Abstract
Background:
The present work was planned to evaluate the antihyperglycemic, lipid-lowering, and antioxidant effect of Lactobacillus casei and Bifidobacterium bifidum in streptozotocin (STZ)-induced diabetic rats.
Methods:
Single daily dose of 1 × 107 cfu/ml of L. casei and B. bifidum alone and in combination of both was given to Wistar rats orally by gavaging for 28 days. Glucose tolerance test, fasting blood glucose (FBG), lipid profile, and glycosylated hemoglobin (HbA1c) were measured from blood. Glycogen from thigh muscles and liver and oxidative stress parameters from pancreas were analyzed.
Results:
Administration of L. casei and B. bifidum alone and in combination of both to diabetic rats decreased serum FBG (60.47%, 55.89%, and 56.49%, respectively), HbA1c (28.11%, 28.61%, and 28.28%), total cholesterol (171.69%, 136.47%, and 173.58%), triglycerides (9.935%, 8.58%, and 7.91%), low-density lipoproteins (53.27%, 53.35%, and 52.91%) and very low-density lipoproteins (10%, 8.58%, and 11.15%, respectively) and increased high-density lipoproteins (13.73%, 15.47%, and 15.47%), and insulin (19.50%, 25.80%, and 29.47%, respectively). The treatment also resulted in increase in muscle (171.69%, 136.47%, and 173.58%) and liver (25.82%, 6.63%, and 4.02%) glycogen level. The antioxidant indexes in pancreas of diabetic rats returned to normal level with reduction in lipid peroxidation (30.89%, 46.46%, and 65.36%) and elevation in reduced glutathione (104.5%, 161.34%, and 179.04%), superoxide dismutase (38.65%, 44.32%, and 53.35%), catalase (13.08%, 27%, and 31.52%), glutathione peroxidase (55.56%, 72.23%, and 97.23%), glutathione reductase (49.27%, 88.40%, and 110.86%), and glutathione-S-transferase (140%, 220%, and 246.6%, respectively) on treatment with L. casei, B. bifidum, and combination treatment.
Conclusions:
Administration of L. casei and B. bifidum alone and in combination of both ameliorated hyperglycemia, dyslipidemia, and oxidative stress in STZ-induced diabetic Wistar rats.
Keywords: Antihyperglycemic, antioxidant, Bifidobacterium bifidum, dyslipidemia, Lactobacillus casei
INTRODUCTION
Diabetes mellitus (DM) is one of the most progressive metabolic disorders associated with constant high blood glucose level, adversely affecting kidney, retina, pancreas, and other organs.[1,2] High level of blood glucose in DM enhances the oxidative stress and generation of glycoxidation products.[3] DM-induced oxidative stress plays an important role in pathophysiology of organ damage.[4] Increase in oxidative stress and free radicals as well as reduction in activities of free radical scavenging enzymes in DM has been demonstrated in animal models[5] and human subjects.[6] Reactive oxygen species (ROS) are involved in β-cell dysfunction,[7] β-cell apoptotic pathways,[8] impaired insulin synthesis, and insulin resistance.[9] The increase in blood glucose level or glycated products enhances lipid peroxidation (LPO), which in turn may further increase the possibilities of formation of advanced glycation end-products.[10]
Controlling blood glucose level is paramount in improving quality of life and preventing DM-related microvascular and macrovascular complications in diabetic patients. In earlier times, before the discovery of insulin, nutritional therapy has helped in the management of DM-related complications. Insulin and other oral/injectable hypoglycemic agents are currently being used for controlling Type 1 DM as well as Type 2 DM (T2DM). Recently, Glucagon-like peptide-1 analogs (exenatide and liraglutide) as well as dipeptidyl peptidase-4 inhibitors (sitagliptin and saxagliptin) have been developed for management of T2DM.[11] The high cost of treatment and several side effects such as abrupt hypoglycemia, lactic acidosis, multiple organ damage, and digestive discomfort, associated with the prolonged use of present-day antidiabetic drugs, have necessitated the search for safer and alternate methods for the management of DM.
Probiotics are microbial dietary supplements that benefit the host through their effect on the intestinal tract.[12] Lactobacilli and bifidobacteria are important probiotic strains useful in the promotion of human health.[13] Probiotics are projected as potential modulators of the gut microbial flora in a beneficial manner, antioxidant, anti-inflammatory, and antihyperlipidemic effects. Consumption of probiotics is reported to protect pancreatic β-cells from oxidative damage, delaying the onset of T2DM and prevented microvascular and macrovascular complications in DM.[14,15] Probiotic-containing foods have been reported to suppress oxidative stress. It has been shown that Lactobacillus casei decreased the oxidative stress[16] and suppressed the effector functions of CD4+ T-cells, accompanied by reducing the proinflammatory molecules,[17] thus having antioxidant and immunomodulatory effects.
Considering the antioxidative potential of probiotics, the present study was planned to evaluate the antihyperglycemic, antioxidant, and lipid-lowering effect of L. casei, Bifidobacterium bifidum alone and in combination in diabetic Wistar rats.
METHODS
Bacterial strains
L. casei (NCDC-017) and B. bifidum (NCDC-231) used in the present study were obtained from the Division of Dairy Microbiology, National Dairy Research Institute, Karnal, India.
Animals
Male Wistar rats, weighing about 150–200 g, were used in the present study. Animals were obtained from the Animal Research Division, Central Drug Research Institute, Lucknow (India). The Institutional Animal Ethical Committee wide reference no. BU/Pharma/IAEC/11/037 approved the use of animals for this project.
Chemicals
Streptozotocin (STZ) was purchased from Sigma Aldrich (St. Louis, MO, USA). Total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TGs), glycosylated hemoglobin (HbA1c), very low-density lipoprotein (VLDL), and fasting blood glucose (FBG) were assayed using standard kits purchased from various firms. Muscle and liver glycogen and antioxidant enzymes were estimated using chemicals of high purity.
Induction of diabetes
Freshly prepared STZ solution in 0.1 M citrate buffer, pH 4.5 was injected (50 mg/kg bodyweight) intraperitoneally to overnight starved rats. To establish the diabetic state, FBG and postparandial glucose were measured regularly, and till, stable hyperglycemia was achieved. Animals with stabilized FBG equal to/more than 250 mg/dL were used in the present study.
Preparation of bacterial stock for dosing
Lyophilized L. casei and B. bifidum were cultured in de Mann Rogosa Sharpe (MRS) broth at 37°C in anaerobic condition for 48 h. One loopful of this culture was suspended in 1 ml of sterilized distilled water. The volume of this suspension was made to 10 ml with sterilized distilled water. Five successive serial dilutions of 1/10 each were prepared in distilled water. From the last (sixth) dilution, 100 μl of suspension was plated on MRS agar. The plate showed 56 colonies after incubation. The last dilution concentration was calculated as 56 × 107 cfu/ml. From this plate, one colony was picked up aseptically and suspended in 1 ml of sterilized distilled water to obtain 1 × 107 cfu/ml concentration.
Dosing of bacterial strain
Single daily dose of L. casei and B. bifidum 1 × 107 cfu/ml suspended in 1 ml of distilled water was given to rats orally by gavaging for 28 days.
Experimental design
The experimental groups with six rats each were prepared as per given schedule.
At the end of the experiment (on 28th day), the overnight fasted rats were sacrificed under mild ether anesthesia. Blood was drawn by heart puncture and collected in ethylenediaminetetraacetic acid (EDTA) vials for estimation of FBG, HbA1c and without EDTA vials for serum isolation for performing lipid profile tests and serum insulin. The liver and thigh muscle were removed, washed with ice-cold saline, and used for glycogen estimation. The pancreas was removed, washed with ice-cold saline, homogenized, and used for biochemical estimations.
Glucose tolerance test
One day before the end of the study, the rats were fasted overnight and FBG was measured by withdrawing blood from tail vein. This FBG was taken as 0 h value for glucose tolerance test (GTT). One milliliter of aqueous solution of glucose (2 mg/ml) was given orally to fasted rats, and blood glucose level was measured at the intervals of 1 h, up to 3 h. The percentage fall in blood glucose level observed between 0 and 1, 2, and 3 h, among various groups compared to diabetic control, was used for assessing glucose tolerance. Glucose concentrations were measured by glucose oxidase method.
Estimation of fasting blood glucose, lipid profile, and glycosylated hemoglobin
FBG (glucose oxidase method), TC, TG, and HDL levels were measured by kit method (Span Diagnostic Reagent Kit, India) and VLDL and LDL were calculated by Friedewald's formula. HbA1c was measured using Euro diagnostic system kit. Liver and muscle glycogen were estimated spectophotometerically as per the standard protocol,[18] and serum insulin levels was measured by rat-specific insulin ELISA kit of Qayee-Bio.
Biochemical estimations from pancreas
Preparation of homogenate
Pancreas was homogenized thoroughly in 0.1 M phosphate buffer (pH 7.4 + 150 mM KCl) to make 10% homogenate. A portion of this homogenate was used for estimation of LPO and GSH and remaining was centrifuged at 9000 rpm for 20 min to obtain supernatant S9 fraction. The (S9) fraction was used for enzyme estimation.
Estimation of oxidative stress parameters
LPO was estimated as described by Ohkawa et al.[19] The activity of superoxide dismutase (SOD) was assayed by the method of Kakkar et al.[20] Catalase (CAT) was assayed by Sinha.[21] The glutathione peroxidase (GPx) and glutathione-S-transferase (GST) activities were measured by the methods of Rotruck et al.[22] and Habig et al.,[23] respectively.
Statistical calculations
Statistical calculations were done using GraphPad InStat software Inc., version 3.06, San Diego, USA. Results were represented as mean ± standard error of mean of observed values. One-way analysis of variance was calculated, and the treatment groups were compared with control group using Dunnett's test.
RESULTS
Effect on fasting blood glucose, glycosylated hemoglobin, insulin, and lipid profile
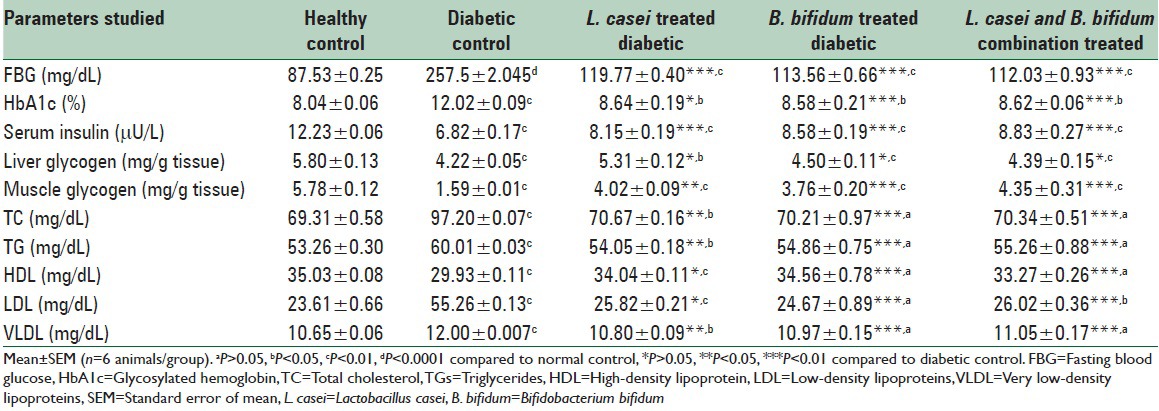
Significant (P < 0.0001, 194.2%) increase in FBG was observed in diabetic rats as compared to healthy control, confirming the diabetic state. Significant (P < 0.01) decrease in FBG level was observed on L. casei, B. bifidum, and combination treatment (60.47%, 55.89%, and 56.49%, respectively) as compared to diabetic rats. Significant (P < 0.01) increase in level of HbA1c (49.50%) in diabetic rats was observed as compared to control group. Nonsignificant (P > 0.05) decrease in L. casei (28.11%) and significant (P < 0.01) decrease in B. bifidum (28.61%) and combination (28.28%) treated rats were observed in HbA1c as compared to diabetic control. Serum insulin level was also significantly (P < 0.01) increased in L. casei, B. bifidum, and combination treated rats (19.50%, 25.80%, and 29.47%, respectively) as compared to diabetic control. There was a significant (P < 0.01) increase in the level of serum TC, TG, LDL, and VLDL and significant (P < 0.01) decrease in serum HDL in diabetic rats as compared to control group. Contrarily, there was a significant (P < 0.01) decrease (171.69%, 9.935%, 10%) in TC, TG, VLDL, nonsignificant (P > 0.05) decrease in LDL (53.27%), and a significant increase in serum HDL (13.73%) as compared to diabetic control after L. casei administration while in case of B. bifidum treatment, a significant (P < 0.01) decrease in TC, TG, LDL, VLDL (136.47%, 8.58%, 53.35%, 8.58%) and significant (P < 0.01) increase in HDL (15.47%) and in rats fed with combination of probiotic stains showed significant (P < 0.01) decrease in TC, TG, LDL, VLDL (173.58%, 7.91%, 52.91%, 11.15%) and significant (P < 0.01) increase in HDL (15.47%) as compared to diabetic rats [Table 1].
Table 1.
Effect of Lactobacillus casei and Bifidobacterium bifidum treatment on fasting blood glucose, glycosylated hemoglobin, serum insulin, muscle and liver glycogen, and lipid profile in normal and diabetic Wistar rats
Effect on muscle and liver glycogen content
Significant increase in muscle glycogen was observed on administration of L. casei (P < 0.05, 171.69%), B. bifidum (P < 0.01, 136.47%), and combination (P < 0.01, 173.58%) as compared to diabetic control. Significant (P > 0.05) increase in liver glycogen was observed in L. casei, B. bifidum, and combination treated rats (25.82, 6.63, and 4.02%, respectively) treated rats as compared to diabetic control [Table 1].
Effect on glucose tolerance
Diabetic rats treated with combination of L. casei and B. bifidum produced highest fall of 66.42% followed by B. bifidum (54.87%) and L. casei (31.85%) as compared to diabetic control between 0 and 1 h during GTT [Figure 1].
Figure 1.
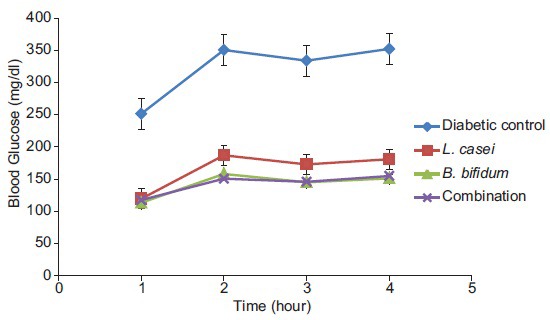
Effect of Lactobacillus casei and Bifidobacterium bifidum treatment on glucose tolerance test in diabetic rats
Effect on oxidative stress in pancreas
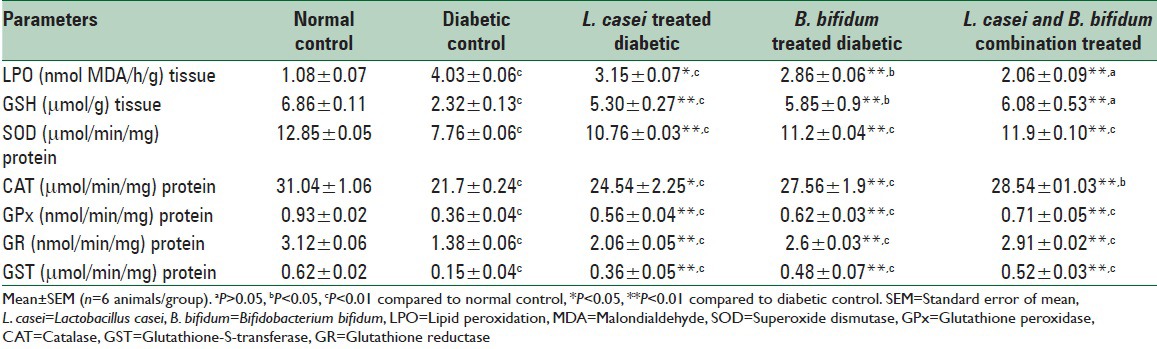
Diabetic rats showed a significant increase (P < 0.01) in the level of LPO (306.4%) as compared to healthy rats. Administration of L. casei significantly (P < 0.05) decreased the level of LPO (30.89%) whereas B. bifidum and combination of both significantly (P < 0.01) decreased the level of LPO (46.46% and 65.36%, respectively) in the pancreatic tissue as compared to diabetic control. Significant (P < 0.01) decrease in the concentration of GSH (68.22%) was observed in the diabetic rats as compared to healthy rats. Administration of L. casei, B. bifidum, and combination of both showed significant (P < 0.01) increase in the level of GSH in the pancreas (104.5%, 161.34%, and 179.04%, respectively) in STZ-induced diabetic rats as compared to diabetic control. The level of SOD in diabetic rats was significantly (P < 0.01) decreased (39.61%) as compared to healthy rats whereas administration of L. casei, B. bifidum, and combination of both in diabetic rats showed significant (P < 0.01) increase in the level of SOD in the pancreas (38.65%, 44.32%, and 53.35%, respectively). The diabetic rats showed significant (P < 0.01) decrease in the concentration of CAT (30.09%) as compared to normal rats and after administration of L. casei showed significant (P < 0.05, 13.08%) increase whereas B. bifidum and combination of both showed significant (P < 0.01) increase in STZ-induced diabetic rats in the pancreatic tissue (27% and 31.52%, respectively) when compared to diabetic control. There was a significant (P < 0.01) decrease in the concentration of GPx (61.29%) in the diabetic rats as compared to normal rats while the oral administration of L. casei, B. bifidum, and combination of both in STZ-induced diabetic rats showed significant (P < 0.01) increase in the pancreatic tissue (55.56%, 72.23%, and 97.23%, respectively) as compared to diabetic control. There was a significant (P < 0.05) decrease in the concentration of glutathione reductase (GR) (55.76%) in the diabetic rats as compared to normal rats while the oral administration of L. casei, B. bifidum, and combination of both in STZ-induced diabetic rats showed significant (P < 0.01) rise in the level of GR in the pancreas (49.27%, 88.40%, and 110.86%, respectively) as compared to diabetic control. The level of GST in diabetic rats was significantly (P < 0.01) decreased in diabetic rats (75.8%) as compared to normal rats whereas oral administration of L. casei, B. bifidum, and combination of both in STZ-induced diabetic rats showed significant (P < 0.01) increase in the level of GST in the pancreas (140%, 220%, and 246.6% respectively) as compared to diabetic control [Table 2].
Table 2.
Effect of Lactobacillus casei, Bifidobacterium bifidum and combination of both bacterial strains on oxidative stress of pancreatic tissue in Wistar rats
DISCUSSION
Probiotics have been reported to confer beneficial effects in various clinical conditions.[24,25] Recently, Lactobacilli and bifidobacteria show beneficial effects in T2DM.[26] Yadav et al.[27] reported that diet fortified with dahi, containing L. acidophilus and L. casei, significantly delayed high fructose-induced glucose intolerance, hyperglycemia, hyperinsulinemia, and dyslipidemia in rats.
In the present study, the diabetic rats showed elevated level of FBG during 28 days experiment while administration of L. casei and B. bifidum at 1 × 107 cfu/ml resulted in a significant decrease in FBG. The findings indicated that L. casei and B. bifidum lowered FBG in 28-day treatment and helped in the management of DM. Lowering in FBG has been reported on probiotic supplement, containing L. plantarum DSM 15313, to high-fat diet fed C57BL/6J mice[28] and probiotic yogurt containing L. acidophilus La5 and Bifidobacterium lactis Bb12.[29]
High amount of blood glucose in DM reacts with other biomolecules to form advanced glycated end-products (AGEs), chiefly HbA1c. The formation/activation of AGEs, transcription factors, and protein kinase C result in increase in oxidative stress.[30,31,32] The increase in the level of HbA1c in the diabetic rats observed in the present study may be due to increase in blood glucose level. Administration of B. bifidum and L. casei alone and in combination of both to diabetic rats significantly reduced HbA1c in diabetic rats. This reduction in HbA1c indicated decreased glycation of proteins, due to lowering of blood glucose for longer period.
The concentrations of lipids, such as TC, TG, and LDL, were significantly high, and HDL was low in diabetic rats compared to control group. The variations in lipid level are due to derangements in metabolic and regulatory mechanisms during diabetic state.[33] Significant decrease in the level of serum TC, TG, LDL, and VLDL and increase in level of HDL were observed in diabetic rats after administration of B. bifidum, L. casei alone and in combination of both. Several mechanisms for the decrease in cholesterol concentration by probiotics have been proposed. It may be due to decrease in cholesterol absorption from intestine[34,35,36] or by enzymatic deconjugation of bile acids by bile salt hydrolase, interfering with the enterohepatic circulation of bile salts. Bifidobacterium sp. removed cholesterol from a broth containing bile salts, by assimilating cholesterol.[37] Consumption of fermented milk containing L. acidophilus and fructooligosaccharides significantly decreased TC concentration after 3 weeks.[38] Studies also indicated that probiotics improve HDL concentration.[39] Impairment in insulin secretion resulted in increased metabolism of lipids in adipose tissue and their release in plasma. A significant increase in insulin level in L. casei, B. bifidum, and combination treated group was observed as compared to untreated diabetic rats. It has been reported that probiotic bacteria improved insulin sensitivity by attenuating local inflammation.[40] The increased number of Bifidobacterium species improves glucose tolerance and insulin secretion.[41] Higher level of blood glucose induces insulin to synthesize glycogen in liver.[42] Depleted insulin levels in diabetes rats have resulted in reduction of muscle and liver glycogen level, in the present study. The decrease in glycogen may be either due to slowdown of the insulin-activated glycogenesis pathway or inactivation of the glycogen synthetase due to oxidative stress induced by diabetic state. Our study showed a significant increase in tissue glycogen in L. casei, B. bifidum, and combination treated rats possibly due to the reactivation of glycogen synthase system or the insulin activated glycogenesis pathway.
Treatment with the probiotic bacteria improved glucose tolerance compared to untreated diabetic rats in GTT. The study indicated enhancement in glucose utilization on treatment with L. casei and B. bifidum. The increase in serum insulin level, muscle, and liver glycogen content and decrease in cholesterol may be collectively responsible for the improvement in glucose tolerance after treatment with L. casei and B. bifidum.
Antioxidants have been attributed for the alleviation of DM complications.[43] The autooxidation of glucose in DM generates high amount of oxygen-free radicals. These oxygen-free radicals are responsible for oxidative deterioration of polyunsaturated lipids named as LPO.[44] In the present study, the level of LPO was increased in pancreas after STZ-exposure to rats which might be due to an increase in the generation of free radicals by STZ. Administration of L. casei and B. bifidum alone and in combination of both significantly decreased the elevated level of LPO in diabetic rats. The above result suggests that probiotics exerted antioxidant activity and protected pancreas from LPO. Significant lowering in TBARS level in pancreatic tissue has been reported in diabetic rats fed with probiotic dahi.[45] Reduced glutathione (GSH) is an important molecule involved in cellular defense against ROS.[46] GSH is a scavenger of free radicals as well as a cosubstrate for peroxide detoxification by GPx.[47]
The DM state decreases antioxidant capacity of tissues and increases the deleterious effects of free radicals. The decrease in GSH level either due to high rate of utilization through GPx activity or lower production has been reported during oxidative stress.[48] The decrease in GSH level in the present study may be due to excessive free radical generation either by STZ exposure or by high glucose level. Administration of probiotic bacterial strain resulted in increase in GSH level in pancreatic tissue in diabetic rats. The elevation in GSH level may be due to increase in the biosynthesis of GSH or reduction in oxidative stress or may be both.
Excessive free radicals and glycation decreased the activity of GPx.[49] In our study, diabetic rats fed with L. casei, B. bifidum, and combination group showed highly significant increase in GPx activity compared to untreated diabetic rats. The probable reason for this increase may be the enhanced GSH biosynthesis and reduction in free radicals in diabetic rats on treatment with L. casei and B. bifidum. Ejtahed et al.[29] reported an increase in GPx level after consumption of probiotic yogurt in a study. SOD, an important enzyme in cellular defense, catalyzes the dismutation of superoxide radicals.[50] This enzyme detoxifies the superoxide anion, thus converting it into H2O2 and water. Administration of B. bifidum and L. casei alone and in combination of both showed highly significant increase in SOD in pancreatic tissue which may be either due to the increase in the activity of CAT and GPx or due to radical-induced activation. CAT is a hemeprotein enzyme involved in cellular defense, which catalyzes the reduction of hydrogen peroxides and protects the tissues from highly reactive hydroxyl radicals.[51] An excess amount of glucose in DM may lead to glycation of CAT, resulting in decrease of its activity.[29] In our study, administration of B. bifidum and L. casei alone and in combination, to diabetic rats, showed significant increase in CAT activity in pancreas. This increase in SOD and CAT level may be due to the reduction of glycation of these enzymes or reduction of reactive oxygen-free radicals. GR is an enzyme that reduces glutathione disulfide to the sulfhydryl form of GSH. GST catalyzes the conjugation of GSH with endogenous as well as xenobiotic substrates having electrophilic functional groups.[52] Exposure of rats to STZ caused significant reduction in GST and GR activities in the present study. The reduction in activities of these enzymes may be due to the increase in ROS and LPO as well as decrease in the level of GSH. Administration of B. bifidum and L. casei alone and in combination of both to diabetic rats showed significant increase in GR and GST activities in pancreatic tissue, indicating increase in antioxidant defense mechanism of the cell. It has been reported earlier that shrimps fed with Pediococcus acidilactici exhibited higher antioxidant defenses and lower oxidative stress level compared to the control group.[53]
CONCLUSIONS
The present study showed that administration of Lactobacillus casei and Bifidobacterium bifidum alone and in combination of both ameliorated hyperglycemia, dyslipidemia, and oxidative stress in STZ-induced diabetic Wistar rats.
Financial support and sponsorship
Dr. Rambir Singh is thankful to the University Grants Commission, Government of India, for providing financial support under major research project scheme Ref. No. 40-269/2011 (SR).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are thankful to Bundelkhand University, Jhansi, India for providing necessary facilities for this research.
REFERENCES
- 1.Lencioni C, Lupi R, Del Prato S. Beta-cell failure in type 2 diabetes mellitus. Curr Diab Rep. 2008;8:179–84. doi: 10.1007/s11892-008-0031-0. [DOI] [PubMed] [Google Scholar]
- 2.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. doi: 10.2337/diacare.28.5.995. [DOI] [PubMed] [Google Scholar]
- 3.Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990;173:932–9. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- 4.Baynes JW, Thorpe SR. The role of oxidative stress in diabetic complications. Curr Opin Endocrinol. 1999;3:277–84. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Bhardwaj P, Sharma P. Antioxidant and toxicological evaluation of Cassia sopherain streptozotocin-induced diabetic Wistar rats. Pharmacognosy Res. 2013;5:225–32. doi: 10.4103/0974-8490.118767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turk HM, Sevinc A, Camci C, Cigli A, Buyukberber S, Savli H, et al. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002;39:117–22. doi: 10.1007/s005920200029. [DOI] [PubMed] [Google Scholar]
- 7.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 8.Simmons RA. Developmental origins of diabetes: The role of oxidative stress. Free Radic Biol Med. 2006;40:917–22. doi: 10.1016/j.freeradbiomed.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Hicks M, Delbridge L, Yue DK, Reeve TS. Increase in crosslinking of nonenzymatically glycosylated collagen induced by products of lipid peroxidation. Arch Biochem Biophys. 1989;268:249–54. doi: 10.1016/0003-9861(89)90586-9. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Arif T, Khan I, Sharma P. Phytochemicals in antidiabetic drug discovery. J Biomed Ther Sci. 2014;1:1–33. [Google Scholar]
- 12.Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, et al. New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol. 2003;37:105–18. doi: 10.1097/00004836-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 14.Amdekar S, Singh V, Kumar A, Sharma P, Singh R. Lactobacillus acidophilus protected organs in experimental arthritis by regulating the pro-inflammatory cytokines. Indian J Clin Biochem. 2014;29:471–8. doi: 10.1007/s12291-013-0396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav H, Shalini J, Francesco M. Probiotics mediated modulation of gut flora might be biotherapeutical approach obesity and type 2 diabetes. Metabolomics. 2011;1:3. doi: 10.4172/2153-0769.1000107e. doi.org/10.4172/2153-0769.1000107e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villarini M, Caldini G, Moretti M, Trotta F, Pasquini R, Cenci G. Modulatory activity of a Lactobacillus casei strain on 1,2-dimethylhydrazine-induced genotoxicity in rats. Environ Mol Mutagen. 2008;49:192–9. doi: 10.1002/em.20367. [DOI] [PubMed] [Google Scholar]
- 17.So JS, Kwon HK, Lee CG, Yi HJ, Park JA, Lim SY, et al. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol. 2008;45:2690–9. doi: 10.1016/j.molimm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Seifter S, Dayton S, Novic B, Muntwyler E. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950;25:191–200. [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 21.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 22.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 23.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 24.Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62–8. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Mengheri E. Health, probiotics, and inflammation. J Clin Gastroenterol. 2008;42(Suppl 3(Pt 2)):S177–8. doi: 10.1097/MCG.0b013e31817eedc4. [DOI] [PubMed] [Google Scholar]
- 26.Aronsson L, Huang Y, Parini P, Korach-André M, Håkansson J, Gustafsson JÅ, et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4) PLoS One. 2010;5:E13087. doi: 10.1371/journal.pone.0013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav H, Jain S, Sinha PR. Anti-diabetic effect of probiotic dahi containing Lactobacillus acidophilus, Lactobacillus casei and Lactococcus lactis bacteria in high fructose diet fed rats. Nutrition. 2007;72:62–8. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Andersson U, Bränning C, Ahrné S, Molin G, Alenfall J, Onning G, et al. Probiotics lower plasma glucose in the high-fat fed C57BL/6J mouse. Benef Microbes. 2010;1:189–96. doi: 10.3920/BM2009.0036. [DOI] [PubMed] [Google Scholar]
- 29.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–43. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Chattopadhyay RR. Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract: Part V. J Ethnopharmacol. 1999;67:373–6. doi: 10.1016/s0378-8741(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 31.Alyssain D, Ibrahim K. A minor haemoglobin fraction and the level of fasting blood glucose. J Facul Med Baghdad. 1981;23:373–80. [Google Scholar]
- 32.Sheela CG, Augusti KT. Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium sativum Linn. Indian J Exp Biol. 1992;30:523–6. [PubMed] [Google Scholar]
- 33.Rajalingam R, Srinivasan N, Govindarajulu P. Effects of alloxan induced diabetes on lipid profiles in renal cortex and medulla of mature albino rats. Indian J Exp Biol. 1993;31:577–9. [PubMed] [Google Scholar]
- 34.Pereira DI, Gibson GR. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl Environ Microbiol. 2002;68:4689–93. doi: 10.1128/AEM.68.9.4689-4693.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liong MT, Shah NP. Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. Int Dairy J. 2005;15:391–8. [Google Scholar]
- 36.Ooi LG, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int J Mol Sci. 2010;11:2499–522. doi: 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahri K, Crociani J, Ballongue J, Schneider F. Effects of three strains of bifidobacteria on cholesterol. Lett Appl Microbiol. 1995;21:149–51. doi: 10.1111/j.1472-765x.1995.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 38.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. 2011;94:3288–94. doi: 10.3168/jds.2010-4128. [DOI] [PubMed] [Google Scholar]
- 39.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76:1249–55. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- 40.Naydenov K, Anastasov A, Avramova M, Mindov I, Tacheva T, Tolekova A, et al. Probiotics and diabetes mellitus. Trakia J Sci. 2012;10:300–6. [Google Scholar]
- 41.Yin YN, Yu QF, Fu N, Liu XW, Lu FG. Effects of four bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol. 2010;16:3394–401. doi: 10.3748/wjg.v16.i27.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klover PJ, Mooney RA. Hepatocytes: Critical for glucose homeostasis. Int J Biochem Cell Biol. 2004;36:753–8. doi: 10.1016/j.biocel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, et al. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2003;67:1421–4. doi: 10.1271/bbb.67.1421. [DOI] [PubMed] [Google Scholar]
- 44.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–24. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 45.Ivorra MD, Payá M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–75. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 46.Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res. 2008;75:189–95. doi: 10.1017/S0022029908003129. [DOI] [PubMed] [Google Scholar]
- 47.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winterbourn CC. Biothiols in Health and Disease. 1995:117–34. [Google Scholar]
- 49.Anuradha CV, Selvam R. Effect of oral methionine on tissue lipid peroxidation and antioxidants in alloxan induced diabetic rats. J Nutr Biochem. 1993;4:212. doi: 10.1016/0955-2863(90)90027-i. [DOI] [PubMed] [Google Scholar]
- 50.Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 51.Hodgson EK, Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: Inactivation of the enzyme. Biochemistry. 1975;14:5294–9. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- 52.Ji X, Zhang P, Armstrong RN, Gilliland GL. The three-dimensional structure of a glutathione S-transferase from the mu gene class. Structural analysis of the binary complex of isoenzyme 3.3 and glutathione at 2.2. A resolution. Biochemistry. 1992;31:10169–84. doi: 10.1021/bi00157a004. [DOI] [PubMed] [Google Scholar]
- 53.Castex M, Lemaire P, Wabete N, Chim L. Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol. 2010;28:622–31. doi: 10.1016/j.fsi.2009.12.024. [DOI] [PubMed] [Google Scholar]


