Abstract
Context:
The dermal papilla (DP) is a condensation of mesenchymal cells at the proximal end of the hair follicle, which determines hair shaft size and regulates matrix cell proliferation and differentiation. DP cells have the ability to regenerate new hair follicles. These cells tend to aggregate both in vitro and in vivo. This tendency is associated with the ability of papilla cells to induce hair growth. However, human papilla cells lose their hair-inducing activity in later passage number. Ovine DP cells are different from human DP cells since they do not lose their aggregative behavior or hair-inducing activity in culture. Nonetheless, our understanding of ovine DP cells is still limited.
Aim:
The aim of this study was to observe the expression of established DP markers in ovine cells and their association with aggregation.
Subjects and Methods:
Ovine DP cells from three different sheep were compared. Histochemistry, immunoflourescence, and polymerase chain reaction experiments were done to analyze the DP markers.
Results:
We found that ovine DP aggregates expressed all the 16 markers evaluated, including alkaline phosphatase and versican. Expression of the versican V0 and V3 isoforms, neural cell adhesion molecule, and corin was increased significantly with aggregation, while hey-1 expression was significantly decreased.
Conclusions:
Overall, the stable expression of numerous markers suggests that aggregating ovine DP cells have a similar phenotype to papillae in vivo. The stability of their molecular phenotype is consistent with their robust aggregative behavior and retained follicle-inducing activity after prolonged culture. Their phenotypic stability in culture contrasts with DP cells from other species, and suggests that a better understanding of ovine DP cells might provide opportunities to improve the hair-inducing activity and therapeutic potential of human cells.
Keywords: Aggregation, dermal papilla cells, ovine, versican
INTRODUCTION
The production of hair fiber depends on a series of epithelial/mesenchymal interactions within the hair follicle.[1] Dermal papilla (DP) cells have been reported to have the ability to regenerate new hair follicles when grafted into the skin. These cells tend to aggregate both in vitro and in vivo; moreover, this tendency is associated with the ability of papilla cells to induce hair growth.[2,3] The possible therapeutic use of papilla cell grafts to treat hair loss is limited because human papilla cells lose their aggregative and hair-inductive ability as they multiply in culture. In contrast, we have previously found that papilla cells from sheep are more stable in vitro and retain these abilities after extensive multiplication.[4] Thus, a comparison of ovine cells with papilla cells from other species should provide insights into the molecular requirements for papilla cell aggregation and hair induction. Herein, we describe the expression of established papilla markers from other species in ovine cells in different states of aggregation.
Several molecular markers have been used as an indicator of hair inductiveness, many of which are also associated with aggregative behavior. An important example is versican. During anagen, versican is expressed in DP in human hair follicles;[5] furthermore, versican expression can be found in anagen hair follicles of mouse, but the expression is absent in telogen hair follicles.[6] Thus, versican contributes to the induction and maintenance of anagen.[6] Versican expression is lost after passaging DP-derived primary cell cultures in mice. Versican-expressing pelage DP cells have the ability to induce hair follicles after primary culture if the concentration of versican-positive cells can be enriched by flow cytometry, suggesting that the versican expression correlates with hair inductivity.[7] Versican expression in human dermal cells is induced by ascorbic acid 2-phosphate, which can also enhance hair follicle initiation and growth.[6] In contrast, in the DP of vellus-like hair follicles, expression of versican was diminished in androgenetic alopecia.[5]
Four different isoforms of versican are known, generated by alternative mRNA splicing.[8,9] In the present study, we investigated the expression of the four versican isoforms and several other DP markers in ovine cell aggregates. We compared ovine cells at different stages of aggregation (subconfluent, early aggregates, and late aggregates) using polymerase chain reaction (PCR) to quantify mRNA abundance and to gain a better understanding of the upregulation or downregulation of markers with aggregation. We also used immunoflurescence and histochemistry to observe the expression of these markers in monolayer cells compared to neighboring aggregates in the same culture.
SUBJECTS AND METHODS
Ovine dermal papilla cells, specimen, and culture conditions
Ovine DP cell cultures were initiated in an earlier study.[4] First, the hair follicle was separated from other tissues with a needle and forceps. Then, a needle was used to make a cut through approximately three-quarters of the follicle, just above the DP. The bulb was pushed to invert the end-bulb through this cut and expose the DP. The basal stalk was cut to isolate the DP. Finally, the DP was transferred to the culture dish. Three lines of ovine DP cells were compared for all experiments (designated “DPC-9,” “DPC-13,” and “DPC-15”), each isolated from a different sheep. Frozen stocks of cells were thawed and grown in minimum essential medium (MEM), 20% (v/v) lamb serum (LS) supplemented with 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 12.5 ng/ml amphotericin B, (components from Invitrogen, Carlsbad, CA, and Sigma, St. Louis, MO, USA). After reaching 80–100% confluency, the cells were passaged and reseeded at approximately 50,000–100,000 cells/cm2. For all experiments, cells were used between passage six and ten. For histochemistry and immunofluorescence, cells were seeded onto glass coverslips coated with rat collagen I (Sigma, St. Louis, USA) in 6-well plates or in 4-well glass chamber slides (Nunc, Rochester, NY, USA), also coated with collagen. For RNA extraction, cells were seeded into 100 mm culture dishes for the subconfluent state and 60 mm culture dishes for the early and late aggregation states.
Histochemistry for alkaline phosphatase
Medium was removed, the cells were washed in phosphate-buffered saline (PBS), and then fixed in acetone at −20°C for 10 min followed by three ice-cold PBS brief washes. Subsequent three NTMT (100 mM NaCl/100 mM tris-Cl (pH 9.5)/50 mM MgCl2/l % (v/v) tween-20) washes were performed for 5 min each, and then the alkaline phosphatase substrate, 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate 4-toluidine (NBT/BCIP, Roche. Manheim, Germany) was added for an overnight incubation at room temperature. Then, the cells were briefly washed with NTMT before mounting in fluorescence-mounting medium and photographing.
Immunofluorescence
Several markers were used as indicators of hair inductiveness in this study [Table 1]. Their function is described in more detail in the discussion. For immunofluorescence, the medium was removed, the cells were washed in PBS, fixed with ice-cold acetone for 10 min, and blocked in 1% (w/v) bovine serum albumin (BSA) in PBS/0.2% tween-20 (PBST) for 2 h. Then, the specimen was briefly washed in PBS three times before incubating with primary antibody diluted in 1% BSA/PBST at 4°C overnight. The primary antibodies used were mouse versican antibody (1:2000 dilution, DSHB, clone 12C5), goat vimentin antibody (1:50 dilution, Abcam, ab11256), mouse α-smooth muscle actin (SMA) (1:450 dilution, Santa Cruz, sc-32251), goat delta antibody (1:450 dilution, Santa Cruz, sc-12531), goat fibroblast growth factor (FGF)-7 antibody (1:200 dilution, R and R Systems, AF-251-NA), rabbit bone morphogenetic protein (BMP)-2 antibody (1:400 dilution, Bioss, bs-1012R), rabbit corin antibody (1:400 dilution, Bioss, bs-7685R), rabbit fibronectin antibody (1:400 dilution, ThermoFisher Scientific, PAI-23693), rabbit secreted frizzled-related protein (SFRP)-2 antibody (1:450 dilution, Novus Biologicals, NPBI-56609), rabbit transforming growth factor (TGF)-β2 antibody (1:400 dilution, Bioss, bs-4909R), goat tenascin (1:400 dilution, Santa Cruz, sc-54348), goat alkaline phosphatase (1:450 dilution, Santa Cruz, sc-15065), and rabbit prominin-1 antibody (1:500 dilution, Abnova, PAB12663). The next step was to wash the cells in PBS three times for 5 min each. The cells were protected from light as much as possible while incubating the specimen with the secondary antibody for 2 h, followed by three PBS washes for 5 min each. Secondary antibodies were anti-mouse IgG1 (1:200 dilution, Life Technologies, A-21121) for versican, anti-goat IgG (1:200 dilution, Life Technologies, A-11058) for vimentin, delta, FGF-7, alkaline phosphatase, and tenascin, anti-mouse IgG (1:200 dilution, Life Technologies, A-21203) for α-SMA, and anti-rabbit IgG (1:200 dilution, Life Technologies, A-21206) for SFRP-2, corin, BMP-2, TGF-β2, fibronectin, and prominin-1. DAPI (0.02 μg/ml) was added for 10 min, then the specimen was washed again in PBS three times for 5 min each and mounted in fluorescence-mounting medium before photographs were taken.
Table 1.
The dermal papilla markers used

Polymerase chain reaction analysis
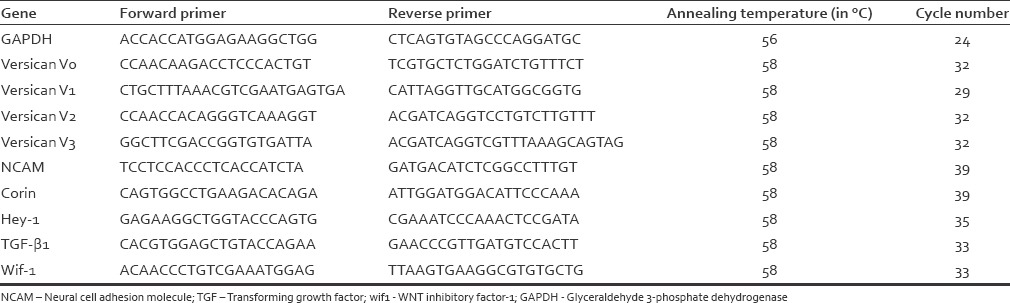
Reverse transcription (RT)-PCR analysis was performed to evaluate several markers [Table 1], similar to a study by Higgins et al.[12] Cells were briefly washed three times with PBS. RNA extraction and purification was performed using an RNeasy Micro Kit (Qiagen). RNA was quantified by measuring optical absorbance at 260 nm, and 400 ng was reverse transcribed with an Omniscript RT Kit (Qiagen) or a ProtoScript II First Strand cDNA Synthesis Kit (New England Biolabs), according to the manufacturers’ instructions. Then, PCR amplification (95°C for 3 min; 25–37 cycles of 95°C for 30 s, 56° or 58°C for 30 s, 68°C for 80 s; and 68°C for 5 min) was performed with primers as shown in Table 2. Completed reactions (10 μl) were electrophoresed in 1.5% agarose gels and stained with ethidium bromide or SYBR safe (Thermo Fisher Scientific) to visualize the results. Each PCR was repeated once for first culture and two times for second culture.
Table 2.
Polymerase chain reaction primers and amplification conditions
Quantification and statistical analysis
Electrophoresis gels were photographed using a Doc-Print VX2 camera. The density of PCR product bands was quantified using Bioprofil Photo-Capt software (Vilber Lourmat, Australia). Values for each DP marker were divided by the corresponding value for the housekeeping gene, GAPDH, to normalize RNA input. Values from replicate electrophoresis gels were then standardized to a mean of 1.0 to compensate for differences in photographic exposure. Thus, the values from replicate experiments could be plotted on a common scale to illustrate the relative changes in marker abundance with aggregation state and the reproducibility of marker expression patterns. Statistical analysis was done in Microsoft Excel. Nonparametric tests were applied because the number of samples was small. Wilcoxon signed rank test was used to analyze the three different ovine strains.
RESULTS
Localization of marker expression
Ovine DP cells spontaneously formed dense, often spherical, aggregates as previously described.[4] Aggregates were typically 100–300 μm in diameter [Figures 1–3]. The localization of 13 DP markers was evaluated by immunofluorescence. All localization experiments were repeated three times with three different strains of ovine DP cells. Similar results were obtained for all replicates.
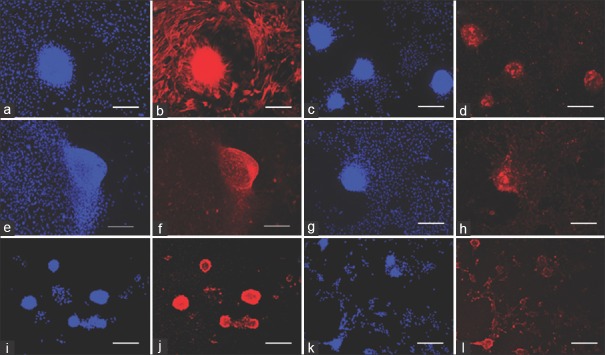
Figure 1.
Marker labeling of ovine dermal papilla cells. (a and b): actin. (c and d): delta. (e and f): vimentin. (g and h): Fibroblast growth factor-7. (i and j): alkaline phosphatase, (k and l): tenascin. DAPI: blue, antigen of interest: red. Scale bar = 200 μm
Figure 3.
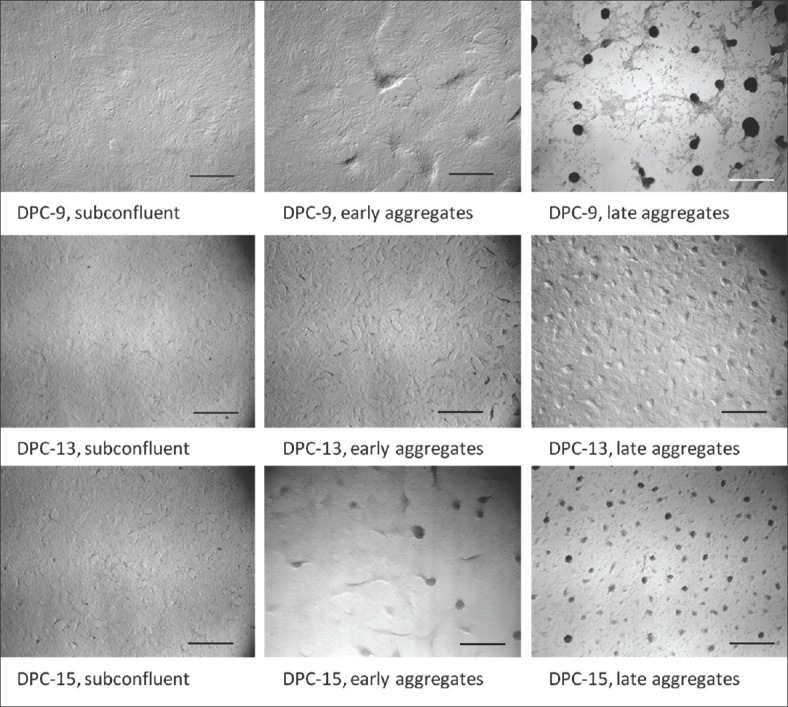
Phase contrast photomicrographs showing the aggregation state of ovine dermal papilla cells immediately before RNA extraction for polymerase chain reaction. Scale bar = 1000 μm
From all the DP cell markers evaluated, the expression of versican, BMP-2, prominin-1, and SFRP-2 was specific for aggregates [Figure 2]. A weaker BMP-2 signal was seen in small clusters of cells that represent early aggregates, but not in scattered monolayer cells [Figure 2c and d]. Alkaline phosphatase, corin, tenascin, FGF-7, α-SMA, delta, fibronectin, and TGF-β2 were expressed in both monolayer cells and aggregates [Figures 1 and 2], albeit to different degrees. The intensity of the color was higher in the aggregates. The greater color intensity in the aggregates is likely to be at least partly due to greater cell density. Vimentin is a major component of the cytoskeleton of mesenchymal cells.[21] Its expression level per cell would not be expected to change with aggregation state, and so it was used as a control for the effect of cell density on color intensity [Figure 1e and f].
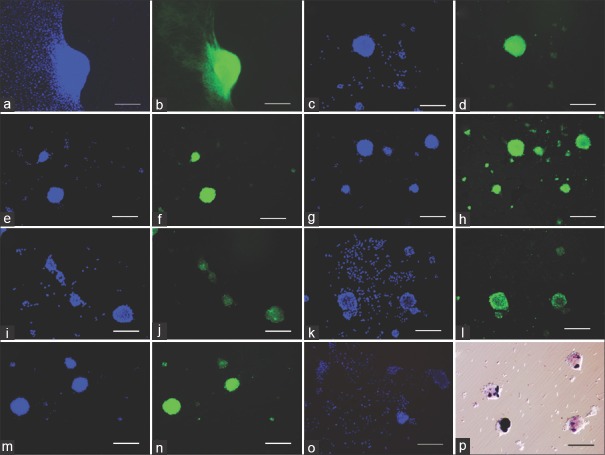
Figure 2.
Marker labeling of ovine dermal papilla cells. (a and b): versican. (c and d): Bone morphogenetic protein-2. (e and f): corin. (g and h): fibronectin. (i and j): prominin-1, (k and l) secreted frizzled-related protein-2, (m and n) transforming growth factor-β2, (o) negative control for anti-goat secondary antibody, (p) alkaline phosphatase. (a-o) Immunofluorescence, DAPI: blue, antigen of interest: green. (p) histochemistry, phase contrast image. Scale bar = 200 μm
The expression of alkaline phosphatase was examined with histochemical staining [Figure 2p] as well as immunofluorescence [Figure 2i and j]. Expression appeared less extensive when evaluated by histochemical staining. It detects alkaline phosphatase enzymatic activity, whereas the immunofluorescence stain detects protein antigens. Variations of staining in both monolayer cells and aggregates were observed. There were aggregates which were not stained. On the other hand, few monolayer cells expressed alkaline phosphatase enzymatic activity [Figure 2p].
Regulation of markers during aggregation
The regulation of marker expression during the formation of aggregates was characterized by using PCR to evaluate mRNA abundance. Three different stages of aggregation were compared, namely, subconfluent cells, early aggregates, and late aggregates [Figure 3]. For subconfluent cells, RNA was extracted 1 day after the cells were seeded, before aggregation had begun. For early aggregates, RNA was extracted after one to 2 days, when the cells had reached confluence and were beginning to cluster, forming swirl or wave patterns. For late aggregates, the cultures were continued until the cells had formed numerous fully-developed spheroid aggregates, 5–7 days after seeding.
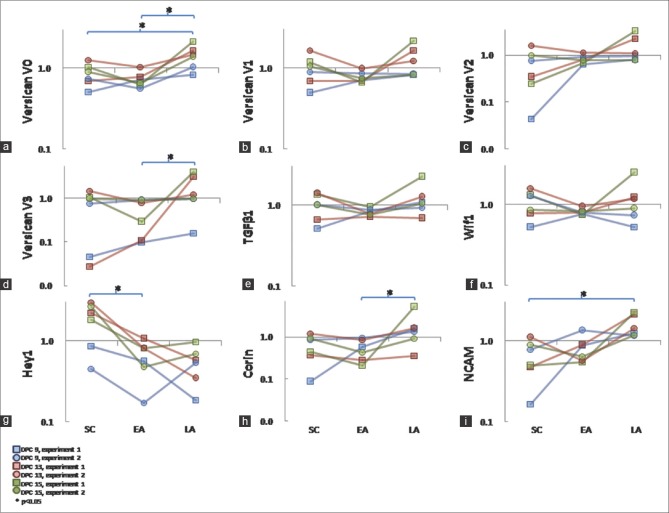
PCR analysis allowed the expression of the four versican isoforms to be evaluated individually. The V0 and V1 isoforms were more abundantly expressed [Figure 4]. Expression of corin, neural cell adhesion molecule (NCAM), TGF-β1, wif1, and hey-1 was also evaluated. Expression of all markers could be detected by PCR in all the three aggregation states. To quantify expression, the cultures were repeated twice for each of the three different strains of ovine DP cells, and the mean expression level from six PCR repeats was calculated. Versican V0, V3, corin, and NCAM showed statistically significant increases in expression with increasing aggregation [Figure 5a, d, h, and i]. In contrast, hey-1 showed a significant decrease [Figure 5g]. The expression of versican V1, V2, TGF-β1, and wif1 did not change [Figure 5b, c, e, and f].
Figure 4.
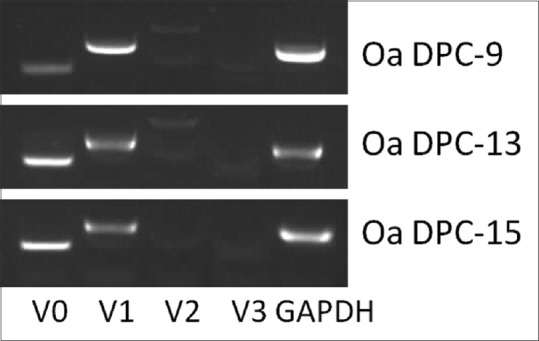
Reverse transcription-polymerase chain reaction analysis of versican isoforms in three strains of cultured dermal papilla cells at the late aggregate stage. The housekeeping gene GAPDH was included as a positive control. Product length: V0 230 bp, V1, 395 bp, V2 627 bp, V3 186 bp, GAPDH 367 bp
Figure 5.
RNA abundance evaluated by polymerase chain reaction. Three strains of ovine dermal papilla cells (red, green, and blue lines) were compared in duplicate (circles, squares) at different stages of aggregation (subconfluent, SC; early aggregates, EA; late aggregates, LA). Expression was normalized to GAPDH and it is presented on a log-scale. Values within each duplicate were standardized to a mean of 1.0, allowing the duplicates to be plotted on a common scale. Thus, these data represent the relative changes in marker abundance with aggregation state. (a): versican V0. (b): versican V1. (c): versican V2. (d): versican V3. (e): Transforming growth factor-β1. (f): wif-1. (g): Neural cell adhesion molecule. (h): corin, (i): hey1. Asterisks denote statistically significant differences at P < 0.05
DISCUSSION
We evaluated the expression of 16 DP markers in ovine cells to compare their phenotype with papillae in vivo and cells from other species. Versican is a well-established DP cell marker and it is involved in the regulation of cell adhesion, migration, proliferation, and differentiation.[22,23,24] Versican protein expression is associated with the hair growth cycle.[5,25] Previous research found that hair regeneration ability was lost in mouse DP cells that did not express versican.[7] Decreased versican expression is correlated with increased passage number of cultured human DP cells and the reduction of both aggregative ability in vitro and hair induction in vivo.[26] In three strains of ovine DP cells, this study confirmed that versican expression is specific for aggregates, and it is not expressed in the cells which were still in a monolayer state[4] [Figure 2].
Versican is a chondroitin sulfate proteoglycan predominantly found in the ECM. Four different isoforms of versican are known, generated by alternative mRNA splicing. Isoforms differ in their chondroitin sulfate-binding domains. The V0 isoform includes both the chondroitin sulfate α and β domains, V1 contains only the β domain, V2 contains only the α, and V3 contains neither domain.[8,9] Each isoform has different biological functions in cell apoptosis and proliferation, and its expression is also regulated differently. For example, in fibroblasts, V1 upregulated proliferation and contributed to protection against cell apoptosis, whereas V2 inhibited cell proliferation.[27] We quantified the expression of the V0, V1, V2, and V3 isoforms in three strains of ovine cells at different stages of aggregation: subconfluent, early aggregates, and late aggregates. V0 and V3 increased significantly with aggregation, while V1 and V2 did not change [Figure 5]. Thus, V0 and V3 are more likely to be directly involved in the mechanism of aggregation. However, V1 and V2 might still be required for the ability to aggregate. A previous study by Soma et al.[5] revealed that the expression of V0 and V1 was prominent in vitro, while V2 and V3 were mainly expressed in vivo in human hair follicles. This change in isoform expression might be related to the loss of hair induction in cultured human DP cells.[5] We did not evaluate versican isoform expression in vivo in sheep. However, based on the stability of hair induction in ovine cells,[4] one might expect that they would change less. Taking our results and those of Soma et al.[5] together, V3 appears to be the versican isoform, most likely to be involved in DP cell aggregation and hair induction.
Aside from versican V0 and V3, only corin and NCAM showed statistically significant increases in mRNA expression with an increased aggregation. There was some expression of corin in monolayered cells and aggregates. Protein expression of SFRP-2, BMP-2, and prominin-1 was confined to aggregates. Histochemical staining for alkaline phosphatase was confined to aggregates whereas immunohistochemical staining was somewhat more extensive. These markers, in addition to versican V0 and V3, are most likely to be directly involved in the mechanism of aggregation.
The expression patterns of these markers are also consistent with the previous studies in other species. Corin is a type II transmembrane protein that interacts with Wnt proteins.[28] Expression is restricted to mouse DP cells during pelage follicle morphogenesis. Another study showed that human corin expression was higher in vivo and in aggregates compared to monolayer cells.[29] NCAM regulates the motility of cells[30] and has been observed in DP cells of the lanugo hair follicle.[31,32] Previous studies of NCAM expression in human DP cells have given contradictory results. Higgins et al.[29] found that expression was higher in monolayered DP cells compared to aggregates and papillae in vivo,[29] while Miao et al.[32] found that it was upregulated in high-passage human DP cell spheroids grown in three-dimensional (3D) matrigel cultures compared to two-dimensional cultures.[32] One difference between these studies was that Miao et al. grew cells on a solid substrate (matrigel-coated plates) to promote aggregation, whereas Higgins et al. grew cells in suspended hanging drops of the medium. In our study, ovine DP cells aggregated on collagen-uncoated plates, and our results were more consistent with those of Miao et al. Thus, the culture environment appears to affect NCAM expression, as well as aggregation status per se.
SFRP-2 functions to increase mesenchymal stem cell survival, inhibit differentiation, increase proliferation, and decrease apoptosis.[33,34] A previous study showed that the expression of SFRP-2 could only be found in human DP cell aggregates, but not in two-dimensional cultures of the same cells.[12] BMP-2 expression was specific for intact human DP, and the expression was restored with 3D culture of DP cells.[12] In this study, only aggregates of ovine DP cells expressed this gene. Hence, it was possible that the ovine DP cells did not lose their aggregative behavior partly because they still expressed this gene in later passages. BMP-2 can upregulate the Id (inhibitor of differentiation) of gene expression and has an effect on cell–matrix interactions.[35] Prominin-1 is a plasma membrane protein and it is specifically localized to plasma membrane protrusions, membrane-to-membrane interactions, and lipid composition (cholesterol). It is expressed in neuroepithelial and hematopoietic stem cells.[36] Mice DP that expressed prominin-1 could regrow new hair compared to prominin-1 (-) cells.[37] A previous study also found that the expression of prominin-1 in human did not change with spheroid cultures.[12] The expression of prominin-1 was found only in the aggregates. Alkaline phosphatase expression is seen in DP in vivo and it is regulated during the murine hair cycle.[38,39,40] Mouse DP cells which express alkaline phosphatase can be cultured and then implanted to grow new hair whereas alkaline phosphatase-negative CTS cells do not have this hair inductivity property.[41] Thus, the association of corin, NCAM, SFRP-2, BMP-2, prominin-1, and alkaline phosphatase with ovine DP cell aggregation is consistent with the stable hair-inductive phenotype of these cells.[12,29,32,37,41,42]
Unlike the other markers, expression of hey-1 in ovine DP cells was significantly decreased with aggregation. This result contrasts with human DP cells, in which Higgins et al. found that hey-1 expression was restored in the cultures of 3D spheres compared to monolayer cells.[12] However, there were also differences in the culture conditions used in these studies. Higgins et al. grew human DP cells in suspended hanging drops of DMEM supplemented with 10% (v/v) FCS. We grew ovine DP cells in MEM with 20% (v/v) LS, attached to a polystyrene substrate. It is not clear whether the differences in hey-1 expression reflect the species of DP cells, the culture conditions, or both. Nevertheless, hey-1 is the downstream of the Notch signaling pathway, which is active in the dermal condensates of developing follicles, but it is downregulated in mature DP.[18,43,44] The reduced hey-1 expression in mature ovine DP cell aggregates is, therefore, consistent with this in vivo expression pattern. It could be concluded that the hey-1 is needed in the development of dermal condensates in vivo, is downregulated in mature DP, is needed in cultured DP cells to regain their hair inductive properties, but is also downregulated in aggregates in vitro as they mature and become more similar to DP in vivo.
We did not find evidence that the remaining markers show changes in expression in association with ovine DP cell aggregation. Expression of these markers persisted regardless of the aggregation state. As for the versican V1 and V2 isoforms, these markers might still be required for the ability to aggregate. In other species, expression of these markers is often lost in DP cells that have lost their hair-inducing ability or their aggregative behavior. The expression of FGF-7 in human DP cells was lost in two-dimensional culture, but was restored with 3D culture.[12] Expression of α-SMA was increased in 3D matrigel cultures compared to dissociated human DP cells.[32] It is notable that these markers were expressed stably in ovine cells, but lost in other species.
CONCLUSION
Overall, the stable and robust expression of numerous papilla markers in ovine cells may help to explain why aggregation and hair induction appear to be more stable in DP cells from sheep compared to other species. Further comparison of ovine with human papilla cells might provide opportunities to stabilize the hair-inducing activity of human cells, and so enhance their therapeutic potential. For example, growth factors and other signaling molecules secreted by ovine DP cells are likely one mechanism that promotes their aggregation.[45,46] Once identified, such signaling molecules could be used as culture additives to stabilize human DP cell aggregation.
Financial support and sponsorship
This work was funded by the Epworth Hospital Dermatology Department. The first author was funded by Australia Awards Scholarship.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The first author was supported by an Australia Awards Scholarship. Our thanks to Associate Prof. Robert Kapsa et al., in whose Lab at St. Vincent Hospital, Melbourne, the majority of these experiments were done.
REFERENCES
- 1.Philpott M, Paus R. Principles of hair follicle morphogenesis. In: Chuong CM, editor. Molecular basis of epithelial appendage morphogenesis. Austin, Texas, USA: R.G. Landers Company; 1998. pp. 75–110. [Google Scholar]
- 2.Jahoda C, Oliver RF. The growth of vibrissa dermal papilla cells in vitro. Br J Dermatol. 1981;105:623–7. doi: 10.1111/j.1365-2133.1981.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 3.Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–2. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 4.Rufaut NW, Nixon AJ, Goldthorpe NT, Wallace OA, Pearson AJ, Sinclair RD. An in vitro model for the morphogenesis of hair follicle dermal papillae. J Invest Dermatol. 2013;133:2085–8. doi: 10.1038/jid.2013.132. [DOI] [PubMed] [Google Scholar]
- 5.Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J Dermatol Sci. 2005;39:147–54. doi: 10.1016/j.jdermsci.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Kim SR, Cha SY, Kim MK, Kim JC, Sung YK. Induction of versican by ascorbic acid 2-phosphate in dermal papilla cells. J Dermatol Sci. 2006;43:60–2. doi: 10.1016/j.jdermsci.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc Natl Acad Sci U S A. 1999;96:7336–41. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemire JM, Braun KR, Maurel P, Kaplan ED, Schwartz SM, Wight TN. Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1630–9. doi: 10.1161/01.atv.19.7.1630. [DOI] [PubMed] [Google Scholar]
- 9.Ito K, Shinomura T, Zako M, Ujita M, Kimata K. Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J Biol Chem. 1995;270:958–65. doi: 10.1074/jbc.270.2.958. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Yoon J, Shin SH, Zahoor M, Kim HJ, Park PJ, et al. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One. 2012;7:e34152. doi: 10.1371/journal.pone.0034152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahoda CA, Reynolds AJ, Chaponnier C, Forester JC, Gabbiani G. Smooth muscle alpha-actin is a marker for hair follicle dermis in vivo and in vitro. J Cell Sci. 1991;99(Pt 3):627–36. doi: 10.1242/jcs.99.3.627. [DOI] [PubMed] [Google Scholar]
- 12.Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A. 2013;110:19679–88. doi: 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama M, Amagai M, Smith LT, Hashimoto K, Shimizu H, Nishikawa T. Epimorphin expression during human foetal hair follicle development. Br J Dermatol. 1999;141:447–52. doi: 10.1046/j.1365-2133.1999.03037.x. [DOI] [PubMed] [Google Scholar]
- 14.Havlickova B, Bíró T, Mescalchin A, Tschirschmann M, Mollenkopf H, Bettermann A, et al. A human folliculoid microsphere assay for exploring epithelial- mesenchymal interactions in the human hair follicle. J Invest Dermatol. 2009;129:972–83. doi: 10.1038/jid.2008.315. [DOI] [PubMed] [Google Scholar]
- 15.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–23. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–57. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue K, Aoi N, Yamauchi Y, Sato T, Suga H, Eto H, et al. TGF-beta is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J Cell Mol Med. 2009;13:4643–56. doi: 10.1111/j.1582-4934.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell BC, Passmore EA, Nesci A, Dunn SM. The Notch signalling pathway in hair growth. Mech Dev. 1998;78:189–92. doi: 10.1016/s0925-4773(98)00177-4. [DOI] [PubMed] [Google Scholar]
- 19.Couchman JR, Gibson WT. Expression of basement membrane components through morphological changes in the hair growth cycle. Dev Biol. 1985;108:290–8. doi: 10.1016/0012-1606(85)90033-8. [DOI] [PubMed] [Google Scholar]
- 20.Bin S, Li HD, Xu YB, Qi SH, Li TZ, Liu XS, et al. BMP-7 attenuates TGF-ß1-induced fibroblast-like differentiation of rat dermal papilla cells. Wound Repair Regen. 2013;21:275–81. doi: 10.1111/wrr.12015. [DOI] [PubMed] [Google Scholar]
- 21.Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–59. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–8. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- 24.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343–54. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 25.du Cros DL, LeBaron RG, Couchman JR. Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol. 1995;105:426–31. doi: 10.1111/1523-1747.ep12321131. [DOI] [PubMed] [Google Scholar]
- 26.Feng M, Yang G, Wu J. Versican targeting by RNA interference suppresses aggregative growth of dermal papilla cells. Clin Exp Dermatol. 2011;36:77–84. doi: 10.1111/j.1365-2230.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- 27.Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, et al. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–40. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–35. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 29.Higgins CA, Richardson GD, Ferdinando D, Westgate GE, Jahoda CA. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp Dermatol. 2010;19:546–8. doi: 10.1111/j.1600-0625.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- 30.Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, et al. NCAM regulates cell motility. J Cell Sci. 2002;115(Pt 2):283–92. doi: 10.1242/jcs.115.2.283. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan ED, Holbrook KA. Dynamic expression patterns of tenascin, proteoglycans, and cell adhesion molecules during human hair follicle morphogenesis. Dev Dyn. 1994;199:141–55. doi: 10.1002/aja.1001990207. [DOI] [PubMed] [Google Scholar]
- 32.Miao Y, Sun YB, Liu BC, Jiang JD, Hu ZQ. Controllable production of transplantable adult human high-passage dermal papilla spheroids using 3D matrigel culture. Tissue Eng Part A. 2014;20:2329–38. doi: 10.1089/ten.tea.2013.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfaro MP, Vincent A, Saraswati S, Thorne CA, Hong CC, Lee E, et al. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem. 2010;285:35645–53. doi: 10.1074/jbc.M110.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 36.Corbeil D, Röper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–60. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 38.Hardy MH. The histochemistry of hair follicles in the mouse. Am J Anat. 1952;90:285–337. doi: 10.1002/aja.1000900302. [DOI] [PubMed] [Google Scholar]
- 39.Handjiski BK, Eichmüller S, Hofmann U, Czarnetzki BM, Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994;131:303–10. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- 40.Chase HB, Rauch R, Smith VW. Critical stages of hair development and pigmentation in the mouse. Physiol Zool. 1951;24:1–8. doi: 10.1086/physzool.24.1.30152098. [DOI] [PubMed] [Google Scholar]
- 41.McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–75. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 42.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–25. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopan R, Weintraub H. Mouse notch: expression in hair follicles correlates with cell fate determination. J Cell Biol. 1993;121:631–41. doi: 10.1083/jcb.121.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon-Thomson C, Botto SA, Cam GR, Moore GP. Notch pathway gene expression and wool follicle cell fates. Aust J Exp Agric. 2008;48:648–56. [Google Scholar]
- 45.Rushan X, Fei H, Zhirong M, Yu-Zhang W. Identification of proteins involved in aggregation of human dermal papilla cells by proteomics. J Dermatol Sci. 2007;48:189–97. doi: 10.1016/j.jdermsci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Luo Y, Hao F, Zhong B, Mai Y, Jiang X. The biological activities of conditioned medium derived from human dermal papilla cells cultured in vitro. Chin J Dermatol. 2004;37:648–50. [Google Scholar]