptor antagonist proglumide exhibiting analgesic activity in vivo.
Methods
Chemicals
Meloxicam was obtained from Abcam (Melbourne, VIC, Australia). Oxycodone was obtained from Mundipharma Pty Ltd (Sydney, NSW, Australia). All other chemicals were obtained from Sigma Aldrich (Castle Hill, NSW, Australia) unless otherwise specified.
Animals
Ethical approval for in vivo experiments in animals was obtained from the University of Queensland animal ethics committee. Experiments involving animals were conducted in accordance with the Animal Care and Protection Regulation Qld (2012), the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th edition (2013) and the International Association for the Study of Pain Guidelines for the Use of Animals in Research.
For behavioural assessment, we used adult male C57BL/6 mice (Animal Resources Centre, Canning Vale, WA, Australia) aged 6–8 weeks. Animals were housed in groups of 2–4 per cage under 12-h light-dark cycles and had access to standard rodent chow and water ad libitum. A red polycarbonate Mouse House (Tecniplast, Italy) and shredded paper nesting material were supplied for enrichment. Animals were acclimatised to the testing room (ambient temperature of 21℃–23℃) for at least 1 h prior to behavioural testing each day. General health and well-being was monitored daily, and no animals were withdrawn from the study. Sample sizes of each experiment are detailed in the figure legends of the corresponding figure.
Induction of burn injury
To induce a mild burn injury, the plantar skin of the left hind paw of mice was applied for 25 s with firm pressure to a Peltier plate (Hot/Cold Plate, Ugo Basile, Comerio, Italy) set at 52.5℃ with the mice under isoflurane (3%) anaesthesia as previously described.11 Sham control mice underwent the same procedure with the plate set at 22℃ (room temperature). Behavioural assessment was performed at the time points indicated.
Spontaneous pain
Spontanous pain was observed as licking, lifting and/or flinching of the affected hind paw. Mice were placed in individual polyvinyl circular containers (25 cm in diameter) on a surface padded with some tissue paper at room temperature. Mice were allowed to roam freely and spontaneous pain was measured in 5-min intervals, as counted by a blinded observer.
Electronic von Frey
Mechanical allodynia was assessed using the electronic von Frey system (MouseMet Electronic von Frey, TopCat Metrology, UK). All measurements were conducted by a blinded observer. Mice were habituated in the electric von Frey apparatus for at least 10 min prior to testing. The electronic von Frey filament was placed against the plantar skin of the hind paw and the pressure increased at a rate of ∼1 g/s through rotation of the device. The MouseMet Software automatically recorded the force at which paw withdrawal occurred. The paw withdrawal force (PWF) was determined by the average of three tests, separated by at least 2 min each.
Hargreaves test
Thermal allodynia was assessed using the Hargreaves apparatus (Plantar Analgesia Meter, IITC, CA, USA). All measurements were conducted by a blinded observer. Mice were habituated in the Hargreaves apparatus in individual polyvinyl boxes (10 × 10 × 10 cm) placed on glass heated to 25℃ for at least 30 min prior to testing. A radiant light heat source (50℃) was focused on the plantar skin of the hind paw, and the latency to a withdrawal response was recorded. The mean time to withdrawal was determined from the average of three tests, separated by at least 2 min. A cut-off time of 20 s was used to avoid tissue damage.
Gait analysis
Gait analysis was assessed using the CatWalk XT analysis system (Noldus Information Technology, The Netherlands) as previously described.12 Mice were placed individually at one end of the elevated glass walkway and allowed to walk freely to the other end, until three successful runs were recorded. Mice received no prior training. Runs that took longer than 10 s or with a speed variance >90% were deemed unsuccessful and discarded. The green intensity of the walkway background was set at 0.10 and camera gain at 20.00. Recordings were analysed using the CatWalk XT software with the parameters described in Table 1.
Table 1.
Description of CatWalk XT parameters used to analyse burn-induced gait abnormalities.
| Paw pressure parameters | |
| Mean intensity | The mean light intensity for an individual paw during an entire run expressed in arbitrary units (AU). |
| Mean intensity of the 15 most intense pixels | The mean intensity of the 15 pixels of a paw with the highest reflected light intensity during an entire run expressed in arbitrary units (AU). |
| Max intensity | The maximum light intensity for an individual paw during a run expressed in arbitrary units (AU). |
| Paw print area parameters | |
| Print area | Representative of the average contact area of an individual paw with the glass plate during a run expressed in cm2. |
| Maximum contact area | The maximum surface area of the print the animal placed on the glass plate during a run expressed in cm2. |
| Dynamic paw parameters | |
| Stand | The average duration of contact of an individual paw with the glass plate during a run expressed in seconds. |
| Swing | The average duration in seconds of no contact of a paw with the glass plate. |
| Duty cycle | Stand as a percentage of Step Cycle: Duty Cycle = Stand / (Stand + Swing) × 100% |
| Interlimb coordination | |
| Regularity index | Percentage of normal step sequences calculated by the formula (NSSP × 4)/PP × 100%, where NSSP is the number of Normal Step Sequence Patterns and PP is the total number of Paw Placements. |
Paw thickness
Paw thickness was measured along the distal-proximal axis at the metatarsal level using a digital vernier caliper (Kincrome, VIC, Australia) whilst the mice was under isoflurane (3%) anaesthesia. The paw thickness of the ipsilateral paw was normalised to the value of the contralateral paw for each animal.
Drug treatment
The analgesic efficacy of clinical compounds was assessed three days after the induction of burn injury. All compounds were administered by intraperitoneal injection in a volume of 10 µL/g using a 30-gauge needle. Animals were randomised to receive either meloxicam (5 mg/kg) once daily, gabapentin (100 mg/kg) three times daily, amitriptyline (3 mg/kg) once daily, proglumide (30 mg/kg) once daily or matched vehicle control, with the first dose administered at the time of the burn injury. Oxycodone (1, 3 and 10 mg/kg) was administered once only on the day of testing. All compounds were diluted in phosphate-buffered saline, except meloxicam, which was diluted in phosphate-buffered saline with 10% dimethyl sulphoxide. Behavioural assessment was performed at least 30 min after the final dose of all compounds by a blind investigator unaware of the treatments received by each individual animal.
Motor assessment
Motor performance was assessed using the Parallel Rod Floor Apparatus with ANY-Maze Software (Stoelting Co., Wood Dale, USA). A single dose of oxycodone (10 mg/kg), gabapentin (100 mg/kg), amitriptyline (3 mg/kg) or proglumide (30 mg/kg) was administered as described above by intraperitoneal injection 30 min prior to behavioural assessment. Mice were placed in the Parallel Rod Floor Apparatus, and the ANY-Maze software recorded the distance travelled and number of foot slips over a 1-min period. The ataxia index was calculated as the ratio of the total number of foot slips to the total distance travelled (in m).
Data analysis
Data were plotted and analysed by GraphPad Prism, version 6.0. Statistical significance was defined as P < 0.05 and was determined by unpaired t-test assuming equal variance. Data are expressed as the mean ± standard error of the mean (SEM).
Sample preparation for RNA-Seq analysis
A previously published procedure13 was used to assess gene expression changes after burn injury. On the third day following burn or sham injury, mice were euthanised with carbon dioxide followed by cervical dislocation. The spine was immediately removed, and the dorsal side of the vertebra dissected to expose the spinal cord. The entire spine was then immersed in ice-cold Dulbecco’s Modified Eagle’s Medium. The spinal cord was then removed and ipsilateral L3, L4 and L5 DRGs were extracted with forceps. The DRGs were immediately placed into RNALater (Thermo Fisher Scientific, Scoresby, VIC, Australia) and excess myelin was removed. DRGs were then cut into small pieces in RNALater, and RNA was extracted using the RNeasy Mini Kit (Qiagen, Melbourne, Australia) according to manufacturer’s instructions, including on-column DNase digestion. Samples from four individual animals were pooled together for one biological replicate. Three biological control replicates and three burn-injury biological replicates were analysed.
RNA-Seq
Library construction and bioinformatics analysis were conducted by the Institute for Molecular Bioscience Sequencing Facility (The University of Queensland, Brisbane, QLD, Australia). RNA-Seq was conducted on the Illumina NextSeq 500 platform as 75-nucleotide single-end runs, with libraries prepared using TruSeq stranded total RNA library preparation. Reads were mapped using the STAR aligner (version star/2.3.0 e)14 and samtools.15 Reads were mapped to the Ensembl Mus musculus mm10 genome (version GRCm38). Count tables were generated using HTSeq_count from HTSeq package,16 and differential expression were analysed using the R (version 3.2.2) package DESeq2.17
Bioinformatics analysis
Plots were generated using the R packages gplots, ggplots2 and pheatmap. Differentially expressed genes from DESeq2 analysis were ranked by adjusted P value. Genes with adjusted P < 0.05 were used for gene ontology (GO) analysis, which was conducted using Cytoscape 3.2.118 with the ClueGO 2.1.7 plugin.19 ClueGO was used with the following settings: Ontologies/Pathways used Mus musculus GO (Biological Processes, version from 30 April 2015). Evidence: all. GO tree internal three to eight levels. GO term restrictions: three minimum genes and 1% of genes. Network connectivity (Kappa score) = 0.4. STRING diagrams were generated using STRING v10.20 Following alignment, count output from HTSeq was analysed using the DESeq2 statistical package.
Results
Behavioural characterisation of a mouse model of burn injury-induced pain
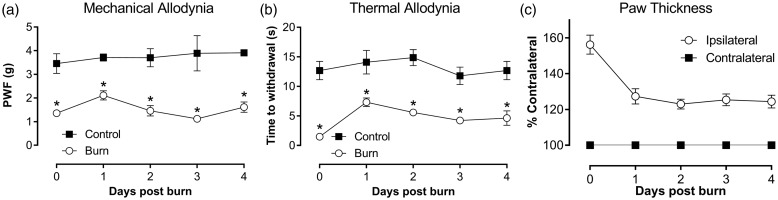
Previously, we reported a novel mouse model of burn-induced pain,11 and here we present for the first time detailed characterisation of this model. Exposure of the left hind paw in mice to a metal surface set at a temperature of 52.5℃ for 25 s resulted in a first-degree or superficial burn, as evidenced by the immediate development of localised redness and swelling, without the presence of blisters. Mild spontanous pain was present immediately after burn injury (9.8 ± 2.7 pain behaviours/5 min) and persisted for up to 6 h, precluding behavioural assessment of mechanical and thermal allodynia until after this time. Once spontanous pain subsided, a significant reduction in mechanial and thermal thresholds was observed compared with sham control animals, which was observed within 6 h of the burn injury and persisted for at least four days post burn injury (Figure 1(a) and (b)). Inflammation, as evidenced by a statistically significant increase in the thickness of the ipsilateral paw relative to the contralateral paw, developed rapidly, peaking immediately after burn injury (paw thickness 6 h: 156 ± 5%) and persisted for at least four days (Figure 1(c)).
Figure 1.
Characterisation of a mouse model of burn injury. (a) Time course of the development of mechanical allodynia assessed by electronic von Frey. (b) Time course of the development of thermal allodynia assessed using the Hargreaves method. (c) Time course of development of inflammation, as measured by paw thickness normalised to the contralateral paw. Statistical significance was determined using t-test, *P < 0.05 compared with sham control. All data are represented as mean ± SEM; n = 3 for sham control group and n = 18 for burn group.
Gait analysis using the CatWalk XT in a mouse model of burn injury-induced pain
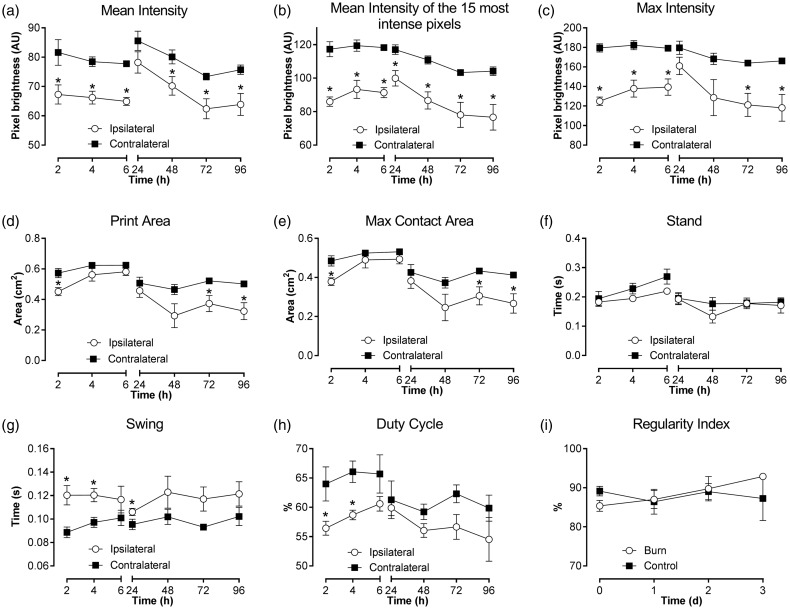
While gait analysis has previously been utilised to quantify pain-related behaviours in other rodent models of inflammatory and neuropathic pain,21,22 gait abnormalities have not previously been assessed in rodent models of burn injury. Here, we provide for the first time a detailed characterisation of gait analysis following a unilateral burn injury in mice using the CatWalk XT. All paw pressure parameters (mean intensity, mean intensity of the 15 most intense pixels, max intensity) were significantly reduced in the ipsilateral hind paw compared with the contralateral hind paw (Figure 2(a)–(c)), signifying a difference in weight bearing between the hind paws. Paw print area parameters (print area, max contact area) were minimally affected; perhaps confounded by the presence of swelling in the ipsilateral hind paw (Figure 2(d) and (e)). Some differences in dynamic paw parameters (stand, swing and duty cycle) were detected in the first 24 h, but lost statistical significance after 48 h (Figure 2(f)–(h)). Interlimb coordination, as measured by the regularity index, remained unchanged from sham controls, indicating that burn-injured mice followed a normal step sequence (Figure 2(i)). As the paw pressure parameter ‘mean intensity of the 15 most intense pixels’ (Figure 2(b)) was the most sensitive parameter to detect differences between ipsilateral and contralateral hind paws in burn-injured mice, this parameter was used to assess the effects of compounds on weight-bearing behaviour.
Figure 2.
Characterisation of gait abnormalities in a unilateral mouse model of burn injury using select parameters from the CatWalk XT. Time course of changes in (a–c) paw pressure parameters, (d, e) paw print area parameters, and (f–h) dynamic paw parameters in the ipsilateral and contralateral hind paw in burn injured animals. (i) No changes in interlimb coordination as measured by the regularity index were detected between burn and sham controls. Statistical significance was determined using t-test, *P < 0.05 compared to contralateral paw or sham control as indicated. All data are presented as mean ± SEM with 5–10 mice per group.
Analgesic efficacy of clinically used analgesics in a mouse model of burn pain
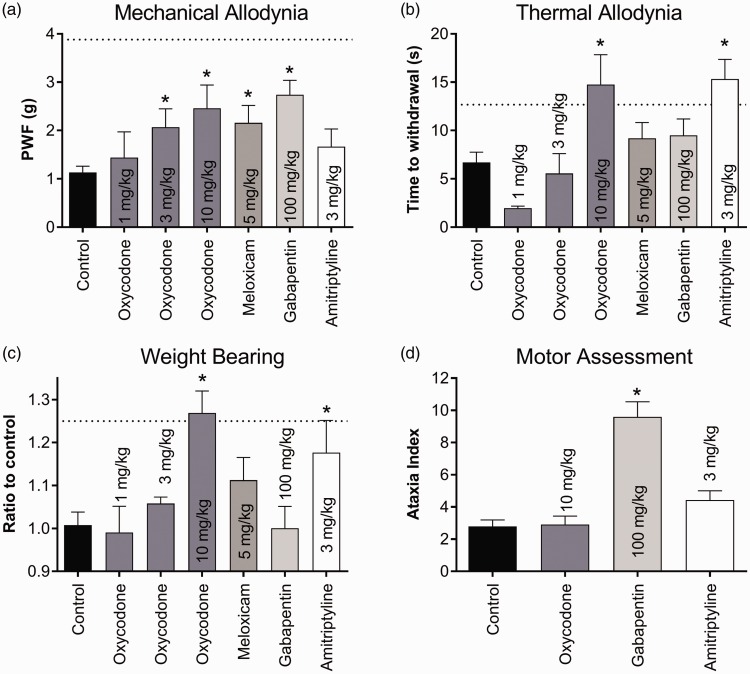
To further characterise the model, we next assessed the analgesic efficacy of a range of clinically used compounds, as systematic assessment of clinically used analgesics has not previously been reported in burn-induced pain. Oxycodone (3 and 10 mg/kg), meloxicam and gabapentin all significantly alleviated mechanical allodynia compared with vehicle control; however, PWF did not return to baseline values (PWF: oxycodone (3 mg/kg), 2.1 ± 0.4 g; oxycodone (10 mg/kg), 2.5 ± 0.5 g; meloxicam (5 mg/kg), 2.2 ± 0.4 g; gabapentin (100 mg/kg), 2.7 ± 0.3 g; vehicle control, 1.1 ± 0.5 g; P < 0.05; Figure 3(a)), while amitriptyline and oxycodone (1 mg/kg) had no significant effect. Both oxycodone (10 mg/kg) and amitriptyline abolished thermal allodynia, significantly increasing the time of withdrawal to a radiant light stimulus compared to vehicle control (time to withdrawal: oxycodone (10 mg/kg), 12.1 ± 3.5 s; amitriptyline (3 mg/kg), 15.3 ± 2.0 s; vehicle control, 6.7 ± 1.1 s; P < 0.05; Figure 3(b)), while meloxicam, gabapentin and oxycodone at doses less than 3 mg/kg had no significant effect. Weight bearing on the ipsilateral hind paw was significantly increased by oxycodone (10 mg/kg) and amitriptyline (ratio to control: oxycodone (10 mg/kg), 1.27 ± 0.05%; amitriptyline (3 mg/kg), 1.18 ± 0.07%; vehicle control, 0.99 ± 0.06%; P < 0.05; Figure 3(c)) but not by meloxicam, gabapentin or doses of oxycodone less than 3 mg/kg. The observed anti-allodynic effects of oxycodone and amitriptyline were not due to motor impairment, as only gabapentin (100 mg/kg) significantly increased the ataxia index compared with vehicle control (ataxia index: vehicle control, 2.8 ± 0.4; gabapentin, 9.6 ± 0.9. P < 0.05; Figure 3(d)).
Figure 3.
Activity of clinically used analgesic compounds in a mouse model of burn-induced pain. (a) Oxycodone (3 and 10 mg/kg), meloxicam, gabapentin and amitriptyline partially but significantly alleviated mechanical allodynia as assessed by electronic von Frey. (b) Only oxycodone (10 mg/kg) and amitriptyline significantly reversed thermal allodynia assessed by the Hargreaves method. (c) Oxycodone (10 mg/kg) and amitriptyline led to a significant increase in weight bearing of the ipsilateral hind paw, measured using the “Mean Intensity of the 15 most intense pixels” parameter in the Catwalk XT. (d) Only gabapentin (100 mg/kg) had a significant effect on motor impairment, significantly increasing the ataxia index compared to vehicle control. The same mice were used for each of the behavioural tests. Statistical significance was determined using t-test. *P < 0.05 compared to vehicle control. Dotted lines represent the mean value for the contralateral paw. All data are represented as mean ± SEM with 5–17 mice per group.
Transcriptomic analysis of lumbar DRG neurons following a unilaterial hindpaw burn
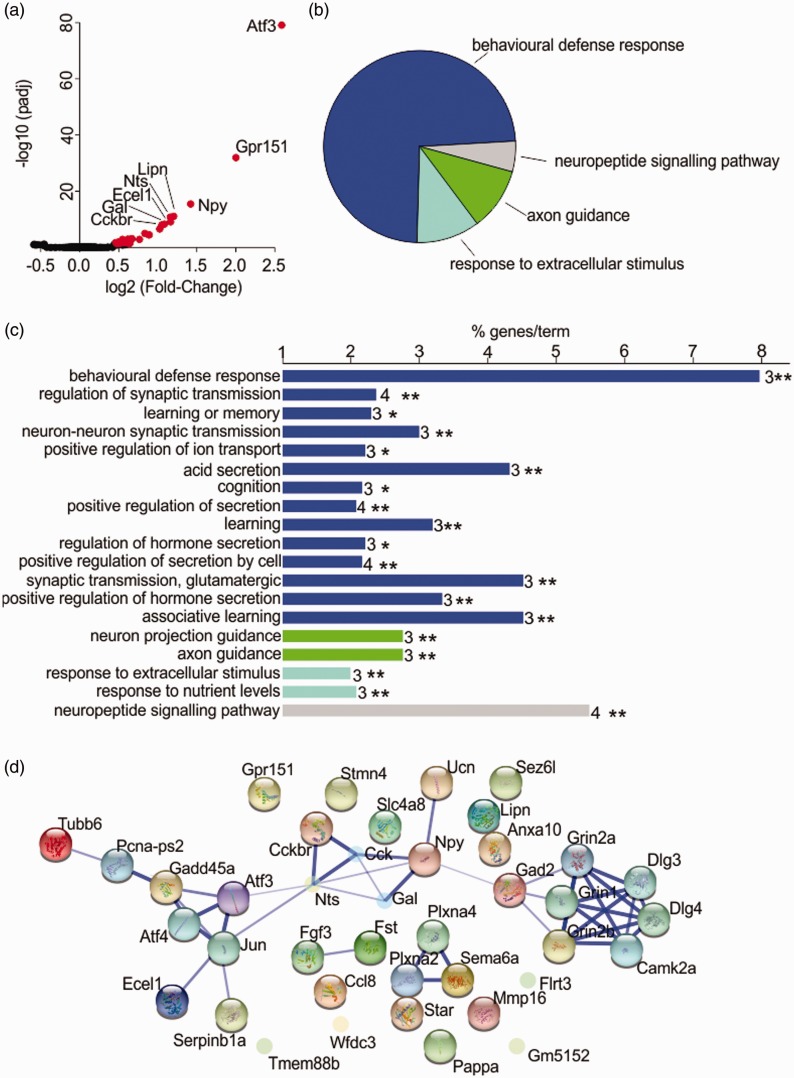
In order to gain further insight into the molecular mechanisms underlying burn injury-induced pain, we conducted a transcriptomic analysis of gene expression changes in whole DRGs using the Illumina NextSeq 500 platform. A total of 4302 billion reads, 72% of which were uniquely mapped to the Mus musculus genome, were generated from all samples. A total of 30 genes were differentially expressed at P < 0.05 in whole DRGs after the burn injury (Table 2, for full list see Supplementary File 1), with the levels of gene expression ranging from at least log2FoldChange 0.46 (Anxa10) to 2.59 (Atf3). A volcano plot revealed significant up-regulation, but no down-regulation of genes in comparison with control DRGs (Figure 4(a)); six of these genes are associated with a known function in pain.31 These pain genes included neuropeptides with a known role in nociceptive signalling (Table 2), specifically neuropeptide Y (Npy), neurotensin precursor (Nts) and galanin (Gal); as well as the gastrin and CCK receptor (Cckbr), the ionotropic N-Methyl D-Aspartate (NMDA) 2B receptor (Grin2b) and glutamic acid decarboxylase 2 (Gad2). Atf3, the most up-regulated gene, codes for a protein associated with neuronal injury, supporting the hypothesis that at least some sensory projections are directly injured in a burn.13 This observation was further confirmed by GO analysis (Figure 4(b) and (c)), which identified Behavioural Defense Response, Neuropeptide Signalling Pathway, Axon Guidance and Response to Extracellular Stimulus as the four main functional clusters of altered gene expression. Additional abundant GO biological process terms, determined as the percentage of associated genes, were glutamatergic synaptic transmission, associative learning and acid secretion (Figure 4(c)).
Table 2.
Table of genes differentially expressed in DRG neurons following burn injury.
| Gene | log2Foldchange | Adjusted P value | Role in pain |
|---|---|---|---|
| Atf3 | 2.59 | 8.73 E-80 | Unknown |
| Gpr151 | 2.00 | 1.11 E-32 | Unknown |
| Npy | 1.42 | 3.41 E-16 | Known analgesic effect23 |
| Lipn | 1.20 | 8.11 E-12 | Unknown |
| Nts | 1.16 | 1.66 E-11 | Involved in visceral nociception and stress-related analgesia24 |
| Ecel1 | 1.17 | 7.99 E-10 | Unknown |
| Gal | 1.09 | 5.12 E-09 | Knockout animals show reduced nociceptive behaviours25 and chronic administration causes pain hypersensitivity26 |
| Cckbr | 1.05 | 9.87 E-09 | Knockout animals show reduced sensitivity in neuropathic pain27 |
| Tmem88b | 1.03 | 2.71 E-07 | Unknown |
| Sema6a | 0.83 | 9.84 E-06 | Unknown |
| Sez6l | 0.88 | 3.11 E-05 | Unknown |
| Star | 0.89 | 3.40 E-05 | Unknown |
| Flrt3 | 0.67 | 0.00069 | Unknown |
| Stmn4 | 0.62 | 0.00112 | Unknown |
| Grin2b | 0.77 | 0.00130 | Overexpression enhanced inflammatory pain responses.28 Knockouts enhanced acute nociceptive responses29 |
| Pappa | 0.55 | 0.00130 | Unknown |
| Fst | 0.66 | 0.00178 | Unknown |
| Fgf3 | 0.64 | 0.00314 | Unknown |
| Ucn | 0.55 | 0.00665 | Unknown |
| Serpinb1a | 0.50 | 0.01034 | Unknown |
| Plxna4 | 0.46 | 0.01738 | Unknown |
| Tubb6 | 0.63 | 0.01738 | Unknown |
| Gm5152 | 0.47 | 0.02848 | Unknown |
| Mmp16 | 0.57 | 0.02848 | Unknown |
| Gad2 | 0.62 | 0.03045 | Knockout animals showed sensitised pain behaviours30 |
| Ccl8 | 0.63 | 0.03961 | Unknown |
| Gadd45a | 0.48 | 0.03961 | Unknown |
| Wfdc3 | 0.64 | 0.03961 | Unknown |
| Slc4a8 | 0.518428 | 0.041841 | Unknown |
| Anxa10 | 0.47941 | 0.049852 | Unknown |
Note. Genes were arranged according to adjusted P value (calculated using the in-build false discovery rate control in the DESeq2 package, using the Benjamini-Hockberg method).
Figure 4.
Transcriptomic analysis of gene expression changes in DRGs after burn injury. (a) Volcano plot showing log of the fold change vs log10 of the adjusted P value. Genes with adjusted P values of < 0.05 are coloured red. (b) Gene Ontology analysis. Functional categories were assigned to genes within Cytoscape according to the Biological Processes pathways, version 30 April 2015. Visualisations were drawn with the Cytoscape plugin ClueGO. Genes were clustered into one main functional group and three smaller groups. Overview pie chart representative of the number of Gene Ontology interactions in each functional group is shown. (c) GO Biological Processes pathway terms for the differentially expressed genes. Numbers after the bar indicate the number of genes associated with each term. The X-axis indicates the percentage of associated genes for each term. Asterisks indicate the P value of the association after Bonferroni stepdown adjustment; * = P < 0.05, ** = P < 0.01. (d) Network analysis showing interactions between differentially expressed genes, generated with STRING version 10 and with the ‘More’ option (add 10 more gene partners) utilised once only. Diagram is presented in confidence view, and the thickness of edges connecting genes is determined by the strength of associations based on evidence.
To gain additional insight into the molecular mechanisms contributing to altered neuronal function and pain after burn injury, we also conducted network analysis with STRING (Version 10). Confirming the findings of GO analysis, glutamatergic transmission and neuronal secretion formed distinctive networks and clusters (Figure 4(d)), with Grin2b and Gad2 functionally linked to Npy, Gal, Nts and Cckbr signalling, consistent with the established roles of these genes in pain and nociception. A second functional cluster linked Atf3, Ecel1 and Tubb6; while several genes significantly up-regulated after burn injury could not be linked to functional networks, reflecting the poorly defined physiological roles of genes including Tmem88b, Sezl6, Lipn, Anxa10, Ccl8 and Gpr151 in sensory neurons and pain.
Accession number
Gene Expression Omnibus accession number: GSE75691. Data include raw.fastq files and count tables produced with HTSeq as.txt files.
Analgesic effects of the CCK receptor antagonist proglumide in a mouse model of burn injury
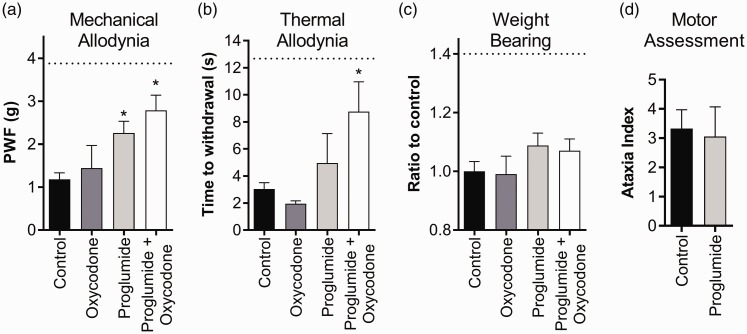
The CCK 2 receptor (Cckbr), a G protein-coupled receptor known as a pain target involved in reducing opioid effectiveness, was found to be up-regulated in the transcriptome following the burn injury. We therefore assessed the analgesic effects of the CCK receptor antagonist proglumide, which is already available in the clinic. Proglumide was tested alone and in combination with a subtherapeutic dose of oxycodone (1 mg/kg), as CCK receptor antagonists are known to augment the analgesic effects of opioids in humans.32,33 Proglumide alone significantly alleviated mechanical allodynia compared with vehicle control, albeit only partial analgesia was achieved (PWF: proglumide (30 mg/kg), 2.3 ± 0.3 g; vehicle control, 1.2 ± 0.1 g; P < 0.05; Figure 5(a)). Co-administration of proglumide with oxycodone significantly reversed mechanical allodynia further (PWF: proglumide (30 mg/kg) + oxycodone (1 mg/kg), 2.3 ± 0.3 g), although the combination was not statistically significant from proglumide alone (P = 0.13). Proglumide alone had no significant effect on thermal allodynia. However, in combination with oxycodone, the time to withdrawal in response to a radiant light heat source was significantly increased (time to withdrawal: proglumide (30 mg/kg) + oxycodone (1 mg/kg), 8.8 ± 2.1 s; vehicle control, 3.0 ± 0.5 s; P < 0.05; Figure 5(b)). Interestingly, neither proglumide alone nor proglumide in combination with oxycodone significantly increased weight bearing in the ipsilateral hind paw (ratio to control: proglumide (30 mg/kg), 1.09 ± 0.04%; proglumide (30 mg/kg) + oxycodone (1 mg/kg), 1.07 ± 0.04%; vehicle control, 1.00 ± 0.03 %; P > 0.05; Figure 5(c)). Proglumide had no significant effect on the ataxia index compared to vehicle control (ataxia index: vehicle control, 3.3 ± 0.6; proglumide (30 mg/kg), 3.1 ± 1.0; P > 0.05; Figure 5(d)).
Figure 5.
Analgesic activity of the CCK antagonist proglumide in burn-induced pain, both alone and in combination with a low-dose opioid. (a) Both proglumide (30 mg/kg) alone and proglumide (30 mg/kg) in combination with oxycodone (1 mg/kg) significantly alleviated burn-induced mechanical allodynia. (b) Proglumide (30 mg/kg) alone had no significant effect on burn-induced thermal allodynia; however, thermal allodynia was significantly reversed when administered in combination with oxycodone (1 mg/kg). (c) Neither proglumide (30 mg/kg) alone nor proglumide (30 mg/kg) in combination with oxycodone (1 mg/kg) significantly improved weight bearing behaviour of the ipsilateral hind paw, as detected by the ‘Mean Intensity of the 15 most intense pixels’ parameter using the Catwalk XT. (d) Proglumide (30 mg/kg) had no significant adverse effect on motor behaviour as assessed by the parallel rod floor test. The same mice were used for each of the behavioural tests. Statistical significance was determined using t-test, *P < 0.05 compared to vehicle control. Dotted lines represent the mean value for the contralateral paw. All data are represented as mean ± SEM with 4–8 mice per group.
Discussion
In this study, we characterised a mouse model of burn injury-induced pain using transcriptomic and pharmacological approaches. This model appears suitable for analgesic efficacy profiling of novel treatments that may provide improved management approaches for this under-treated and difficult-to-manage condition. In contrast to previously reported mouse models,13 this model causes a superficial or first-degree burn without the presence of blisters or broken skin, while still producing robust mechanical and thermal allodynia as well as detectable differences in weight-bearing behaviour.
Mild burns affecting less than 10% of total body surface area represent the overwhelming majority in the clinical setting and account for approximately 80% of all hospital or emergency service admissions,34 making this model relevant to many patients. Clinically used analgesics and adjuvants known to have some efficacy in burn pain, such as oxycodone and gabapentin,35,36 reduced mechanical and thermal allodynia as well as gait abnormalities, supporting the translational validity of our model. Specifically, gabapentin and amitriptyline, two drugs used as chronic pain adjuvant treatments, were effective at alleviating mechanical and thermal allodynia, respectively. However, both drugs were only partially anti-allodynic, consistent with effects seen in the clinic.10,37,38 The model identified both gabapentin and amitriptyline as potentially effective compounds, although systematic clinical trials assessing efficacy of adjuvants in human burn patients are lacking.
Opioids form the mainstay of therapy for burn-induced pain, albeit the drug of choice varies. Oxycodone, a commonly used µ-opioid receptor agonist, successfully reversed mechanical allodynia, thermal allodynia as well as weight-bearing parameters. Notably, burn injury-induced pain behaviours were relatively opioid-resistant compared with doses required to elicit analgesia in simple nociceptive mice models (1–2 mg/kg).39,40 A requirement for higher opioid doses to achieve effective analgesia has been noted for human burn patients,41 suggesting that the pathophysiological mechanisms underpinning burn-injury induced pain incorporate elements that lead to decreased efficacy of opioids. This phenomenon can either lead to ineffective analgesia due to the clinician’s unwillingness to prescribe beyond standards opioid doses or place patients at higher risk of opioid adverse effects.
In this study, we have used RNA-seq analysis with the view to explore gene expression changes that could provide insight into the altered sensory neuron function after burn injury. Although transcriptomic analysis has been widely used to comprehensively analyse differential gene expression, global gene expression changes that occur in DRGs after a mild peripheral burn have not been explored previously. However, changes in expression of cytokines and inflammation-related genes that occur after burn injury have been assessed in cultured keratocytes from burn patients,42 and RNA from circulating leukocytes of patients critically injured in burn injuries have also been analysed.43 As expected based on the different cell types studied, none of the differentially expressed genes identified in these studies overlapped with those identified in our DRG transcriptome; consequently, GO terms enriched in these studies were also distinct. However, compared with a study which analysed whole porcine skin following a bromine-induced chemical burn,44 our GO analysis yielded similar pathways and biological functions. These included the terms ‘axonal guidance’ and ‘cell signalling’, suggesting convergent molecular changes in both burn models. However, while “cancer” was a biological function highly enriched in the porcine skin, this was not the case in our study, possibly due to the nature of the bromine burn. Similar term enrichment was also found in a transcriptomic analysis of skeletal muscle both at the burn site and distant from the injury in mice,45 which – similar to our findings – also identified the enriched terms ‘stress response’ and ‘defense response’.
Overall, we detected 30 genes that were differentially up-regulated in DRGs following a first-degree burn. This number is likely a reflection of the relatively mild injury induced. The biological processes enriched in our dataset exhibited an inflammatory and regenerative profile consistent with peripheral nerve injury and regeneration, as well as synaptic signalling and neuropeptide secretion indicative of functional sensitisation. Up-regulation of genes associated with neuronal damage, including Atf3,46 Ecel147 and Star,48 support the view that at least some sensory projections are directly injured during a burn.13 Interestingly, although voltage-gated sodium channels (NaV) have previously been identified as crucial for burn-induced thermal allodynia at the behavioural level,13 gene expression levels of all genes coding for NaV channels were unchanged.
Potential pain-related genes identified from the burn-injury transcriptome included neuropeptide Y (Npy), neurotensin precursor (Nts), galanin (Gal), CCK 2 receptor (Cckbr), NMDA 2B receptor (Grin2b) and glutamic acid decarboxylase 2 (Gad2). Npy, Nts and Gal in particular are well known to be involved in nociceptive signalling pathways. Up-regulation of Npy has been consistently observed in inflammatory and neuropathic pain models and appears to play an important role in spinal nociceptive transmission as well as recovery from hyperalgesia.49 In contrast, the roles of Nts and Gal in pain are more complex, with both peptides reported to mediate pro-algesic as well as analgesic effects.50–52 NMDA receptors are widespread throughout the nervous system with NMDA2b, the protein encoded by Grin2b, implicated in glutamate-mediated hyperalgesia53 as well as use-dependent spinal sensitisation and long-term potentiation.54 Ketamine, a non-selective NMDA receptor antagonist, has also been used in burn pain with some success.8,38 While NMDA receptors are typically associated with hyper-excitability and excitatory neurotransmission, glutamic acid decarboxylase (GAD) converts glutamate to the inhibitory neurotransmitter γ-aminobutyric acid (GABA), and altered GAD expression is likely to result in dysregulation of excitatory and inhibitory neurotransmission. Accordingly, epigenetic suppression of Gad2 expression in central pain pathways has been linked to the development of persistent pain,30 while peripheral overexpression of Gad2 led to attenuation of neuropathic pain behaviours.55
Up-regulation of the gene encoding the CCK-2 receptor, a gastrin and cholecystokin receptor, was particularly interesting in light of our observation that high opioid doses are required to elicit analgesia in our burn pain model, since overexpression of the CCK-2 receptor is known to be associated with enhanced nociception and neuropathic pain27,56 and its activation interferes with opioid-induced analgesia.57,58 We thus investigated the analgesic and opioid-sparing effect of proglumide, the inhibitor of CCK receptors, on the basis that this compound is already in clinical use and efficacy in our model could be directly translated to improved management of burn pain in the clinic. Indeed, proglumide was efficacious in mechanical allodynia when administered as monotherapy, or together with oxycodone at doses that did not produce analgesic effects alone (1 mg/kg). This also corroborates with existing evidence that proglumide works synergistically with opioids to increase analgesic efficacy in other pain conditions.32,33 Interestingly, co-administration with low-dose oxycodone further improved PWF values, verging on statistical significance (P = 0.13). Co-administration of proglumide and oxycodone also alleviated thermal allodynia, although proglumide alone had no significant effect on thermal withdrawal thresholds or gait, and CCK receptors-mediated analgesia is likely to be modality-specific. Proglumide also did not induce any motor deficits in the animals, suggesting that CCK receptors could be potential targets for burn pain.
In light of clear clinical guidelines for opioid use in burn patients and previously described effects of CCK receptor inhibition on opioid analgesia and tolerance,32,33 combination treatment with proglumide and opioids may lead to clinical benefits for burn patients. However, it would be interesting to assess analgesic synergy with CCK receptor inhibitors and adjuvant treatments such as amitriptyline and gabapentin in future studies. In addition, as proglumide is a relatively non-selective CCK receptor antagonist, further studies using more selective antagonists such as L-365,260 are needed to better understand the involvement of CCK-2 specifically in burn-induced pain.
Mechanical and thermal allodynia are both abnormal stimulus-induced sensations that may mimic elevated pain experienced by patients during procedures such as dressing changes.4 However, stimulus-evoked allodynia may be poorly reflective of spontaneous or background pain and we thus evaluated the ability of the CatWalk XT system to detect burn-induced gait abnormalities as a surrogate for non-stimulus evoked pain. Consistent with previous studies, we found a good correlation between the development of altered CatWalk XT parameters, specifically those relating to paw contact with the glass walkway, with mechanical and thermal allodynia detected by more traditional behavioural assessment. However, gait abnormalities were only alleviated by high-dose oxycodone and amitriptyline, suggesting that these parameters are not simply a reflection of mechanical allodynia but represent a distinct pain modality. It is possible that this discrepancy arises from interference of local inflammation and paw swelling with normal gait. Accordingly, it is currently unclear whether analgesic effects detected using the CatWalk XT system can be translated to clinical efficacy in human burn patients more readily than effects detected using conventional tests such as the von Frey or Hargreaves apparatus. The at times poor predictive power of conventional assessment of evoked nociceptive behaviours suggests that effects on gait should be assessed routinely, as these parameters may reflect clinically meaningful measures of pain and analgesia.
In conclusion, we have described an optimised mouse model of burn-induced pain associated with mechanical allodynia, thermal allodynia, inflammation and gait abnormalities, all of which develop immediately after the thermal insult and last for at least four days. While rat models based on similar procedures have been reported,38,59,60 an advantage of murine models lies in the ability to evaluate the pathophysiological mechanisms using knockout animals, particularly in the absence of highly selective pharmacological modulators of novel burn pain targets identified in our transcriptome. Gene expression changes in DRG neurons define burn pain as a mixed inflammatory and neuropathic condition characterised by deficits in neuropeptide signalling, axon guidance and glutamatergic synaptic transmission. Proglumide, an antagonist of CCK receptors, effectively reversed burn-induced mechanical allodynia and produced additive analgesia with low-dose oxycodone. These data provide insight into the molecular nature of burn pain and may assist with future investigation into novel targets and potential analgesic compounds to improve clinical management of burn pain.
Supplementary Material
Acknowledgements
The authors would like to thank the Sequencing Facility at the Institute for Molecular Bioscience, University of Queensland, for their technical help with sequencing and transcriptomic analysis.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project is funded by an Australian Research Council Future Fellowship (IV), a NHMRC Senior Principle Research Fellowship (RJL), the University of Queensland Centre for Pain Research Funds and a UQ Research Scholarship (KY).
References
- 1.Mock C, Peck M, Peden M, et al. A WHO plan for burn prevention and care, Geneva, Switzerland: World Health Organization, 2008. [Google Scholar]
- 2.Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2011; 37: 1087–1100. [DOI] [PubMed] [Google Scholar]
- 3.Summer GJ, Puntillo KA, Miaskowski C, et al. Burn Injury pain: the continuing challenge. J Pain 2007; 8: 533–548. [DOI] [PubMed] [Google Scholar]
- 4.Carrougher GJ, Ptacek JT, Sharar SR, et al. Comparison of patient satisfaction and self-reports of pain in adult burn-injured patients. J Burn Care Rehabil 2003; 24: 1–8. [DOI] [PubMed] [Google Scholar]
- 5.Choinière M, Melzack R, Rondeau J, et al. The pain of burns: characteristics and correlates. J Trauma 1989; 29: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 6.Fischer TZ, Waxman SG. Extraterritorial temperature pain threshold abnormalities in subjects with healed thermal injury. J Rehabil Res Dev 2012; 49: 515–522. [DOI] [PubMed] [Google Scholar]
- 7.Abdi S, Zhou Y. Management of pain after burn injury. Curr Opin Anaesthesiol 2002; 15: 563–567. [DOI] [PubMed] [Google Scholar]
- 8.Retrouvey H, Shahrokhi S. Pain and the thermally injured patient—a review of current therapies. J Burn Care Rehabil 2015; 36: 315–323. [DOI] [PubMed] [Google Scholar]
- 9.Gray P, Williams B, Cramond T. Successful use of gabapentin in acute pain management following burn injury: a case series. Pain Med 2008; 9: 371–376. [DOI] [PubMed] [Google Scholar]
- 10.Wibbenmeyer L, Eid A, Liao J, et al. Gabapentin is ineffective as an analgesic adjunct in the immediate postburn period. J Burn Care Res 2014; 35: 136–142. [DOI] [PubMed] [Google Scholar]
- 11.Deuis JR and Vetter I. The thermal probe test: A novel behavioral assay to quantify thermal paw withdrawal thresholds in mice. Temperature 2016. [DOI] [PMC free article] [PubMed]
- 12.Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete Freund’s adjuvant-induced arthritis using the CatWalk system. BMC Musculoskelet Disord 2013; 14: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shields SD, Cheng X, Uceyler N, et al. Sodium channel Nav1.7 is essential for lowering heat pain threshold after burn injury. J Neurosci 2012; 32: 10819–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxf) 2013; 29: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics (Oxf) 2009; 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics (Oxf) 2015; 31: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics (Oxf) 2009; 25: 1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 2015; 43: D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuma T, Kamoda H, Miyagi M, et al. Comparison of catwalk analysis and von Frey testing for pain assessment in a rat model of nerve crush plus inflammation. Spine 2013; 38: E919–E924. [DOI] [PubMed] [Google Scholar]
- 22.Chiang C-Y, Sheu M-L, Cheng F-C, et al. Comprehensive analysis of neurobehavior associated with histomorphological alterations in a chronic constrictive nerve injury model through use of the CatWalk XT system. J Neurosurg 2014; 120: 250–262. [DOI] [PubMed] [Google Scholar]
- 23.Naveilhan P, Hassani H, Lucas G, et al. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature (Lond) 2001; 409: 513–517. [DOI] [PubMed] [Google Scholar]
- 24.Gui X, Carraway RE, Dobner PR. Endogenous neurotensin facilitates visceral nociception and is required for stress-induced antinociception in mice and rats. Neuroscience 2004; 136: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 25.Kerr BJ, Gupta Y, Pope R, et al. Endogenous galanin potentiates spinal nociceptive processing following inflammation. Pain 2001; 93: 267–277. [DOI] [PubMed] [Google Scholar]
- 26.Kerr BJ, Cafferty WBJ, Gupta YK, et al. Galanin knockout mice reveal nociceptive deficits following peripheral nerve injury. Eur J Neurosci 2000; 12: 793–802. [DOI] [PubMed] [Google Scholar]
- 27.Kurrikoff K, Koks S, Matsui T, et al. Deletion of the CCK2 receptor gene reduces mechanical sensitivity and abolishes the development of hyperalgesia in mononeuropathic mice. Eur J Neurosci 2004; 20: 1577–1586. [DOI] [PubMed] [Google Scholar]
- 28.Wei F, Wang G-D, Kerchner GA, et al. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci 2001; 4: 164–169. [DOI] [PubMed] [Google Scholar]
- 29.Wainai T, Takeuchi T, Seo N, et al. Regulation of acute nociceptive responses by the NMDA receptor GluRa2 subunit. Neuroreport 2001; 12: 3169–3172. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Cai Y-Q, Zou F, et al. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 2011; 17: 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaCroix-Fralish ML, Ledoux JB, Mogil JS. The pain genes database: an interactive web browser of pain-related transgenic knockout studies. Pain 2007; 131: 3e1–3e4. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein ZP, Yucht S, Battista E, et al. Proglumide as a morphine adjunct in cancer pain management. J Pain Symptom Manage 1998; 15: 314–320. [DOI] [PubMed] [Google Scholar]
- 33.McCleane GJ. The cholecystokinin antagonist proglumide enhances the analgesic efficacy of morphine in humans with chronic benign pain. Anesth Analg 1998; 87: 1117–1120. [PubMed] [Google Scholar]
- 34.Wasiak J, Spinks A, Ashby K, et al. The epidemiology of burn injuries in an Australian setting, 2000–2006. Burns 2009; 35: 1124–1132. [DOI] [PubMed] [Google Scholar]
- 35.Sharar SR, Carrougher GJ, Selzer K, et al. A comparison of oral transmucosal fentanyl citrate and oral oxycodone for pediatric outpatient wound care. J Burn Care Rehabil 2002; 23: 27–31. [DOI] [PubMed] [Google Scholar]
- 36.Cuignet O, Pirson J, Soudon O, et al. Effects of gabapentin on morphine consumption and pain in severely burned patients. Burns 2006; 33: 81–86. [DOI] [PubMed] [Google Scholar]
- 37.Werner MU, Perkins FM, Holte K, et al. Effects of gabapentin in acute inflammatory pain in humans. Reg Anesth Pain Med 2001; 26: 322–328. [DOI] [PubMed] [Google Scholar]
- 38.Oatway M, Reid A, Sawynok J. Peripheral antihyperalgesic and analgesic actions of ketamine and amitriptyline in a model of mild thermal injury in the rat. Anesth Analg 2003; 97: 168–173. [DOI] [PubMed] [Google Scholar]
- 39.Minami K, Hasegawa M, Ito H, et al. Morphine, oxycodone, and fentanyl exhibit different analgesic profiles in mouse pain models. J Pharmacol Sci 2009; 111: 60–72. [DOI] [PubMed] [Google Scholar]
- 40.Yang P-P, Yeh G-C, Huang EY-K, et al. Effects of dextromethorphan and oxycodone on treatment of neuropathic pain in mice. J Biomed Sci 2015; 22: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bittner EA, Shank E, Woodson L, et al. Acute and perioperative care of the burn-injured patient. Anesthesiology (Hagerst) 2015; 122: 448–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gragnani A, Cezillo MVB, da Silva IDCG, et al. Gene expression profile of cytokines and receptors of inflammation from cultured keratinocytes of burned patients. Burns 2014; 40: 947–956. [DOI] [PubMed] [Google Scholar]
- 43.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med 2011; 208: 2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price JA, Rogers JV, McDougal JN, et al. Gene expression analysis of bromine-induced burns in porcine skin. Toxicol Lett (Shannon) 2008; 182: 69–78. [DOI] [PubMed] [Google Scholar]
- 45.Padfield KE, Zhang Q, Gopalan S, et al. Local and distant burn injury alter immuno-inflammatory gene expression in skeletal muscle. J Trauma Inj Infect Crit Care 2006; 61: 280–292. [DOI] [PubMed] [Google Scholar]
- 46.Kiryu-Seo S, Kato R, Ogawa T, et al. Neuronal injury-inducible gene is synergistically regulated by ATF3, c-Jun, and STAT3 through the Interaction with Sp1 in damaged neurons. J Biol Chem 2008; 283: 6988–6996. [DOI] [PubMed] [Google Scholar]
- 47.Kiryu-Seo S. Identification and functional analysis of damage-induced neuronal endopeptidase (DINE), a nerve injury associated molecule. Anat Sci Int 2006; 81: 1–6. [DOI] [PubMed] [Google Scholar]
- 48.Mirzatoni A, Spence RD, Naranjo KC, et al. Injury-induced regulation of steroidogenic gene expression in the cerebellum. J Neurotrauma 2010; 27: 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solway B, Bose SC, Corder G, et al. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A 2011; 108: 7224–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobner PR. Neurotensin and pain modulation. Peptides (N Y) 2006; 27: 2405–2414. [DOI] [PubMed] [Google Scholar]
- 51.Liu H-X, Hökfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci 2002; 23: 468–474. [DOI] [PubMed] [Google Scholar]
- 52.Xu XJ, Hökfelt T, Wiesenfeld-Hallin Z. Galanin and spinal pain mechanisms: past, present, and future. Experientia Supplementum 2010; 102: 39–50. [DOI] [PubMed] [Google Scholar]
- 53.Gabra BH, Kessler FK, Ritter JK, et al. Decrease in N-Methyl-D-aspartic acid receptor-NR2B subunit levels by intrathecal short-hairpin RNA blocks group I metabotropic glutamate receptor-mediated hyperalgesia. J Pharmacol Exp Ther 2007; 322: 186–194. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen LM, Gjerstad J. Spinal cord long-term potentiation is attenuated by the NMDA-2B receptor antagonist Ro 25-6981. Acta Physiol 2008; 192: 421–427. [DOI] [PubMed] [Google Scholar]
- 55.Kim J, Kim SJ, Lee H, et al. Effective neuropathic pain relief through sciatic nerve administration of GAD65-expressing rAAV2. Biochem Biophys Res Commun 2009; 388: 73–78. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Gardell S, Zhang D, et al. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain 2009; 132: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benedetti F, Amanzio M, Thoen W. Disruption of opioid-induced placebo responses by activation of cholecystokinin type-2 receptors. Psychopharmacology 2011; 213: 791–797. [DOI] [PubMed] [Google Scholar]
- 58.Grastilleur S, Mouledous L, Bedel J, et al. Role of kinin B2 receptors in opioid-induced hyperalgesia in inflammatory pain in mice. Biol Chem Hoppe Seyler 2013; 394: 361–368. [DOI] [PubMed] [Google Scholar]
- 59.Sorkin L, Svensson CI, Jones-Cordero TL, et al. Spinal p38 mitogen-activated protein kinase mediates allodynia induced by first-degree burn in the rat. J Neurosci Res 2009; 87: 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jun JH, Yaksh TL. The effect of lntrathecal gabapentin and 3-isobutyl gamma-aminobutyric acid on the hyperalgesia observed after thermal injury in the rat. Anesth Analg 1998; 86: 348–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.